Abstract
Background & Aims
IκB kinase-β (IKKβ) mediates activation of the nuclear factor-κB (NFκB), which regulates immune and inflammatory responses. Although NFκB is activated in cells from patients with inflammatory diseases or cancer, little is known about its roles in development and progression of esophageal diseases. We investigated whether mice that express an activated form of IKKβ in the esophageal epithelia develop esophageal disorders.
Methods
We generated ED-L2-Cre/Rosa26-IKK2caSFL mice, in which the ED-L2 promoter activates expression of Cre in the esophageal epithelia, leading to expression of a constitutively active form of IKKβ (IKKβca) in epithelial cells but not inflammatory cells or the surrounding stroma (IKKβca mice). Mice lacking the Cre transgene served as controls. Some mice were given intraperitoneal injections of neutralizing antibodies against granulocyte macrophage colony-stimulating factor (GMCSF) or tumor necrosis factor (TNF), or immunoglobulin G1 (control), starting at 1 month of age. Epithelial tissues were collected and analyzed by immunofluorescence, immunohistochemical, and quantitative real-time PCR assays. Transgenes were overexpressed from retroviral vectors in primary human keratinocytes.
Results
IKKβca mice developed esophagitis and had increased numbers of blood vessels in the esophageal stroma, compared with controls. Esophageal tissues from IKKβca mice had increased levels of GMCSF. Expression of IKKβca in primary human esophageal keratinocytes led to 11-fold overexpression of GMCSF and 200-fold overexpression of TNF. Incubation of human umbilical vein endothelial cells with conditioned media from these keratinocytes increased endothelial cell migration by 42% and promoted formation of capillary tubes; these effects were blocked by a neutralizing antibody against GMCSF. Injections of anti-GMCSF reduced angiogenesis and numbers of CD31+ blood vessels in esophageal tissues of IKKβca mice but did not alter the esophageal vasculature of control mice and did not alter recruitment of intraepithelial leukocytes to esophageal tissues of IKKβca mice. Injections of anti-TNF prevented development of esophagitis in IKKβca mice.
Conclusions
Constitutive activation of IKKβ in the esophageal epithelia of mice leads to inflammation and angiogenesis, mediated by TNF and GMCSF, respectively.
Keywords: microenvironment, immune regulation, transcription factor, gene regulation
Introduction
The mucosal integrity of the esophagus is regularly challenged by its continuous exposure to irritant factors such as refluxates of gastro-duodenal contents, alcohol, cigarette smoke, and hot beverages. Defective esophageal epithelial homeostasis has been linked to inflammation and injury and is implicated in the pathogenesis of esophageal diseases, resulting in significant morbidity, mortality, and health care expenditures1, 2. For example, esophageal cancer is the sixth most common cause of cancer-related death worldwide3, and most of these cancers arise from squamous mucosa4. Moreover, patients receiving irradiation for thoracic malignancies typically develop an acute esophagitis which can necessitate hospitalization or treatment interruption and eventually lead to stricture or ulceration5. Overall, a greater understanding of the mechanisms and consequences of esophageal inflammation and injury will lead to new therapeutic approaches for these diseases.
Dysregulation of signaling by the NFκB transcription factor family is associated with numerous inflammatory diseases and cancers, including reflux esophagitis6-10, and members of the NFκB family are pivotal for the maintenance of homeostasis by controlling diverse biological processes, such as inflammation, cell survival, and cell growth11. However, the direct consequences of NFκB activation on the esophageal microenvironment are not known, and the complex mechanisms by which NFκB signaling leads to the development and/or progression of esophageal diseases have yet to be determined.
The protein kinase IKKβ (IKK2), a member of the IκB kinase (IKK) complex, is responsible for activation of the NFκB signaling pathway12, 13. To date, most studies of epithelial IKKβ signaling have focused on processes intrinsic to epithelial cells, or linked to immune regulation. Yet epithelial inflammation has consequences outside of the epithelium, and our knowledge of the mechanisms by which epithelial IKKβ signaling modulates endothelial cells, immune cell recruitment, and other components of the stroma is still limited. Moreover, the effects of IKKβ activation on esophageal epithelial homeostasis have not been been studied in vivo.
Here, we have utilized mice with activation of IKKβ specifically in the epithelia of the esophagus to demonstrate that epithelial IKKβ activation leads to esophagitis and to changes in the stromal vasculature. Moreover, epithelial IKKβ activation stimulates secretion of pro-angiogenic granulocyte-macrophage colony-stimulating factor (GM-CSF) by esophageal keratinocytes, resulting in neovascularization, and GM-CSF blockade prevents the pro-angiogenic effects of activated NFκB signaling in vitro and in vivo. Thus, epithelial IKKβ signaling is an important regulator of esophageal stromal vasculature through GM-CSF and of inflammation through TNF.
Materials and Methods
Generation of ED-L2-Cre/Rosa26-IKK2caSFL Mice
All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Mutant mice were hemizygous for Rosa26-Stop-Floxed-Constitutively active IKK2 (Rosa26-IKK2caSFL, Jackson Laboratories, Bar Harbor, ME) and for the Cre transgene. All mice used for experiments were on a pure c57BL/6 background. For all experiments with ED-L2-Cre/Rosa26-IKK2caSFL (L2/IKKβca) mice, sex-matched littermate IKK2caSFL lacking the Cre transgene served as controls. Additional details are provided in the Supplementary Materials and Methods section.
Cell Culture and Treatment
Mouse and primary human esophageal keratinocytes and primary human esophageal fibroblasts were isolated as described14. Human umbilical vein endothelial cells were purchased from Lonza (Walkersville, MD). Human Embryonic Kidney 293T cells, Phoenix-Ampho, and Phoenix-Eco cells were purchased from ATCC (Manassas, VA). Cells were cultured, transfected or infected, and quantitation was performed as described in the Supplementary Materials and Methods section
Immunofluorescence, Immunohistochemistry, and Microvessel density
Immunofluorescence and immunohistochemistry were performed using standard protocols. For descriptions of protocols and antibodies used, see the Supplementary Materials and Methods section. Blood vessels were visualized by staining endothelial cells with CD31 antibody. Six high-power fields were identified on each slide, and the MVDs were calculated as the total area of vessels in a ×200 field relative to the total stromal area.
Western Blots
Western Blots were performed as described previously15. Additional details are provided in the Supplementary Materials and Methods section.
RNA Analyses
RNA was extracted using the GeneJET RNA purification kit (Thermo Scientific, Pittsburg, PA) following the manufacturer's instructions. Quantitative real-time polymerase chain reaction (PCR) analyses were performed as described15. Additional details are provided in the Supplementary Materials and Methods section.
Tube Formation Assay
Basement membrane extract (Trevigen, Gaithersburg, MD) was added to the wells of a 96-well plate and allowed to solidify at 37°C for 30 minutes. HUVEC (15,000 cells per well) were added in conditioned media from primary human esophageal keratinocytes, in the presence or absence of a neutralizing antibody against human GM-CSF (R&D systems). Cells were incubated at 37°C for 4h. The tubular networks were photographed in the wells using a Qimaging digital camera (Surrey, BC, Canada) attached to an inverted microscope (Leica DMIRB, Buffalo Grove, IL) with 10× objective.
Endothelial Cell Migration Assay
HUVEC (5×104 cells) were added into the top chamber of an 8μM FluoroBlok insert (Corning) in 300μl of serum-free EBM2 media (Lonza) in triplicate. The inserts were placed into the bottom chamber of a 24-well plate containing conditioned media from primary human esophageal keratinocytes. At 24h, cells that migrated through the pores of the membrane to the bottom chamber were stained with calcein 8 μg/ml (Molecular Probes, Eugene, OR) in PBS for 30 min at 37°C. The fluorescence of migrated cells was quantified using a fluorescence microplate reader (Biotek FL×800, Winnoski, VT) at 485 nm excitation and 530 nm emission. Data are expressed as number of cells that migrated through or invaded pores +/− SEM.
In vivo MP1-22E9 and XT3.11 treatment
Starting at 1 month of age, L2/IKKβca mice and littermate controls were given intraperitoneal injections of the GM-CSF neutralizing antibody MP1-22E9, the TNF neutralizing antibody XT3.11, IgG2a control (for MP1-22E9), or IgG1 control (for XT3.11) at doses of 800μg per mouse for MP1-22E9 or 500μg per mouse for XT3.11 (BioXCell, West Hanover, NH). Mice were treated every Monday and Thursday until they reached 1.5 month of age. The following numbers of matched littermate control and mutant mice were injected with neutralizing antibody or control: IgG2a, three pairs; MP1-22E9, three pairs. The following numbers of mutant mice were injected with neutralizing antibody or control: IgG1, four mice; and XT3.11, 4 mice.
Statistical Analyses
Results were expressed as mean ± standard error of the mean, with statistical significance of differences between experimental conditions established at 95%. Two-way analysis of variance (ANOVA) and Student t test were used to indicate the statistical difference between groups using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). Additional details are provided in the Supplementary Materials and Methods section.
Results
To delineate the role of epithelial NFκB/IKKβ signaling in the control of the esophageal microenvironment, we crossed ED-L2/Cre mice16 to mice in which a constitutive active form of IKKβ (IKKβca) and EGFP were inserted into the endogenous ROSA26 locus (IKK2caSFL; Jackson Laboratory)17 (Supplementary 1A). ED-L2/Cre mice express Cre recombinase under the control of the Epstein-Barr Virus (EBV) ED-L2 promoter, which drives transgenic expression specifically within the epithelia of the tongue, esophagus, and forestomach and the skin of the ventral neck15, 18. L2/Cre;ROSA26-IKKβca+/L mice (L2/IKKβca mice) had increased IKKβ signaling in esophageal epithelia, with increased IKKβ expression, and decreased expression of IκBα, a downstream target typically inhibited by IKKβ activation (Supplementary Figure 1B). Recombination efficiency within esophageal epithelia of 1 month-old L2/IKKβca mice was confirmed by immunofluorescence for EGFP (Supplementary Figure 1D). In contrast, no EGFP expression was observed in control mice (Supplementary Figure 1C).
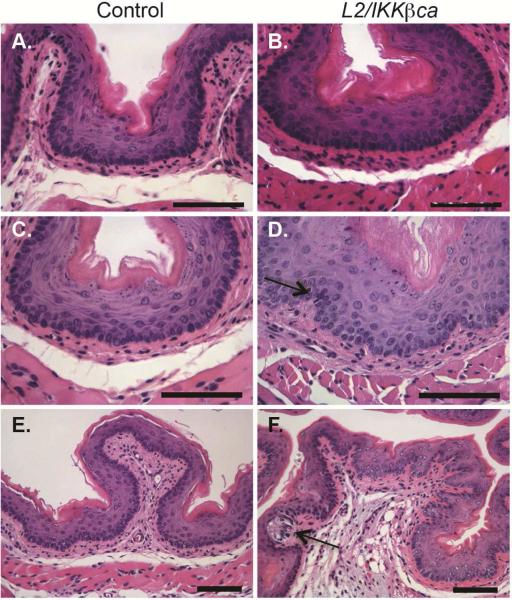
At 1 month of age, L2/IKKβca mice demonstrated mild hyperplasia (Figure 1B), compared to littermate controls (Figure 1A). By 1.5 month of age, compared to control mice (Figure 1C), esophageal mucosa of L2/IKKβca mice became irregular, with the elongation of lamina propria papilla, and the presence of an inflammatory infiltrate (Figure 1D). By 3 months of age, the stromal architecture of L2/IKKβca mice was further disrupted (Figure 1F), compared to controls (Figure 1E), with hydropic changes, indicative of cellular injury (Figure 1F, arrow). BrdU labeling showed increased proliferation in esophageal epithelia of L2/IKKβca mice at 4 weeks of age (Supplementary Figure 2A). Interestingly, TUNEL assay revealed a dramatic increase in apoptotic cells of L2/IKKβca mice (Supplementary Figure 2C) compared to littermate controls (Supplementary Figure 2B) only at 3 months of age, suggesting a response of the epithelium to the inflammation seen at 6 weeks of age or a compensatory response to the increased proliferation. We also observed hyperplasia of the tongue and ventral neck skin but not the forestomach of L2/IKKβca mice (Supplementary Figure 3), and L2/IKKβca mice developed crusted skin lesions and alopecia that necessitated euthanasia by 3 months of age. No lesions were observed in control mice.
Figure 1.
Epithelial specific IKKβ activation alters the esophageal microenvironment. (A-B) At 1 month of age, mice with increased epithelial IKKβ signaling had epithelial hyperplasia (B), compared to littermate controls (A). (n=5 mice per group) (C-D) At 1.5 month of age, control mice (C) demonstrated homogenous basal cells while basal layers of L2/IKKβca mice (D) were expanded and irregular. Moderate inflammatory infiltrates were also observed (arrow). (n=8 mice per group) (E-F). At 3 months of age, hydropic changes (arrow) and stromal remodeling were seen in the esophageal microenvironment of L2/IKKβca mice (F), compared to controls (E). (n=5 mice per group) Scale bars, 25 μm.
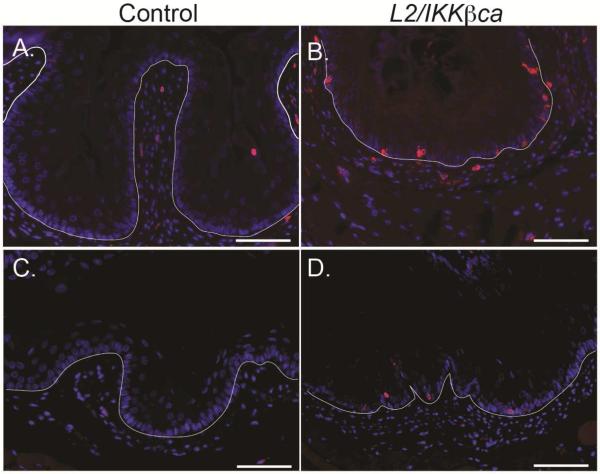
To confirm the presence of esophageal inflammation in L2/IKKβca mice, we stained esophageal mucosa of control and L2/IKKβca mice for the leukocyte marker CD45. Infiltrating leukocytes were observed in the epithelia and lamina propria of L2/IKKβca mice (Figure 2B/D), compared to controls (Figure 2A/C), at 6 weeks (Figure 2A-B) and 3 months (Figure 2C-D) of age. Using PCR arrays, we also observed statistically significantly changes in expression of 13 cytokines, including several key chemokines, in esophageal epithelia isolated from 4 week-old L2/IKKβca mice, compared to epithelia from littermate controls (Supplementary Table 1). Among these, interleukin-10, lymphotoxin A, tumor necrosis factor, interleukin 12B, interleukin 12A, Chemokine (C-X-C motif) ligand 5, granulocyte-macrophage stimulating factor, and thrombopoietin were upregulated in esophageal epithelia of L2/IKKβca mice, while bone morphogenetic protein 2, bone morphogenetic protein 7, macrophage migration inhibitory factor, bone morphogenetic protein 4, and cardiotrophin 1 were downregulated. To characterize the immune cell infiltrate observed in L2/IKKβca mice, we performed FACS analyses on spleen and esophagus from 6 week-old mice. We analyzed the leukocyte fraction (CD45+) for macrophages, natural killer cells (NK), dendritic cells (DC), myeloid-derived suppressor cells (MDSC), T-cells, T helper cells (Th), cytotoxic T cells (CTL), and regulatory T (Treg) cells (Supplementary Table 2). Among these, only MDSCs were significantly different between L2/IKKβca and control mice, when controlling for tissue type, with MDSCs increased by more 6-fold in esophageal epithelia and more than 9-fold in spleens of L2/IKKβca mice compared to controls. No significant changes were observed for macrophages, NK cells, DC, MDSC, T-cells, Th cells, CTL cells, and T-reg cells.
Figure 2.
L2/IKKβca mice have increased esophageal infiltration of immune cells. (A-D) Esophageal mucosa of control mice (A/C) contained few leukocytes, as indicated by staining for CD45, but multiple leukocytes were present in esophageal epithelia and lamina propria of L2/IKKβca mice (B/D) at 6 weeks of age (A-B) (n=8 mice per group) and 3 months of age (C-D) (n=5 mice per group). Scale bars: 25μm.
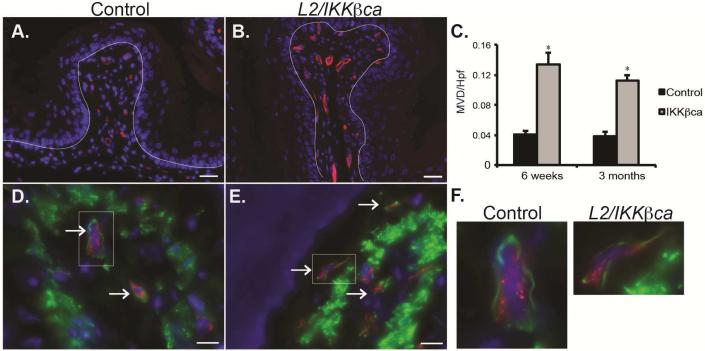
Initial histological studies of esophageal mucosa from L2/IKKβca mice suggested that these mice had alterations in their esophageal vasculature. To delineate the esophageal stromal vasculature of L2/IKKβca mice, we stained esophageal sections for the endothelial cell marker CD31 (Figure 3A-B). Compared to littermate controls, L2/IKKβca mice had a 3- to 4-fold increase in microvessel density (MVD) starting at 1.5 months of age (Figure 3C). Pericytes are mesenchymal-derived cells that support endothelial cell maturation and blood vessel integrity, and coverage of blood vessels by pericytes is reflective of a functional microvasculature19. By co-staining with CD31 for endothelial cells and desmin for pericytes (Figure 3D-F), we demonstrated that pericyte coverage was preserved in L2/IKKβca mice.
Figure 3.
L2/IKKβca mice have increased angiogenesis. (A-B) At 1.5 months of age, L2-IKKβca mice (B) had increased staining for the endothelial cell marker CD31 (red) compared to littermate controls (A). DAPI was used as a nuclear stain, and the dotted line was drawn to demonstrate the location of the basement membrane. (n=8 mice per group) Scale bars, 25 μm. (C) Quantification of microvessel density per high power field (MVD/hpf) revealed a statistically significant (*p<0.05, Student t test) increase in L2/IKKβca mice beginning at 1.5 months of age. (D-E) Co-staining for the endothelial cell marker CD31 (red) and the smooth muscle cell marker desmin (green) revealed no difference in pericyte coverage between 6 week-old control (D) and L2/IKKβca mice (E). (F) Left panel: Insert from picture in 3D, Right panel: Insert from picture in 3E. (n=4 mice per group).
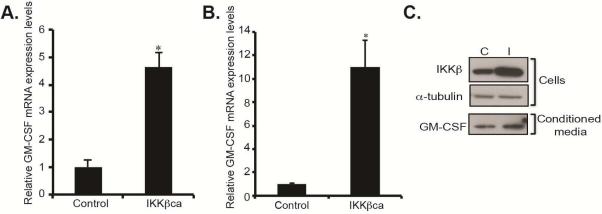
The formation of new vasculature shapes the microenvironment and has been detected early in the pre-malignant stages of cancer and in the context of inflammation and injury20. We hypothesized that pro-angiogenic factors were secreted by esophageal epithelial cells in response to IKKβ activation, leading to changes in the stromal vasculature. To identify these pro-angiogenic factors, we performed mouse cytokine/chemokine PCR arrays on esophageal epithelial peelings from L2/IKKβca mice at 1 month of age, before the onset of changes in the esophageal vasculature. Among the most highly induced pro-angiogenic factors was GM-CSF, which was increased by 4.6-fold in 1 month-old L2/IKKβca mice (Figure 4A). In contrast, expression of VEGF, a well-known inducer of angiogenesis, was not significantly changed (not shown). Immunohistochemistry also demonstrated increased GM-CSF in esophageal epithelia of 4 week-old L2/IKKβca mice (Supplementary Figure 4B) compared to littermate controls (Supplementary Figure 4A). To confirm that changes in GM-CSF mRNA expression were a direct effect of IKKβ activation, we infected primary human esophageal keratinocytes with IKKβca overexpressing retrovirus or control. Compared with control cells, IKKβca overexpressing primary human esophageal keratinocytes had an 11–fold induction in GM-CSF mRNA (Figure 4B). In addition, conditioned media from cultured primary human esophageal keratinocytes expressing IKKβca revealed increased secretion of GM-CSF, compared to controls (Figure 4C). Taken together, these data demonstrate that epithelial IKKβ promotes secretion of pro-angiogenic GM-CSF.
Figure 4.
Activation of epithelial IKKβ signaling leads to increased GM-CSF expression and secretion. (A) By quantitative real-time PCR, GM-CSF mRNA expression levels were induced 4.6-fold in esophageal epithelia of 4 week-old L2/IKKβca mice (n=3). *Significant difference from control at p-value of less than 0.01. (B) In immortalized primary human esophageal keratinocytes retrovirally transduced to express constitutively-active IKKβ, GM-CSF mRNA expression was increased by 11-fold, compared with esophageal keratinocytes with control plasmid (n=4). * Significant difference from control plasmid at p-value of less than 0.01, Student t test. (C) Primary human esophageal keratinocytes with increased IKKβ signaling had increased secretion of GM-CSF in their conditioned media, compared to control cells (n=4).
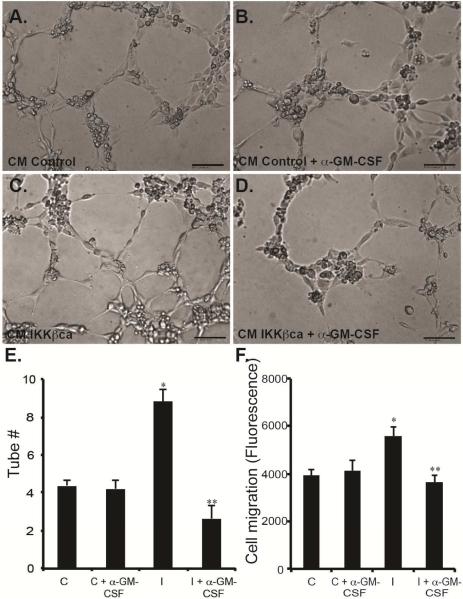
We next sought to determine whether increased angiogenesis with IKKβ activation was a direct result of GM-CSF secretion by esophageal epithelial cells. Treatment of HUVECs with conditioned media from primary human esophageal keratinocytes expressing IKKβca increased capillary tube formation (Figure 5C/E), compared to controls (Figure 5A/E). This increase in tube formation was abolished by treatment with GM-CSF blocking antibody (Figure 5D/E). Importantly, GM-CSF neutralization did not affect tube formation when the assay was performed in the presence of conditioned media from keratinocytes expressing empty vector control (Figure 5B/E). We next performed endothelial cell migration assays in the presence of conditioned media from IKKβca expressing keratinocytes. HUVEC migration was increased by 42% in the presence of conditioned media from esophageal keratinocytes with constitutively active IKKβ, compared to controls, and GM-CSF neutralizationblocked this increased cell migration (Figure 5F). GM-CSF blockade did not alter HUVEC cell migration treated with conditioned media from control esophageal keratinocytes (Figure 5F). Thus, increased epithelial IKKβ signaling promotes angiogenesis via GM-CSF.
Figure 5.
Epithelial-derived GM-CSF promotes angiogenesis in vitro. (A-D) In a tube formation assay, HUVEC cultured in the presence of conditioned media from human esophageal keratinocytes with constitutively-active IKKβ had increased ability to form tubes (C), compared to HUVEC treated with conditioned media from control esophageal keratinocytes (A). (B/D) Treatment with a blocking antibody against GM-CSF abolished the increased tube formation induced by the presence of conditioned media from esophageal keratinocytes with increased IKKβ signaling (D) but did not affect control cells (B). (n=3 in triplicates) (E) Quantification of the number of tubes per high power field in cells treated with control (C) and IKKβca (I) conditioned media, with and without GM-CSF blockade. (F) Transwell assays demonstrated that conditioned media from esophageal keratinocytes with constitutively-active IKKβ increased HUVEC cell migration, compared to control cells. GM-CSF blockade abolished the increased cell migration of HUVEC incubated in the presence of conditioned media from esophageal keratinocytes with active IKKβ. (n=3 in triplicates) *Significant difference from control plasmid at p-value of less than 0.01. ** Significant difference from IKKβca at p-value of less than 0.01, Student t test and ANOVA.
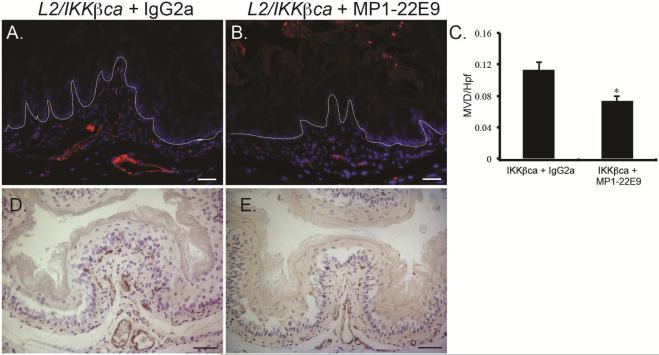
To determine if GM-CSF was also responsible for neovascularization in mice with increased IKKβ signaling, we treated L2/IKKβca mice and littermate controls with the GM-CSF blocking antibody MP1-22E9 or IgG2a control. Control mice treated with IgG or MP1-22E9 had no differences in the esophageal vasculature or homeostasis (not shown). In contrast, compared to L2/IKKβca mice treated with IgG2a (Figure 6A/D), L2/IKKβca mice with GM-CSF neutralization (Figure 6B/E) had a significant reduction in the number of CD31+ blood vessels (Figure 6C) and VWF positive endothelial cells. GM-CSF can induce angiogenesis either by a direct effect on endothelial cells or through the recruitment of macrophages that subsequently release pro-angiogenic factors 21-23. However, GM-CSF inhibition did not appear to alter recruitment of intraepithelial leukocytes, as we observed similar numbers of CD45+ cells in esophageal epithelia of L2/IKKβca mice treated with GM-CSF blocking antibody (Supplementary Figure 5B), compared to mutant mice treated with IgG2a control (Supplementary Figure 5A). Thus, IKKβ activation in keratinocytes induces GM-CSF secretion to triggers stromal neovascularization.
Figure 6.
GM-CSF inhibition decreases angiogenesis in L2/IKKβca mice. (A-E) 4 week-old L2/IKKβca mice and their littermate controls were treated with IgG2a control antibody or MP1-22E9 every Monday and Thursday for 2 weeks. (A-B) By immunofluorescence, CD31 positive blood vessels were decreased in L2/IKKβca mice treated with MP1-22E9 (B) compared to those treated with IgG2a (A). (n=3 mice per group) (C) Quantification of microvessel density per high power field (MVD/hpf) in mice treated with MP1-22E9 or IgG2a. *Significant difference from L2/IKKβca mice treated with IgG2a at p-value of less than 0.01, Student t test. (D-E) Immunohistochemistry revealed decreased numbers of VWF positive endothelial cells in L2/IKKβca mice treated with MP1-22E9 (E), compared to those treated with IgG2a (D). (n=3 mice per group) Scale bars, 25 μm (A-B), 50 μm (D-E).
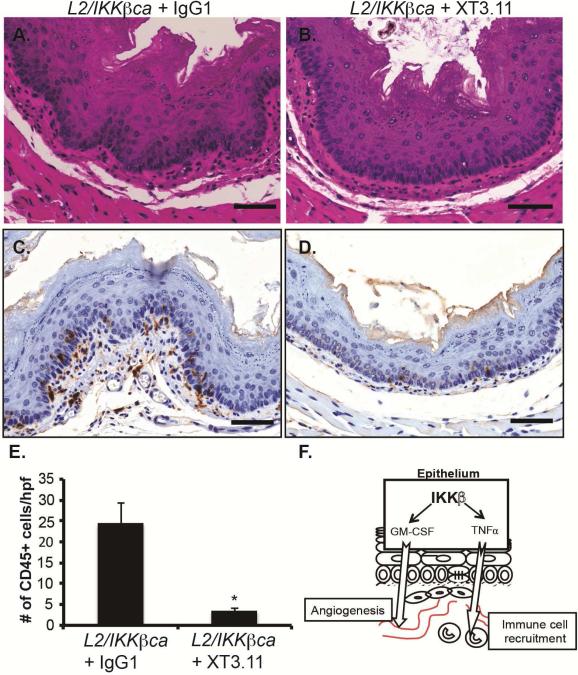
We next sought to determine whether other cytokines identified in our PCR array were responsible for the inflammatory response and immune cell infiltration in L2/IKKβca mice. Among the cytokines we identified as up-regulated in esophageal keratinocytes of mice with constitutively-active IKKβ was pro-inflammatory tumor necrosis factor (TNF), which was increased by 200-fold (Supplementary Table 1). Interestingly, TNF signaling drives accumulation of MDSCs24, 25, which were increased in L2/IKKβca mice. To determine if TNF was required for the inflammatory response and immune cell infiltration following IKKβ activation, we treated L2/IKKβca mice with the TNF neutralizing antibody XT3.11 or IgG1 control. L2/IKKβca mice treated with IgG1 had irregular basal layers (Figure 7A) compared to L2/IKKβca mice treated with XT3.11 (Figure 7B). Staining for the leukocyte marker CD45 showed decreased immune cell infiltration in the esophageal mucosa of L2/IKKβca mice treated with XT3.11 (Figure 7D-E) compared to L2/IKKβca mice treated with IgG1 (Figure 7C/E). Taken together, these data suggest that activation of epithelial IKKβ leads to inflammation and immune cell recruitment via TNF.
Figure 7.
TNF neutralization decreases immune infiltration and inflammation in L2/IKKβca mice. (A-D) 4 week-old L2/IKKβca mice and their littermate controls were treated with IgG1 control or XT3.11 every Monday and Thursday for 2 weeks. (A-B) Esophageal epithelia of L2/IKKβca mice treated with XT3.11 (B) had homogeneous basal cells and regular basal layers compared to L2/IKKβca mice treated with IgG1 control antibody (A). (n=4 mice per group) (C-D) By immunohistochemistry, CD45 positive leukocytes were decreased in L2/IKKβca mice treated with XT3.11 (D) compared to IgG1 control antibody (C). Scale bars: 50μm. (E) Quantification of the number of CD45 positive cells per high power field (# of CD45+ cells/hpf) in the esophageal mucosa of mice treated with IgG1 control or XT3.11. (n=4 mice per group) *Significant difference from L2/IKKβca mice treated with IgG1 control at p value of less than 0.01, Student t test. (F) A model for the role of epithelial IKKβ signaling in inflammation and angiogenesis within the esophagus.
Inflammation is implicated in the development of numerous malignancies26-28 including esophageal squamous cell cancer (ESCC), which often arises in the setting of chronic inflammation15, 29-32. Yet the implications of activated IKKβ/NFκB signaling, GM-CSF upregulation, and increased TNF for human esophageal squamous cell carcinogenesis are not well defined. Using human tissue microarrays (TMA), we examined IKKβ/NFκB, GM-CSF, TNF, angiogenesis, and inflammation in normal and inflamed human esophageal tissues as well as human ESCC. Consistent with important functions during esophageal squamous cell carcinogenesis, p65 NFκB, GM-CSF, TNF, CD45, and microvessel density (MVD) all increased progressively from normal to inflamed human esophageal tissues and were highest in ESCC (Supplementary Figures 6-11 and Supplementary Tables 3-5). Moreover, in human ESCC, p65 NFκB expression correlated with expression of the IKKβ/NFκB targets GM-CSF and TNF (Supplementary Table 6), and TNF levels correlated with CD45 expression. However, none of these individual markers was significantly associated with the histopathological or clinical characteristics of the ESCC patients (Supplementary Table 7).
Discussion
Esophageal homeostasis involves dynamic crosstalk between epithelial cells and underlying stromal cells. Yet, while alterations of the stromal microenvironment occur in esophageal diseases such as esophageal cancer and esophagitis, our knowledge of the molecular mechanisms that mediate changes in the microenvironment and that regulate epithelial-stromal interactions in the context of esophageal diseases is still very limited. Here, by activating IKKβ/NFκB signaling specifically in epithelial cells of the esophagus, we have interrogated the effects of epithelial inflammation on both esophageal epithelia and the stromal microenvironment. Moreover, this model has allowed us uniquely to define the consequences of esophageal inflammation in the early changes of esophageal diseases.
IKKβ activation in esophageal epithelia of mice causes esophagitis, with recruitment of intraepithelial leukocytes by 6 weeks of age and overt esophageal mucosal injury by 3 months of age. Interestingly, inflammation in L2/IKKβca mice develops in the absence of physical changes to increase acid or non-acid reflux. Moreover, this epithelial inflammation is sufficient to drive changes in not only the epithelia but also the surrounding stroma. Notably, increased levels of the pro-inflammatory cytokine TNF precede immune cell infiltration into the esophageal mucosa of L2/IKKβca mice, and TNFα blockade prevents immune cell accumulation in the esophageal mucosa following IKKβ activation. TNF signaling leads to accumulation of MDSCs24, 25, and MDSCs were increased by more than 6-fold in esophageal epithelia of L2/IKKβca mice. Since both TNFα and MDSCs are implicated in chronic inflammation and squamous cell carcinogenesis in mouse esophagus15, 29, we are currently investigating the role of TNF in MDSC recruitment following activation of epithelial IKKβ.
Our findings not only improve our understanding of the early stages of esophagitis but also provide important insights into the development of ESCC. IKKβ/NFκB signaling is elevated in patients with esophagitis, as well as those with esophageal squamous cell carcinoma33, 34. In fact, we demonstrated previously that the IKKβ/NFκB pathway is activated in a mouse that develops esophagitis and esophageal squamous cell carcinoma15. Moreover increased angiogenesis and GM-CSF expression are also observed in ESCC29, 35, and elevated levels of GM-CSF correlate directly with future development of ESCC in high-risk patients36. Here, we demonstrate that p65 NFκB, GM-CSF, and TNF, along with angiogenesis and inflammation increase step-wise during progression from normal esophagus to esophageal inflammation to ESCC. An important caveat is no single factor among those that we examined predicts the histopathological or clinical characteristics of human ESCC, suggesting that other factors contribute to tumor growth, spread, and patient survival. Future studies will be directed towards determining the contributions of IKKβ/NFκB, GM-CSF, and TNF to human ESCC growth and progression and to identifying other key factors, ideally through a large clinical trial allowing the collection of both tissue and biochemical samples. Constitutive epithelial IKKβ activation induces spontaneous tumor formation and accelerates tumor initiation in the intestine37, 38. Unfortunately, because of a severe skin phenotype, L2/IKKβca mice cannot be aged beyond 3 months. Thus, to define the consequences of epithelial IKKβ activation on esophageal carcinogenesis, we are administering chemical carcinogens such as nitroso-compounds to IKKβca mice. Nonetheless, our current study provides compelling evidence for a role of the IKKβ/NFκB pathway and its downstream targets GM-CSF and TNF in the development of esophagitis and ESCC.
Angiogenesis is essential for tissue repair in inflammatory diseases and wound healing, and insufficient vascularization is associated with chronic, non-healing wounds39. However, angiogenesis must be tightly regulated, since uncontrolled neovascularization promotes the development and progression of cancer20. Our studies here demonstrate that constitutive activation of IKKβ in esophageal epithelial cells leads to GM-CSF production, resulting in increased neovascularization. GM-CSF can play a pro-angiogenic role either through the recruitment of macrophages or by acting directly on endothelial cells21-23. Since neovascularization precedes recruitment of intraepithelial leukocytes in the esophagus of L2/IKKβca mice, our data suggest that GM-CSF secretion directly activates esophageal neovascularization. However, given the functions of GM-CSF in myeloid cell differentiation40, we cannot exclude that GM-CSF also regulates angiogenesis indirectly through myeloid cells.
Recombinant GM-CSF promotes wound healing and angiogenesis in multiple tissues and contexts41-44. Of note, GM-CSF improves esophageal mucosal healing and enhances blood vessel formation in patients with severe radiation esophagitis45. However, human studies of radiation-induced esophagitis are challenging, due to difficulties with biopsying the region and attendant risks for the patient. Since whole-body irradiation models are well established in mice, molecular mechanisms of angiogenesis can readily be investigated in L2/IKKβca mice with radiation-induced esophageal injuries.
Another implication of our data is the potential dissociation of IKKβ/NFκB pathway effects in the esophagus on angiogenesis and on inflammation and immune cell recruitment. Theoretically, therapeutic strategies in esophageal diseases could be tailored towards either GM-CSF or TNF in order to specifically target angiogenesis or inflammation. In the future, we plan to delineate the consequences of modulating other cytokines that we have identified to be differentially expressed in L2/IKKβca mice compared to controls, allowing us to define the mechanisms underlying esophagitis and ESCC.
In sum, we propose a model (Figure 7F) in which IKKβ activation in esophageal epithelium promotes esophagitis and angiogenesis, triggering an epithelial-mesenchymal signaling network mediated by cytokine secretion from esophageal epithelial cells. We identify GM-CSF as an important pro-angiogenic factor secreted by esophageal keratinocytes following IKKβ activation that leads directly to stromal neovascularization. Furthermore, we identify TNF as a key pro-inflammatory cytokine necessary for inflammation and immune cell infiltration following IKKβ activation. As such, we define IKKβ, GM-CSF and TNF as potential therapeutic targets for esophagitis and ESCC.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by: NIH NIDDK K99 DK094977, NIH NIDDK R00 DK094977, and a Zell Scholarship from the Robert H. Lurie Comprehensive Cancer Center to MPT; NIH NIDDK R01 DK069984 to JPK; the University of Pennsylvania Center for Molecular Studies in Digestive and Liver Diseases (NIH NIDDK P30 DK050306) through the Molecular Pathology and Imaging Core, the Molecular Biology/Gene Expression Core, the Cell Culture Core, and the Transgenic and Chimeric Mouse Core; NIH NCI P01 CA098101 (“Mechanisms of Esophageal Carcinogenesis”); and by the Robert H. Lurie Comprehensive Cancer Center (NIH NCI CCSG P30 CA060553) through the Northwestern University Mouse Histology and Phenotyping Core and the Northwestern University Center for Advanced Microscopy.
M.-P.T. designed and performed experiments, analyzed and interpreted data, obtained funding and wrote the manuscript; D.W. designed and performed experiments, analyzed and interpreted data; B.K.S. designed and performed experiments, analyzed and interpreted data; C.T-S. and T.K. designed and performed experiments, analyzed and interpreted data; V.L. and J.D.C. analyzed data; A.J.K-S. analyzed and interpreted data and J.P.K. designed experiments, analyzed and interpreted data, obtained funding, and wrote the manuscript.
Abbreviations
- IKKβ
IkappaB kinase beta
- GM-CSF
Granulocyte Macrophage Colony-Stimulating Factor
- NFκB
Nuclear Factor Kappa B
- qPCR
Quantitative real-time PCR
- TBP
TATA box binding protein gene
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- TNF
Tumor necrosis factor
- ESCC
Esophageal squamous cell cancer
- MVD
Microvessel density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest exist.
Transcript Profiling: Not applicable
References
- 1.Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543–52. doi: 10.1016/s1542-3565(05)00153-9. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–38. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 3.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 5.Werner-Wasik M, Paulus R, Curran WJ, Jr., et al. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non-small-cell lung cancer: analysis of the radiation therapy oncology group (RTOG) database. Clin Lung Cancer. 2011;12:245–51. doi: 10.1016/j.cllc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901–9. [PubMed] [Google Scholar]
- 7.Baldwin AS., Jr. Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–9. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Vlantis K, Wullaert A, Sasaki Y, et al. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J Clin Invest. 121:2781–93. doi: 10.1172/JCI45349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 13.Huxford T, Huang DB, Malek S, et al. The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell. 1998;95:759–70. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Goldstein BG, Nakagawa H, et al. Kruppel-like factor 5 activates MEK/ERK signaling via EGFR in primary squamous epithelial cells. Faseb J. 2007;21:543–50. doi: 10.1096/fj.06-6694com. [DOI] [PubMed] [Google Scholar]
- 15.Tetreault MP, Wang ML, Yang Y, et al. Klf4 overexpression activates epithelial cytokines and inflammation-mediated esophageal squamous cell cancer in mice. Gastroenterology. 2010;139:2124–2134. e9. doi: 10.1053/j.gastro.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetreault MP, Yang Y, Travis J, et al. Esophageal squamous cell dysplasia and delayed differentiation with deletion of kruppel-like factor 4 in murine esophagus. Gastroenterology. 2010;139:171–81. e9. doi: 10.1053/j.gastro.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki Y, Derudder E, Hobeika E, et al. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–39. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa H, Wang TC, Zukerberg L, et al. The targeting of the cyclin D1 oncogene by an Epstein-Barr virus promoter in transgenic mice causes dysplasia in the tongue, esophagus and forestomach. Oncogene. 1997;14:1185–90. doi: 10.1038/sj.onc.1200937. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, Simons M. Regulation of vascular integrity. J Mol Med. 2009;87:571–82. doi: 10.1007/s00109-009-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussolino F, Ziche M, Wang JM, et al. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991;87:986–95. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eubank TD, Roberts R, Galloway M, et al. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity. 2004;21:831–42. doi: 10.1016/j.immuni.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roda JM, Sumner LA, Evans R, et al. Hypoxia-inducible factor-2alpha regulates GM-CSF-derived soluble vascular endothelial growth factor receptor 1 production from macrophages and inhibits tumor growth and angiogenesis. J Immunol. 2011;187:1970–6. doi: 10.4049/jimmunol.1100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, Li B, Li X, et al. Transmembrane TNF-alpha promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J Immunol. 2014;192:1320–31. doi: 10.4049/jimmunol.1203195. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Rong L, Zhao X, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122:4094–104. doi: 10.1172/JCI64115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Stairs DB, Bayne LJ, Rhoades B, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–83. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro U, Jr., Posner MC, Safatle-Ribeiro AV, et al. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1174–85. [PubMed] [Google Scholar]
- 31.Liu K, Jiang M, Lu Y, et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–15. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diakowska D. Cytokines association with clinical and pathological changes in esophageal squamous cell carcinoma. Dis Markers. 2013;35:883–93. doi: 10.1155/2013/302862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B, Li YY, Tsao SW, et al. Targeting NF-kappaB signaling pathway suppresses tumor growth, angiogenesis, and metastasis of human esophageal cancer. Mol Cancer Ther. 2009;8:2635–44. doi: 10.1158/1535-7163.MCT-09-0162. [DOI] [PubMed] [Google Scholar]
- 34.O'Riordan JM, Abdel-latif MM, Ravi N, et al. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasiaadenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257–64. doi: 10.1111/j.1572-0241.2005.41338.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Li Z, Cui J, et al. Cellular changes in the tumor microenvironment of human esophageal squamous cell carcinomas. Tumour Biol. 2012;33:495–505. doi: 10.1007/s13277-011-0281-3. [DOI] [PubMed] [Google Scholar]
- 36.Keeley BR, Islami F, Pourshams A, et al. Prediagnostic serum levels of inflammatory biomarkers are correlated with future development of lung and esophageal cancer. Cancer Sci. 2014;105:1205–11. doi: 10.1111/cas.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vlantis K, Wullaert A, Sasaki Y, et al. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J Clin Invest. 2011;121:2781–93. doi: 10.1172/JCI45349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaked H, Hofseth LJ, Chumanevich A, et al. Chronic epithelial NF-kappaB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci U S A. 2012;109:14007–12. doi: 10.1073/pnas.1211509109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–9. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Fang Y, Shen J, Yao M, et al. Granulocyte-macrophage colony-stimulating factor enhances wound healing in diabetes via upregulation of proinflammatory cytokines. Br J Dermatol. 2010;162:478–86. doi: 10.1111/j.1365-2133.2009.09528.x. [DOI] [PubMed] [Google Scholar]
- 42.Gulcelik MA, Dinc S, Dinc M, et al. Local granulocyte-macrophage colony-stimulating factor improves incisional wound healing in adriamycin-treated rats. Surg Today. 2006;36:47–51. doi: 10.1007/s00595-005-3097-1. [DOI] [PubMed] [Google Scholar]
- 43.Ergun SS, Kyran B, Su O, et al. Effects of granulocyte macrophage colony stimulating factor on random flap healing and immune profile in rats with impaired wound healing by glucocorticoids. Ann Plast Surg. 2004;52:80–8. doi: 10.1097/01.sap.0000096449.71981.1b. [DOI] [PubMed] [Google Scholar]
- 44.Memisoglu E, Oner F, Ayhan A, et al. In vivo evaluation for rhGM-CSF wound-healing efficacy in topical vehicles. Pharm Dev Technol. 1997;2:171–80. doi: 10.3109/10837459709022622. [DOI] [PubMed] [Google Scholar]
- 45.Koukourakis MI, Flordellis CS, Giatromanolaki A, et al. Oral administration of recombinant human granulocyte macrophage colony-stimulating factor in the management of radiotherapy-induced esophagitis. Clin Cancer Res. 1999;5:3970–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.