Abstract
Background
The emergence of Zika virus (ZIKV) in the Americas has coincided with an increase in the report of birth of infants with microcephaly. On 1 February 2016, the World Health Organization declared the suspected link between ZIKV and microcephaly a Public Health Emergency of International Concern. However, to date, precise quantification of this association is lacking.
Methods
We retrospectively analysed data from a ZIKV outbreak in French Polynesia in October 2013–April 2014, which was the largest ever documented prior to the outbreak in the Americas. Serological and surveillance data were used to estimate the probability of ZIKV infection for each week of the epidemic. We also conducted an exhaustive search of medical records to identify all microcephaly cases from September 2013–July 2015. Simple models were developed to determine the period during pregnancy when ZIKV infection may increase the risk of microcephaly and estimate the associated risk.
Findings
Sixty-six percent (95% CI: 62, 70) of Polynesians were infected by ZIKV. Of the eight microcephaly cases identified during the 23-month study period, seven (88%) occurred in the four-month period following the ZIKV outbreak. This pattern was best explained by a model that assumed ZIKV infection in the first trimester of pregnancy increased the risk of microcephaly. In this model, the risk of microcephaly associated with ZIKV infection was 95 (95 CI: 34, 191) per 10,000 women infected in the first trimester of pregnancy while the prevalence of microcephaly was 2 (95% CI: 0, 8) per 10,000 neonates. Models where the risk of microcephaly also increased if infection occurred in trimesters 2 and 3 were not significantly worse fitting than this model.
Interpretation
This study provides the first quantitative estimate of the risk of microcephaly in a foetus/neonate whose mother was infected by ZIKV.
Funding
Labex-IBEID, NIH-MIDAS, AXA Research fund and EU-PREDEMICS.
Introduction
Since the first case of Zika virus (ZIKV) disease was identified in Brazil in May 2015, ZIKV, an arthropod-borne virus in the genus of Flavivirus1, has quickly expanded in Brazil and throughout the Americas. As of 19 February 2016, 28 countries of the region have reported cases2. Although ZIKV infection often leads to mild disease, the emergence of ZIKV in the Americas has coincided with a steep increase in Guillain-Barré syndrome, an autoimmune disease causing acute or subacute flaccid paralysis, and the birth of infants with neurological complications such as congenital microcephaly3–5.
Congenital microcephaly is a neurologic abnormality, present at birth, which is defined as an head circumference < −2 standard deviations (SD) below the mean for sex, age and ethnicity6. The term “severe” microcephaly is used for a head circumference < −3 SD7. Microcephaly can either be isolated or combined with other abnormalities. Microcephaly is associated with a reduction in brain volume and often, intellectual and/or motor disabilities including: speech impairment8, poor neurocognitive outcome9 or behavioural issues10. Causes include genetic11 or environmental factors12 that can impact brain development13, such as prenatal viral infections like rubella or cytomegalovirus14, maternal alcohol use15 and hypertensive disorders during pregnancy16. Cases have also been reported following intra-uterine infections with West Nile17, another flavivirus, and chikungunya18 virus.
On 1 February 2016, the World Health Organization declared the suspected link between ZIKV and microcephaly a Public Health Emergency of International Concern19. To reduce the risk of microcephaly, a number of recommendations have been made to pregnant women and women of childbearing age including to avoid travelling to affected countries, to use condom with partners returning from affected countries or to delay pregnancy20,21. Assessments are also ongoing to determine the level of monitoring that is required for pregnant women during ZIKV epidemics. In this crisis, clinical management of pregnant women, individual decisions regarding family planning and the response of the broader Public Health community need to be informed by precise estimates of the risk of microcephaly in a foetus/neonate whose mother has been infected by ZIKV. However, although there is growing evidence that ZIKV can cause microcephaly22,23, to date, a quantification of the association between ZIKV and microcephaly is lacking.
A timely assessment of this association may prove difficult from data gathered in an ongoing epidemic such as in the Americas. First, delays may occur between infection of a mother and diagnosis of microcephaly in her foetus/neonate. It may therefore take time before all cases of microcephaly potentially associated with ZIKV are reported. Second, surveillance systems only detect a small proportion of ZIKV infections24 and the true number of pregnant women that have been infected by ZIKV is therefore unknown. The total number of infections can be estimated by serological cross-sectional surveys only once an epidemic is over. This means there are important uncertainties about both the numerator and the denominator required to calculate the risk of microcephaly per infected pregnant woman.
Here, from the retrospective analysis of a large ZIKV outbreak that took place in French Polynesia25 in October 2013–April 2014, we test and characterize the strength and nature of the association between ZIKV and microcephaly. In particular, we evaluate the risk of microcephaly in a foetus/neonate whose mother has been infected by ZIKV. The French Polynesian outbreak presents a number of interesting properties to support such an assessment. First, it is the largest ZIKV outbreak ever documented prior to the current outbreak in the Americas. Second, French Polynesia has a strong infrastructure for the surveillance of infectious diseases and the detection of complications during pregnancy. Third, sufficient time has elapsed since the end of the outbreak for all cases of microcephaly potentially associated with ZIKV to be detected. Finally, serological data, which are necessary to estimate the number of pregnant women that were infected during the epidemic, are available26,27.
Methods
Our assessment was based on the joint analysis of four datasets that document i) all cases of microcephaly in French Polynesia in the study period (September 1st 2013 – July 31st 2015), ii) the weekly number of consultations for suspected ZIKV infection, iii) seroprevalence for ZIKV antibodies at the start and end of the epidemic and iv) the number of births in French Polynesia.
The general principles of the analysis are as follows. The serological study informs on the overall proportion of persons infected during the epidemic while the epidemic curve indicates the weeks when these people were likely to have been infected. From these two datasets, it is possible to estimate the probability of infection for each week of the epidemic. For a cohort of women whose pregnancy started for example on week 45 of 2013, it is then possible to calculate the expected proportion that were infected by ZIKV during the first, second or third trimester of their pregnancies. With this information, we can estimate expected trends in microcephaly for different scenarios about the ‘period of risk’ during pregnancy when infection of the mother by ZIKV may increase the risk of microcephaly for her foetus/neonate. We ascertain which scenarios are most likely by comparing predicted trends with the observed timing of microcephaly cases.
Further details on the data and the analytical framework are provided below and in the Supplementary Material.
Microcephaly data
For the study period, we retrospectively identified all complications that satisfied the following case definition: infants or foetuses whose head circumference was measured below two standard deviations from normal head circumference adjusted for gestational age and sex. To constitute this dataset, we performed an exhaustive search of medical records of patients who had been referred to the only prenatal diagnosis specialist centre of the territory. Other cases were exhaustively looked for in hospital discharge data (Programme de Médicalisation des Systèmes d’Information) from neonatology ward records. All suspected cases of microcephaly during the study period were reviewed by a group of specialists (MB, PGA, DEG, VA, CG) to identify those retained for the analysis. A description of standard follow up of pregnancies is provided in Supplementary Material.
Surveillance data
Weekly numbers of patient consultations for suspected ZIKV infection were estimated by the local sentinel surveillance system, which has existed since 2005. In normal situations out of epidemic periods, the system relies on 20 sentinel general practitioner (GP) sites. However, capacity can be expanded during epidemics. During the ZIKV outbreak of 2013–2014, an average of 50 sentinel sites, covering 30% of all GPs in the territory, reported each week to the system. The number of consultations for the whole territory was extrapolated from the number of consultations for sentinel sites. The case definition of a suspected ZIKV infection is a patient presenting with a rash and/or fever above 38.5°C, associated with at least two of the following symptoms: conjunctivitis, joint pain and/or muscle pain and limbs oedema. Laboratory confirmation by RT-PCR was obtained for 4.4% of suspected cases.
Serological data
We used data from three serological studies conducted in French Polynesia on: i) 593 Polynesians aged 18–79 years from Tahiti (the largest island in the territory) who donated blood between July 2011 and October 2013, i.e. prior to the epidemic27, ii) 196 persons from the general population aged 7–86 (median: 41) years residing in five of the most inhabited islands, between February and March 2014, i.e. in the second half of the epidemic26 and iii) 476 children from Tahiti aged 6–16 (median: 11) years, between May and June 2014, i.e. after the end of the epidemic26. In each of the three studies, serum samples were tested for evidence of historic ZIKV exposure using indirect IgG ELISAs27.
Demographic data
The annual reported number of births in French Polynesia is 4,182 (for 2013–2014) for a population of 270,000 (December 2013)28.
Statistical analysis
We developed a simple mathematical/statistical model to characterize the association between ZIKV and microcephaly. In the model, we assumed that there is a ‘period of risk’ during pregnancy when infection of the mother by ZIKV may increase the risk of microcephaly for her foetus/neonate: the risk of microcephaly was therefore ρ0 + ρZ if the mother was infected by ZIKV during the ‘period of risk’ and ρ0 otherwise (baseline). Six possible ‘periods of risk’ were considered: trimester 1; trimesters 1 & 2; trimesters 1, 2 & 3; trimester 2; trimesters 2 & 3; trimester 3. In addition, a scenario with ‘no association’ (i.e. no ‘period of risk’) was also considered.
We followed the cohort of nS women who started a pregnancy on week wS. Assuming that the birth rate was constant during the study period, we defined nS = 4,182 / 52 = 80.4. The probability pI (wI) that these women got infected by ZIKV on week wI was assumed to be proportional to the number IwI of consultations for suspected ZIKV infection in that week: where parameter γ was the final attack rate. In our baseline scenario, the final attack rate γ was estimated from the third serological study, i.e. the one performed after the end of the epidemic.
Once we reconstructed temporal trends in ZIKV infection, we were able to use the model to predict trends in microcephaly under the different scenarios about the ‘period of risk’. This also required modelling the duration of pregnancy for microcephaly cases (Figure S1).
For each model variant, maximum likelihood estimates of model parameters were obtained with a simulated annealing algorithm29. Ninety-five percent confidence intervals (95% CI) were derived with the likelihood ratio method30. A likelihood ratio test was used to compare models with a ‘period of risk’ with the ‘no association’ model. Otherwise, the Akaike Information Criterion with a correction for small sample size (AICc) was used31. The best fitting model is the one with the smallest AICc. A difference dAICc ≥4 was considered as indicative of a considerable improvement in model fit31.
In a sensitivity analysis, we also explored scenarios where the final attack rate was 50%, 60%, 70% and 80% and where the weekly number of births was 60 or 100. We also fitted a saturated model where the risk of microcephaly was estimated for each trimester of pregnancy.
Technical details are provided in the Supplementary Material. Data used to fit the mathematical model are available as Supplementary Material. Box 1 summarises key modelling assumptions.
Box 1. Modelling assumptions.
Our estimates of the risk of microcephaly associated with ZIKV infection rely on following assumptions:
There is a ‘period of risk’ during pregnancy when ZIKV infection of the mother may increase the risk of microcephaly for her foetus/neonate.
All microcephaly cases in the study period have been identified.
The number of ZIKV infections in a given week is proportional to the number of consultations for suspected ZIKV infection on that week.
The proportion of women of childbearing age infected by ZIKV during the epidemic is similar to the proportion of children who seroconverted (estimated in a serological study).
The birth rate is constant during the study period and can be estimated from official statistics.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Zika epidemic in French Polynesia
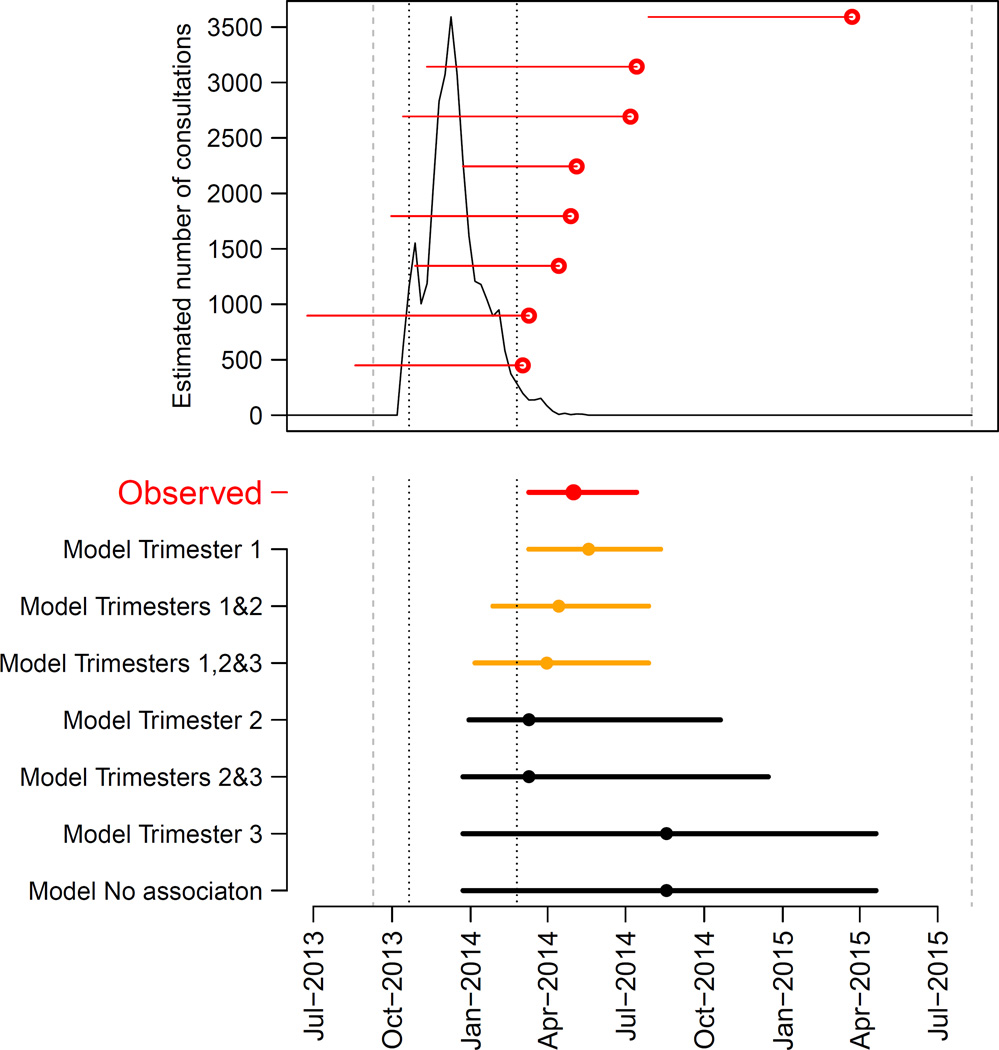
The outbreak began in October 2013 (week 41), peaked in December 2013 and was over in April 2014 (Figure 1A). By the end of the outbreak, public health officials had recorded 8,750 suspected cases of ZIKV infections of which 383 were laboratory confirmed by molecular testing (RT-PCR). It was estimated that more than 31,000 patients consulted for suspected ZIKV infection during this outbreak32 (Figure 1A).
Figure 1. Epidemic of ZIKV and timing of microcephaly cases in French Polynesia, September 2013–July 2015.
A. Estimated number of weekly consultations for suspected ZIKV infection (black line) along with the timing of microcephaly cases. For each microcephaly case, a red horizontal line indicates the estimated start of pregnancy and the date when it ended (delivery / medical abortion) (red dot). Dashed grey lines indicate the start/end of the study period (September 2013 – July 2015). Dotted black lines show the time period when 95% of consultations for suspected ZIKV infection occurred (14 October 2013 – 17 February 2014). B. Timing of microcephaly cases in French Polynesia (in red) along with predictions from seven models (in orange and black). These models make different assumptions about the ‘period of risk’ in pregnancy when ZIKV infection of the mother leads to an increased risk of microcephaly for the foetus/neonate. Dots indicate the median date while horizontal lines show the 15% and 85% percentiles. Models are sorted according to their AICc with the best fitting model at the top. Predictions of models are in orange when fit is judged satisfying (based on dAICc) and in black otherwise.
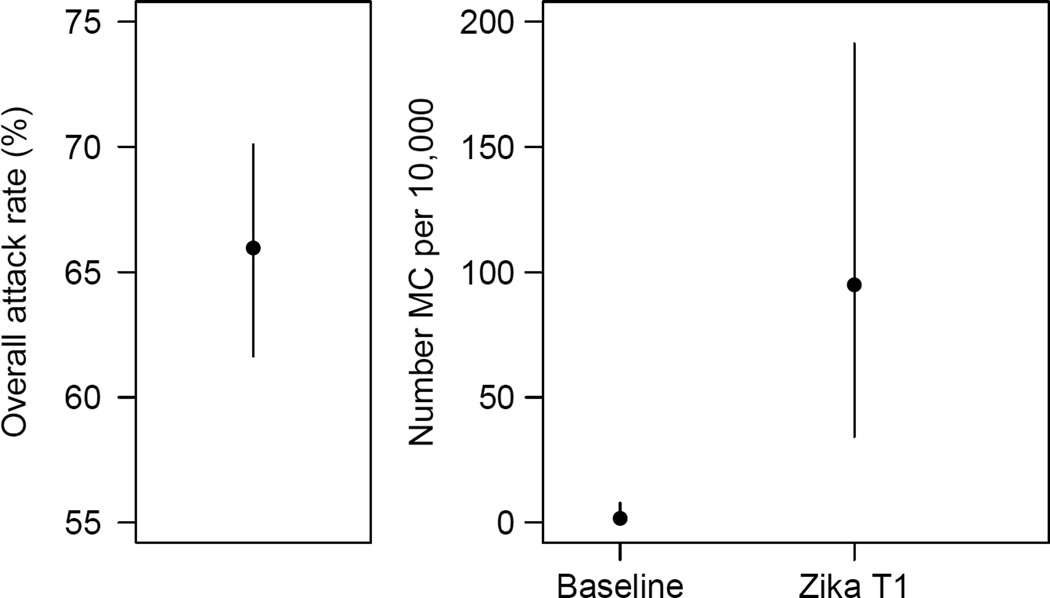
Prior to this outbreak, ZIKV seroprevalence was 0.8%27. It increased to 50% (95% CI 43%, 56%; 97/196) by the second half of the outbreak26 and reached 66% (95% CI: 62, 70; 314/476) after the end of the outbreak26 (Figure 2A).
Figure 2. Final attack rates in the ZIKV epidemic and strength of the association between ZIKV and microcephaly in French Polynesia.
A. Final attack rates (95% CI) in the ZIKV epidemic in French Polynesia. B. Baseline prevalence of microcephaly (number per 10,000 neonates) in French Polynesia and risk of microcephaly associated with ZIKV infection (number per 10,000 women infected in the first trimester of pregnancy).
Cases of microcephaly in the study period
Eight cases of microcephaly satisfying our case definition were identified during the study period (Table 1). Five cases were pregnancies which had been terminated through medical abortion, while three children were born. Median gestational age among foetuses whose pregnancies had been terminated following diagnosis of microcephaly was at 30.1 weeks (IQR: 26.1–31.4). Normal foetal karyotype was obtained among six infants and was not available for the two others.
Table 1. Clinical characteristics of eight cases of microcephaly reported in French Polynesia, 1 September 2013 – 31 July 2015.
| n (%) or median | IQR | ||
|---|---|---|---|
| Mother’s characteristics prior to pregnancy | |||
| Age at beginning of pregnancy (years) | 29.2 | 24.3–34.1 | |
| Infants or foetuses characteristics | |||
| Sex | |||
| Male | 6 (75) | ||
| Female | 2 (25) | ||
| Pregnancy outcome | |||
| Medical termination | 5 (62.5) | ||
| Gestational age at termination (weeks) | 30.1 | 26.1–31.4 | |
| Childbirth | 3 (37.5) | ||
| Gestational age at birth (weeks) | 38.0 | 37.2–39.5 | |
Association between ZIKV and microcephaly
Of the eight microcephaly cases detected during the 23-month study period, seven (88%) were identified in a four-month time period (1 March – 10 July 2014) that immediately followed the ZIKV outbreak (Figure 1A).
Of the six models with a ‘period of risk’ during pregnancy, four explained the observed pattern significantly better than the ‘no association’ model (p-value in the range: 0.0007–0.0172) (Table 2). The two models that did not perform significantly better than the ‘no association’ model assumed the ‘period of risk’ was restricted to trimester 3 only or to trimester 2 and 3.
Table 2. Baseline prevalence of microcephaly in French Polynesia and risk of microcephaly associated with ZIKV infection.
Six scenarios were considered for the ‘period of risk’ during pregnancy when infection of the mother by ZIKV may increase the risk of microcephaly. A last scenario assumed no association between ZIKV and microcephaly.
| ‘Period of risk’ during pregnancy | baseline‡ | ZIKV† | risk ratio | p-value* | dAICcⱡ |
|---|---|---|---|---|---|
| Trimester 1 | 2 (0,8) | 95 (34,191) | 53.4 (6.5,1061.2) | 0.0007 | 0 |
| Trimesters 1 and 2 | 2 (0,8) | 50 (17,101) | 26.4 (3.0,352.0) | 0.0015 | 1.37 |
| Trimesters 1, 2 and 3 | 2 (0,9) | 42 (13,86) | 20.8 (2.1,424.1) | 0.0032 | 2.73 |
| Trimester 2 | 4 (0,12) | 84 (12,196) | 23.2 (1.4,407.8) | 0.0172 | 5.76 |
| Trimesters 2 and 3 | 4 (0,13) | 53 (0,135) | 11.9 (0.0,177.5) | 0.0525 | 7.67 |
| Trimester 3 | 10 (3,18) | 0 (0,251) | 0.0 (0.0,49.3) | 1 | 11.43 |
| No association | 10 (5,18) | - | - | 7.15 |
: Baseline prevalence of microcephaly in French Polynesia, measured by the number of microcephaly cases per 10,000 neonates.
: Risk of microcephaly associated with ZIKV infection, measured by the number of microcephaly cases per 10,000 women infected by ZIKV during the ‘period of risk’.
: Models with a ‘period of risk’ are compared with the model with no association using a likelihood ratio test.
: Quality of fit is assessed with the dAICc criterion where the model with the smallest dAICc is the best fitting model (in bold) and a dAICc difference ≥4 is indicative of a considerably better fit31.
Three models provided satisfying fits to the data (Figure 1B) and had relatively similar model comparison criteria AICc (Table 2). The common feature for these models was that they all included trimester 1 in the ‘period of risk’. The best fitting model was the one where the ‘period of risk’ was restricted to trimester 1. In this model, the risk of microcephaly associated with ZIKV infection was 95 (95 CI: 34, 191) per 10,000 women infected in the first trimester of pregnancy while the prevalence of microcephaly was 2 (95% CI: 0, 8) per 10,000 neonates, consistent with previous estimates from Europe (1.9 per 10,000)33 and Brazil (2 per 10,000)34. This corresponds to a risk ratio of 53.4 (95% CI: 6.5, 1061.2). Two other models could not be ruled out. They had the following ‘periods of risk’: i) trimesters 1 and 2 with a risk of microcephaly of 50 (95% CI: 17, 101) per 10,000 women infected in trimester 1 or 2; ii) trimesters 1, 2 and 3 with a risk of microcephaly of 42 (95% CI: 13, 86) per 10,000 women infected in trimester 1, 2 or 3. Models that assumed trimester 1 was not in the ‘period of risk’ were not supported by the data (Figure 1B, Table 2).
Sensitivity analysis
Estimates in Table 2 were obtained under the assumptions that ZIKV final attack rates in women of childbearing age were similar to the 66% rate observed in children26 and that there were 80 births per week28. We explored sensitivity of our estimates to these assumptions. For the various scenarios we considered and irrespective of the ‘period of risk’, the relative change in estimates was between −20% (attack rate of 80%, birth rate of 100 per week) and +33% (attack rate of 50%, birth rate of 60 per week) (Table 3). This means that for the best fitting model where the ‘period of risk’ is restricted to trimester 1, the estimated risk of microcephaly associated with ZIKV infection remains between 80–130 per 10,000 women infected in the first trimester of pregnancy. Analysis of the saturated model where the risk of microcephaly was estimated per trimester provided further support for our best fitting model (Table S1).
Table 3. Sensitivity of the estimated risk of microcephaly associated with ZIKV infection to modelling assumptions.
The table gives the number of microcephaly per 10,000 women infected during the assumed ‘period of risk’, for different assumptions about final attack rates and birth rates. Our baseline scenario (in bold) corresponds to a final attack rate of 66% based on a serological study performed after the end of the epidemic26 and 80.4 births per week based on official statistics28. The change relative to the baseline scenario is also indicated.
| ‘Period of risk’ in pregnancy | |||||
|---|---|---|---|---|---|
| Trimester 1 | Trimesters 1 and 2 | Trimesters 1, 2 and 3 | Change relative to baseline | ||
| Final attack rate | |||||
| 50% | 125 (45,251) | 66 (22,133) | 55 (17,113) | 32% | |
| 60% | 104 (38,209) | 55 (19,111) | 46 (14,94) | 9% | |
| 66% (baseline) | 95 (34,191) | 50 (17,101) | 42 (13,86) | 0% | |
| 70% | 90 (32,179) | 47 (16,95) | 40 (12,81) | −5% | |
| 80% | 78 (28,157) | 41 (14,83) | 35 (11,71) | −18% | |
| Weekly number of births | |||||
| 60 | 127 (46,256) | 67 (23,136) | 56 (17,115) | 33% | |
| 80.4 (baseline) | 95 (34,191) | 50 (17,101) | 42 (13,86) | 0% | |
| 100 | 76 (28,154) | 40 (14,82) | 34 (10,158) | −20% | |
Discussion
The large outbreak of ZIKV that took place in French Polynesia in 2013–2014 offers a unique opportunity to better quantify and characterize the association between ZIKV and microcephaly. Of the eight cases of microcephaly that were reported during our 23-month study period, seven occurred in the four-month period that immediately followed the ZIKV outbreak. Such temporal clustering of cases gives strong support to the proposed association between ZIKV and microcephaly. To make a more nuanced assessment of this association, we developed mathematical models that predicted temporal trends in microcephaly under different scenarios about the link with ZIKV. Key conclusions of this analysis are threefold. First, models that assumed that infection of the mother increased the risk of microcephaly explained observed patterns significantly better than the ‘no association’ model. Second, in the best fitting models, the ‘period of risk’ during which ZIKV infection resulted in an increased risk of microcephaly always included the first trimester of pregnancy and the best fit was obtained when the ‘period of risk’ was restricted to this trimester. Third, thanks to the availability of serological data, we were able to estimate the risk of microcephaly per infected pregnant woman.
We estimated that the risk of microcephaly was about 1% if the mother was infected by ZIKV in the first trimester of pregnancy. This risk may seem low compared to other viral infections associated to birth defects. For example, 13% of primary cytomegalovirus infections in pregnancy result in symptomatic congenital disease of the newborn36; the risk of congenital rubella syndrome ranges between 38% and 100% if the mother is infected during the first trimester of pregnancy37; global adverse foetal outcomes occur for 10% of pregnant women infected by parvovirus B19. However, a major difference between ZIKV and these infections is that the incidence of ZIKV can be very high during outbreaks (e.g. 66% of the population in French Polynesia26 and 73% on the island of Yap24). This contrasts with estimates for cytomegalovirus (1–4% in pregnant women)38, rubella (<10 cases in pregnant women per year in France)39 or parvovirus B19 (0.61–1.24% in women of childbearing age)40. So, although ZIKV infection is associated with a relatively low foetal risk, the fact that it can infect a substantial proportion of a population makes it an important source of concern for public health. These findings highlight the need to protect pregnant women or women trying to become pregnant from infection with ZIKV. As there is no treatment currently available for ZIKV and development of a vaccine will take time, these women should protect themselves from mosquitos in affected countries and avoid travel to these countries if they can.
Our analysis provides strong support for the hypothesis that infection in the first trimester of pregnancy is associated with an increased risk of microcephaly. Such pattern of risk has also been observed for other intra-uterine viral infections like rubella or cytomegalovirus, where infections during the first trimester or early second trimester are associated with brain damage35. Larger datasets will be required to determine whether infection in the remainder of the pregnancy also increases the risk of microcephaly. It will also be important to determine if the risk of microcephaly varies with the severity of clinical symptoms in the infected mother.
Four datasets that informed on different aspects of this epidemic were used in our analysis. The first one consisted of an exhaustive search of all microcephaly cases in French Polynesia during the study period. We restricted our analysis to cases that satisfied a strict case definition for microcephaly for two reasons. First, the WHO Public Health Emergency of International Concern focused on microcephaly and we therefore felt this concern should be addressed first. Second, the lack of standardised case definition for microcephaly has been identified as an important source of confusion in this epidemic43,44 which may have led to overestimating the number of microcephaly cases in South America44. To ensure the quality of the diagnostics, a group of five specialists reviewed all potential cases and made final decision on the ones to be retained in the analysis. While our analysis was restricted to the link between ZIKV and microcephaly, it will be important to ascertain whether ZIKV may generate other neurological complications. Other types of complications have for example been reported in French Polynesia although the link with ZIKV has yet to be established4. This should be the subject of further investigations in French Polynesia and elsewhere.
The second dataset consisted of data from syndromic Sentinel surveillance, which suffer from a number of limitations. For example, they typically only detect a small proportion of infections. However, this should not affect our analysis because we only used them to determine the timing of the epidemic not the size of the epidemic. This was done by assuming that the proportion of infections occurring on a given week was proportional to the number of consultations for suspected ZIKV infection reported on that week. This assumption might be violated if propensity to consult for ZIKV symptoms or reporting practices changed substantially during the epidemic. Such phenomenon was for example observed in the 2009 H1N1pdm09 influenza pandemic45.
Three seroprevalence studies were used to evaluate the final attack rate of ZIKV. These studies were performed in different populations with different age structures. However, there is little reason to expect large difference in risk between children and adults. The risk of ZIKV exposure in an outbreak on Yap Island was found to be similar across age groups24. In addition, the three estimates of seropositivity are entirely consistent with that expected over the course of an outbreak in a previously naïve population. Finally, the 66% estimate for the final attack rate is consistent with 73% (95% CI: 68%, 77%) found in the ZIKV outbreak on Yap24. Our estimates for the risk of microcephaly were also relatively robust to large scale changes in the overall attack rate (Table 3). Since less than 1% of individuals tested positive for ZIKV before the start of the ZIKV outbreak despite high levels of dengue seropositivity27, cross-reaction in serological assays is unlikely to be an important issue here.
Our analysis also relied on the total number of documented annual birth in French Polynesia. French Polynesia is an overseas collectivity of the French Republic and as such population statistics there are of a quality relatively similar to that seen in mainland France, including carefully tabulated birth statistics. Since birth counts were only available on an annual basis, we made the assumption of a constant birth rate during the study period. In practice small variations in weekly number of births may be expected but estimates were relatively robust to such variations (Table 3). Because we were interested in assessing the risk of microcephaly due to ZIKV in foetuses that could have been expected to be live born in the absence of ZIKV, it was more appropriate to use statistics on live births than on live births and medical abortions, even though medical abortion was performed for a substantial proportion of our microcephaly cases.
One needs to be cautious when using data from the ZIKV outbreak in French Polynesia to make extrapolations for other settings. First, the spread of an arbovirus like ZIKV is affected by entomological, environmental and climatic factors so that attack rates in other ZIKV outbreaks may be different than what was observed in French Polynesia. Second, we cannot exclude the possibility that the risk of microcephaly associated with ZIKV infection will differ in other populations due to genetic factors.
Much more epidemiologic and experimental research needs to be done to evaluate and understand the role of ZIKV infection in congenital abnormalities such as microcephaly. Research must be undertaken to determine the causal link between ZIKV infection and microcephaly. Experimental studies investigating maternal-foetal transmission should be a priority. In addition, affected and at risk countries should establish cohorts of pregnant women to be followed and tested throughout their pregnancy46. With growing number of countries affected by the current outbreak, a minimum level of standardization across these cohort studies is required. Our study was retrospective in nature but prospective studies evaluating the link between ZIKV and microcephaly are urgently needed. Groups like ISARIC47 and CONSISE48 are working with affected countries, WHO, USCDC and others to generate these protocols rapidly.
This study provides strong statistical support for the suspected association between ZIKV and microcephaly and estimates that the risk of microcephaly increases to about 1% if a mother has been infected by ZIKV during the first trimester of pregnancy. The study therefore emphasizes the need for a strong and prompt response to protect, inform and monitor this at-risk population as well as the need for a strong research agenda to better understand the causal link between ZIKV and microcephaly and to develop effective treatments and vaccines against ZIKV.
Supplementary Material
Research in context.
Evidence before this study
Microcephaly is a condition defined by a measured head circumference below two standard deviations from normal head circumference. Its incidence is estimated between 5.8 per 100,000 live births in the USA and 18.7 per 100,000 live or stillborn births and medical abortions in Europe. Long term outcome of this condition is heterogeneous and has been associated with several neurologic conditions such as epilepsy or intellectual deficiencies. Following current ZIKV epidemic in South America, an outbreak of microcephaly among newborns has been reported in several countries, conducting World Health Organization to declare ZIKV outbreak an international Public Health Emergency. However, the association between ZIKV and microcephaly remains to be quantified.
Added value of this study
From the retrospective analysis of a large ZIKV outbreak that took place in French Polynesia in 2013–2014, we were able to: 1) provide strong statistical support for the association between ZIKV infection and microcephaly; 2) establish that the period of risk (i.e. period in pregnancy when ZIKV infection of the mother leads to an increased risk of microcephaly in the foetus/neonate) was likely to contain the first trimester of pregnancy but may also include the second and third trimester; 3) estimate that the risk of microcephaly associated with ZIKV infection was 95 (95% CI: 34, 191) per 10,000 women infected by ZIKV in the first trimester.
Implications of all the available evidence
Our work provides strong statistical support for the previously suspected link between ZIKV infection during pregnancy and microcephaly. Hence, it emphasises the need for health authorities of affected countries to organise foetal monitoring, promote vector control and provide evidence-driven information for pregnant women.
Acknowledgments
This study has received funding from the French Government's Investissement d'Avenir program, Laboratoire d'Excellence "Integrative Biology of Emerging Infectious Diseases" (grant n°ANR-10-LABX-62-IBEID), the NIGMS MIDAS initiative, the AXA Research Fund and the European Union Seventh Framework Programme (FP7/2007-2013) under Grant Agreement number 278433- PREDEMICS.
Footnotes
Contributors
SC, MB, AF, TD, and HPM conceived and designed the study; MB, PB, and HPM designed the case report forms and collected the epidemiological data; MB, PGA, DEG, VA and CG provided care to the mothers and their children and collected the clinical data; MB, PGA, DEG, VA, and CG reviewed all clinical files of congenital malformation cases and decided on whether they conformed or not with the study case definition; SC and HS developed and ran the mathematical model; SC, TD, HS, MDvK, AF and HPM interpreted the model results; SC, MB, TD, MDvK, AF and HPM wrote the first version of the manuscript. All authors critically reviewed and approved the final version of the manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Gubler D, Kuno GLM. Flaviviruses. In: Knipe DMHP, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields virology. Philadelphia, PA: Lippincott Williams & Wilkins Publishers; 2007. pp. 1153–1252. [Google Scholar]
- 2.WHO. Zika situation report. [Last accessed on 27 Feb 2016];2016 Feb 19; Available at http://www.who.int/emergencies/zika-virus/situation-report/19-february-2016/en/
- 3.WHO. Guillain-Barré syndrome - El Salvador. [Last accessed on Feb 5 2016];2016 Jan 21; Available at http://www.who.int/csr/don/21-january-2016-gbs-el-salvador/en/
- 4.ECDC. Risk assessment: Zika virus epidemic in the Americas: potentially associated with microcephaly and Guillain-Barré syndrome. [Last accessed on 5 Feb 2016];2016 Available at. [Google Scholar]
- 5.Soares de Araujo J, Regis T, Gomes R, et al. Microcephaly in northeast Brasil: a review of 16 208 births between 2012 and 2015. [Last accessed on 8 Feb 2015];Bull World Health Organ. 2016 Available at http://who.int/bulletin/online_first/16-170639.pdf?ua=1 [Submitted] [Google Scholar]
- 6.Ashwal S, Michelson D, Plawner L, Dobyns WB Quality Standards Subcommittee of the American Academy of N, the Practice Committee of the Child Neurology S. Practice parameter: Evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73(11):887–897. doi: 10.1212/WNL.0b013e3181b783f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passemard S, Kaindl AM, Verloes A. Microcephaly. Handb Clin Neurol. 2013;111:129–141. doi: 10.1016/B978-0-444-52891-9.00013-0. [DOI] [PubMed] [Google Scholar]
- 8.Whitehouse AJ, Zubrick SR, Blair E, Newnham JP, Hickey M. Fetal head circumference growth in children with specific language impairment. Arch Dis Child. 2012;97(1):49–51. doi: 10.1136/adc.2009.180331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolk H. The predictive value of microcephaly during the first year of life for mental retardation at seven years. Dev Med Child Neurol. 1991;33(11):974–983. doi: 10.1111/j.1469-8749.1991.tb14813.x. [DOI] [PubMed] [Google Scholar]
- 10.Stoler-Poria S, Lev D, Schweiger A, Lerman-Sagie T, Malinger G. Developmental outcome of isolated fetal microcephaly. Ultrasound Obstet Gynecol. 2010;36(2):154–158. doi: 10.1002/uog.7556. [DOI] [PubMed] [Google Scholar]
- 11.Abuelo D. Microcephaly syndromes. Semin Pediatr Neurol. 2007;14(3):118–127. doi: 10.1016/j.spen.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Tarrant A, Garel C, Germanaud D, et al. Microcephaly: a radiological review. Pediatr Radiol. 2009;39(8):772–780. doi: 10.1007/s00247-009-1266-x. quiz 888-9. [DOI] [PubMed] [Google Scholar]
- 13.von der Hagen M, Pivarcsi M, Liebe J, et al. Diagnostic approach to microcephaly in childhood: a two-center study and review of the literature. Dev Med Child Neurol. 2014;56(8):732–741. doi: 10.1111/dmcn.12425. [DOI] [PubMed] [Google Scholar]
- 14.Noyola DE, Demmler GJ, Nelson CT, et al. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr. 2001;138(3):325–331. doi: 10.1067/mpd.2001.112061. [DOI] [PubMed] [Google Scholar]
- 15.Krauss MJ, Morrissey AE, Winn HN, Amon E, Leet TL. Microcephaly: an epidemiologic analysis. Am J Obstet Gynecol. 2003;188(6):1484–1489. doi: 10.1067/mob.2003.452. discussion 9–90. [DOI] [PubMed] [Google Scholar]
- 16.Olusanya BO. Full-term newborns with congenital microcephaly and macrocephaly in Southwest Nigeria. Int Health. 2012;4(2):128–134. doi: 10.1016/j.inhe.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 17.O'Leary DR, Kuhn S, Kniss KL, et al. Birth outcomes following West Nile Virus infection of pregnant women in the United States: 2003–2004. Pediatrics. 2006;117(3):e537–e545. doi: 10.1542/peds.2005-2024. [DOI] [PubMed] [Google Scholar]
- 18.Gerardin P, Samperiz S, Ramful D, et al. Neurocognitive outcome of children exposed to perinatal mother-to-child Chikungunya virus infection: the CHIMERE cohort study on Reunion Island. PLoS Negl Trop Dis. 2014;8(7):e2996. doi: 10.1371/journal.pntd.0002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. [Last accessed on 5 Feb 2016]; Available at http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/
- 20.Petersen EE, Staples JE, Meaney-Delman D, et al. Interim Guidelines for Pregnant Women During a Zika Virus Outbreak - United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- 21.The Washington Post. As Zika virus spreads, El Salvador asks women not to get pregnant until 2018. [Last accessed on 8 Feb 2016];2016 Available at https://www.washingtonpost.com/world/the_americas/as-zika-virus-spreads-el-salvador-asks-women-not-to-get-pregnant-until-2018/2016/01/22/1dc2dadc-c11f-11e5-98c8-7fab78677d51_story.html. [Google Scholar]
- 22.Mlakar J, Korva M, Tul N, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016 doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 24.Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 25.Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20(6):1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aubry M, Teissier A, Roche C, et al. Serosurvey of dengue, Zika and other mosquito-borne viruses in French Polynesia. Poster presentation 765; 64th Annual Meeting of the American Society of Tropical Medicine and Hygiene; Philadelphia, USA. Oct 25–29 2015. [Google Scholar]
- 27.Aubry M, Finke J, Teissier A, et al. Seroprevalence of arboviruses among blood donors in French Polynesia, 2011–2013. Int J Infect Dis. 2015;41:11–12. doi: 10.1016/j.ijid.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Institut de Statistique de Polynésie Française. Les grands indicateurs de la population issus de l'état-civil. [Last Accessed on 8 Feb 2016]; Available at http://www.ispf.pf/themes/Geographie/Population/Coupdoeil.aspx. [Google Scholar]
- 29.Liu JS. Monte Carlo strategies in scientific computing. New York: Springer-Verlag; 2001. [Google Scholar]
- 30.King AA, Ionides EL, Pascual M, Bouma MJ. Inapparent infections and cholera dynamics. Nature. 2008;454(7206):877-U29. doi: 10.1038/nature07084. [DOI] [PubMed] [Google Scholar]
- 31.Burnham K, Anderson D. Model selection and multimodel inference: A practical information-theoretic approach. 2nd. New York: Springer-Verlag; 2002. [Google Scholar]
- 32.Mallet H, Vial A, Musso D. Bilan de l’épidémie à virus Zika en Polynésie Française 2013–2014. [Last accessed 14 Jan 2016];Bulletin d’information sanitaires, épidémiologiques et statistiques. 2015 Available at http://www.hygiene-publique.gov.pf/IMG/pdf/no13_-_mai_2015_-_zika.pdf.
- 33.Eurocat. European surveillance of congenital anomalies (Eurocat), final activity report 2002–2003. [Last accessed on 8 Feb 2016];2003 Available at http://ec.europa.eu/health/ph_threats/non_com/docs/eurocat_en.pdf. [Google Scholar]
- 34.Butler D. ZIKA VIRUS Microcephaly surge in d oubt. Nature. 2016;530(7588):12–13. doi: 10.1038/nature.2016.19259. [DOI] [PubMed] [Google Scholar]
- 35.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–R162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naing ZW, Scott GM, Shand A, et al. Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. Aust N Z J Obstet Gynaecol. 2016;56(1):9–18. doi: 10.1111/ajo.12408. [DOI] [PubMed] [Google Scholar]
- 37.De Santis M, Cavaliere AF, Straface G, Caruso A. Rubella infection in pregnancy. Reprod Toxicol. 2006;21(4):390–398. doi: 10.1016/j.reprotox.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199–213. doi: 10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vauloup-Fellous C, Bouthry E, Grangeot-Keros L. Infections materno-foetales: difficultés diganostiques et prise en charge maternelle. Annales de Biologie Clinique. 2013;71:5–18. doi: 10.1684/abc.2013.0897. [DOI] [PubMed] [Google Scholar]
- 40.de Jong EP, Walther FJ, Kroes AC, Oepkes D. Parvovirus B19 infection in pregnancy: new insights and management. Prenat Diagn. 2011;31(5):419–425. doi: 10.1002/pd.2714. [DOI] [PubMed] [Google Scholar]
- 41.Haute Autorité de Santé. Suivi et orientation des femmes enceintes en fonction des situations à risque identifiées. [Last accessed on 25 Feb 2016]; Available at http://www.has-sante.fr/portail/jcms/c_547976/fr/suivi-et-orientation-des-femmes-enceintes-en-fonction-des-situations-a-risque-identifiees. [Google Scholar]
- 42.Comité National Technique de Dépistage Prénatal. Rapport du Comité Technique de l'Echographie de dépistage prénatal. [Last Accessed on 25 Feb 2016]; Available at https://www.cfef.org/archives/lettres/DocusCTE/01.html. [Google Scholar]
- 43.Heymann DL, Hodgson A, Sall AA, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387(10020):719–721. doi: 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Victora CG, Schuler-Faccini L, Matijasevich A, Ribeiro E, Pessoa A, Barros FC. Microcephaly in Brazil: how to interpret reported numbers? Lancet. 2016;387(10019):621–624. doi: 10.1016/S0140-6736(16)00273-7. [DOI] [PubMed] [Google Scholar]
- 45.Dorigatti I, Cauchemez S, Ferguson NM. Increased transmissibility explains the third wave of infection by the 2009 H1N1 pandemic virus in England. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(33):13422–13427. doi: 10.1073/pnas.1303117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. [Last Accessed on 8 Feb 2016]; Available at http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committe-ezika/en/ [Google Scholar]
- 47.ISARIC. International Severe Acute Respiratory and Emerging Infection Consortium. [Last accessed on 11 Feb 2016]; Available at https://isaric.tghn.org/ [Google Scholar]
- 48.Concise. Consortium for the standardization of influenza seroepidemiology. [Last accessed on 11 Feb 2016]; Available at https://consise.tghn.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.