Abstract
Investigator-administered nicotine alters neurotensin and substance P levels in Sprague-Dawley rats. This finding suggests a role of the dopamine-related endogenous neuropeptides in nicotine addiction. We sought to extend this observation by determining the responses of neurotensin and substance P systems (assessed using radioimmunoassay) in male and female rats following nicotine self-administration (SA). Male and female Sprague-Dawley were trained to self-administer nicotine, or receive saline infusions yoked to a nicotine-administering rat during daily sessions (1-h; 21 days). Brains were extracted 3 h after the last SA session. Nicotine SA increased tissue levels of neurotensin in the males in the anterior and posterior caudate, globus pallidus, frontal cortex, nucleus accumbens core and shell, and ventral tegmental area. Nicotine SA also increased tissue levels of neurotensin in the females in the anterior caudate, globus pallidus, nucleus accumbens core and shell, but not in the posterior caudate, frontal cortex, or ventral tegmental area. There were fewer sex differences observed in the substance P systems. Nicotine SA increased tissue levels of substance P in both the males and females in the posterior caudate, globus pallidus, frontal cortex, nucleus accumbens shell, and ventral tegmental area. A sex difference was observed in the nucleus accumbens core, where nicotine SA increased tissue levels of substance P in the males, yet decreased levels in the females. The regulation of neuropeptides following nicotine SA may play a role in the susceptibility to nicotine dependence in females and males.
Keywords: Neuropeptide, Dopamine, Addiction, Male, Female, Limbic-Related
Graphical Abstract
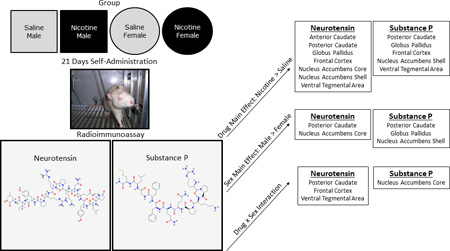
In Sprague-Dawley rats, nicotine self-administration alters neurotensin-like immunoreactivity and substance P-like immunoreactivity differentially in male and female rats. The regulation of neuropeptides following nicotine SA may play a role in the susceptibility to nicotine dependence in females and males.
Introduction
In the past half century, an estimated 20,830,000 Americans have died prematurely from the consequences of smoking (USDHHS, 2014). Despite almost ubiquitous public knowledge that smoking is harmful, nearly 1 in 5 Americans currently smoke cigarettes (USDHHS, 2014). Individuals that do smoke overwhelmingly report a desire to stop completely (68.8%; CDC, 2011) and more than half attempt to quit in a given year (52.4%; CDC, 2011). Unfortunately, very few quit attempts are successful; only 6.2% of smokers effectively quit smoking annually (CDC; 2011). Nicotine is generally considered the primary addictive constituent in tobacco (Benowitz, 2010). Examination of possible neurobiological alterations in the mammalian brain following long-term nicotine SA will inform smoking cessation strategies and hopefully increase the percentage of quit attempts that are successful.
Studies investigating potential sex differences in nicotine dependence have determined that, on average, men are more likely to be smokers than women (20.5% vs 15.3%: CDC, 2014) and appear to smoke more cigarettes per day than women (Peters et al, 2014). While slightly less likely to be smokers, women actually progress to smoking dependence more rapidly, attempt to quit less frequently, relapse at higher rates than men, and show lower rates of efficacy to nicotine replacement therapy (Cepeda-Benito et al, 2004; Ockene, 1993; Perkins, 2001; Perkins and Scott, 2008; Piper et al, 2010; Tunstall et al, 1985; Wetter et al, 1999). Preclinical research suggests that females are more influenced by conditioned reinforcement of cues associated with nicotine and demonstrate greater withdrawal symptoms from nicotine than males (see reviews; O'Dell and Torres, 2014; Perkins, 2009; Perkins et al, 1999; Torres and O'Dell, 2015). Further elucidation of the neurobiological factors that contribute to these sex differences is needed to help identify the basis of gender distinctions and inform the development of gender-sensitive strategies for tobacco cessation.
Numerous studies have examined the effects of nicotine on dopaminergic systems in limbic-related (LR) neural structures, establishing the importance of dopamine in the motivational factors contributing to nicotine addiction (Benwell and Balfour, 1992; Benwell et al, 1994; Calabresi et al, 1989; Corrigall et al, 1994; Corrigall et al, 1992; Damsma et al, 1989; Grenhoff et al, 1986; Imperato et al, 1986; Marshall et al, 1997; Mifsud et al, 1989; Nisell et al, 1997). Studies have also demonstrated that nicotine differentially alters dopaminergic systems in females and males (Dluzen and Anderson, 1997; Harrod et al, 2004; Pogun, 2001). Dluzen and Anderson (1997) showed that peak nicotine-evoked (10 µM in vitro infusion of nicotine) dopamine release tended to be lower in ovariectomized females compared to castrated males and estrogen treatment resulted in differential effects. Estrogen increased nicotine-evoked dopamine release in the females and decreased release in the males. Extracellular dopamine concentrations in the nucleus accumbens have also been reported higher in female rats compared to male rats following systemic nicotine injections (Pogun, 2001). Harrod and colleagues (2004) showed that females exhibited an increase in the number of dopamine transporters in the NAcc following 21 days of nicotine infusions (50 µg/kg/ml). While the impact of nicotine on sex differences in dopaminergic neurotransmission has been established, less is known about the effects of nicotine on neuropeptides that modulate these dopaminergic systems.
Neurotensin, an endogenous tridecapeptide, and substance P, an undecapeptide, inhibit and stimulate, respectively, dopaminergic neuronal function in LR brain regions, and participate in the modulation of dopaminergic pathways vital to nicotine addiction [for a review of the complex dopamine-neurotensin/substance P interactions see Binder et al, (2001); Commons, (2010); Tyler-McMahon et al, (2000)]. Consistent with this suggestion, investigator-administered nicotine significantly altered both neurotensin and substance P systems associated with dorsal and ventral striatal structures. Five doses (2-hour intervals) of 0.8 mg/kg nicotine rapidly reduced both neurotensin-like immunoreactivity (NTLI) and substance P-like immunoreactivity (SPLI) levels in regions related to limbic- and basal ganglia-related structures. The neurotensin-related changes were antagonized by D2, but not D1, antagonists (Alburges et al, 2007); in contrast, the nicotine-induced changes in SPLI levels were blocked by both D1 and D2 antagonists (Alburges et al, 2009). Consequently, in the present study, neurotensin and substance P systems were investigated for sexually diergic responses to nicotine in LR brain regions. This was done in a more chronic fashion by examining tissue levels of NTLI and SPLI following 21 days of nicotine SA and determining sex patterns in these responses.
Materials and Methods
Subjects
Fifty-one Sprague-Dawley rats (32 females; 19 males) were purchased from Harlan Laboratories (Indianapolis, IN, USA) at approximately 9 weeks of age. Rats were housed individually in clear polycarbonate cages (35.5 × 32 × 18 cm; length × width × depth) with TEK-Fresh® cellulose bedding. Rats were allowed to acclimate to the colony room for 3 days. At that time, 90% free feeding weights were calculated and maintained for the duration of the experiment. Rats received ad libitum access to water in the home cages. A 6:00 AM light/6:00 PM dark cycle was maintained in the colony room. All experimental procedures were conducted during light phase of the cycle. Protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
Conditioning sessions were conducted in 10 chambers (ENV-008CT; Med Associates, Georgia, VT, USA). Each chamber measured 30.5×24.1×21 cm and was enclosed in a sound-attenuating cubicle. A variable-speed syringe pump (PMH-100VS; Med-Associates) was located outside each cubicle. Tygon® tubing was threaded from the pump syringe, through a leash, into the chamber to be attached to the catheter port that exited below the scapula of the rat. A recessed receptacle (5.2×5.2×3.8 cm) was centered on 1 sidewall of each chamber. A dipper arm, when raised, provided access to 0.1 ml of 26% (w/v) sucrose in this recessed receptacle. A retractable lever was located on each side of the receptacle. A white cue-light (2.54 cm diameter; 28V, 100-mA) was mounted 7 cm above each lever. A house-light (two white 28V, 100-mA lamps) was located in the cubicle, 10 cm above the Perspex chamber ceiling. An infrared emitter/detector unit was located 4 cm above the rod floor and 14.5 cm from the side wall containing the receptacle. Chamber activity was defined as the number of times this beam was broken.
Drugs
(−)-Nicotine hydrogen tartrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Nicotine was dissolved in 0.9% sterile saline and adjusted to a pH of 7.0 ± 0.2 using a dilute NaOH solution. Nicotine was infused intravenously at 35.74 µl over 1 sec at 0.0071 mg/infusion or approximately 0.03 mg/kg/infusion given an average weight of 236 g. Nicotine dose (calculated from the base form) was based on previous research (Levin et al, 2011a; Palmatier et al, 2007).
Preliminary Lever Training
Following colony acclimation and food restriction, rats were trained to lever press with sucrose (26% w/v). Sessions were initiated by illumination of the house-light and random selection of 1 of the 2 levers for insertion into the chamber. Following a lever press, or a lapse of 15 sec, sucrose was available for 4 sec and the lever was retracted. A timeout also commenced with an average duration of 60 sec and a range of 30–89 sec. Following the timeout, this protocol was repeated with the stipulation that the same lever was not presented more than twice in a row. A session terminated when the rat received 60 sucrose deliveries; 65 to 80 min depending on individual performance. Rats were trained until a lever press was made on at least 80% of lever insertions. All rats met criterion between sessions 3 to 5.
Catheter Surgery and Recovery
Rats were then implanted with indwelling jugular catheters using our standard protocol (Charntikov et al, 2013). Rats were anesthetized using a 2:1 ketamine HCl (100 mg/kg) plus xylazine HCl (20 mg/kg) cocktail (intramuscular; IM). Once anesthetized, rats were prepped for surgery and the indwelling catheter was surgically implanted. Buprenorphine (0.1 mg/kg, SC) and atipamezole (0.5 mg/kg, IM) were administered immediately following surgery to control for pain and to terminate anesthesia, respectively. Buprenorphine was again administered 24 h post-surgery. Rats recovered for 7 days, during which, they stayed in their home cages except for daily flushing of catheters with a cocktail of 0.2 ml cefazolin (50 Units/ml) and heparin (30 Units/ml). Catheters were check for patency on the last day of recovery by IV infusion of 0.05 ml xylazine (20 Units/ml). Rats that displayed motor ataxia within 20 sec were considered patent (Charntikov et al, 2013; Reichel et al, 2008). Patency was again checked following session 20 of the self-administration phase (21 total sessions, see below). Rats that were not patent were excluded from the study. Forty-one rats (22 female; 19 male) recovered from surgery and remained patent for the duration of the experiment.
Post-surgery Training
Following recovery, rats were trained to respond on a variable ratio 3 (VR3) schedule with a sucrose reinforcer. Training consisted of three daily 1 h sessions conducted on consecutive days. Levers were again inserted individually with the condition that the same lever was not inserted more than 2 times consecutively. In contrast to pre-surgery training, lever pressing was required to receive sucrose access. On average, every 3rd lever press (range 1–5) was followed by 4-sec access to sucrose. These procedures produced robust responding with both levers having a similar reinforcement history. By session 3, all rats earned greater than 80% of 60 possible sucrose deliveries.
Self-administration
Following post-surgery training, female and male rats were assigned to 1 of 2 conditions; nicotine self-administration or saline-yoked. This produced 4 separate groups: nicotine-female (n=12), saline-female (n=10), nicotine-male (n=10), and saline-male (n=9). Assignment of active and inactive lever was counterbalanced as much as allowed by the sample size for each group. Before a rat was attached to the leash/tubing and the start of each session, the catheter was flushed with 0.2-ml heparin (30 Units/ml) in sterile saline. After each session, the catheter was flushed with a 0.2 ml of the cefazolin/heparin cocktail. Sessions began with insertion of both levers and priming of the catheter with nicotine or saline [ca. 31 µl (90% of internal catheter volume)] depending on group assignment. Rats in the nicotine self-administration groups received nicotine by pressing the active lever on a VR3 schedule. Responses on the inactive lever were recorded but held no programmed consequence. Following a nicotine infusion, a 20-sec timeout commenced. Timeouts were signaled by illumination of the house-light and extraction of the levers. Following the timeout, both levers were extended and the light was turned off. Rats in the saline-yoked groups were presented with two inactive levers; saline infusions and consequent signaled timeouts were matched to the response pattern of a nicotine self-administering partner. That is, responding on the two levers was recorded, but the saline rats did not control the infusions. Self-administration sessions (1 h) were conducted daily for 21 consecutive days. Brains were extracted 3 h after the last self-administration session, rapidly frozen with dry ice, and stored at −80°C for neurotensin/substance P processing.
Brain Dissection
After treatments, brains were removed rapidly, frozen immediately on dry ice and stored at −80° C until dissected and analyzed. For regional studies, brain areas were dissected from consecutive 1-mm thick coronal slices as previously described (Alburges et al. 2007; Alburges et al. 2009; Alburges et al., 2011). Based on the atlas of Paxinos and Watson (1986), regions were removed and subsequently stored at −80° C until assayed for NTLI and SPLI levels.
Radioimmunoassay
Antiserum was raised against the neurotensin carboxy-terminus in New Zealand White rabbits and expresses no cross-reactivity with 1000-fold excess concentrations of other endogenous neuropeptides, including dynorphin, metenkephalin, cholecystokinin, substance P or substance K. The substance P antiserum was also raised in New Zealand White rabbits as previously described (Letter et al, 1987). This antiserum recognizes the substance P carboxy terminus and expresses no cross-reactivity with 1,000-fold excess concentrations with dynorhpin A, metenkephalin, neurotensin, or substance K.
Neuropeptide (NTLI or SPLI) concentrations within brain regions were determined by solid-phase radioimmunoassay (RIA) as previously described (Alburges et al, 2009; Alburges et al, 2011). Briefly, dissected tissue was homogenized in 300 µL 10 mM HCl, boiled for 10 min, then centrifuged at 17000 g for 30 min. A small amount of supernatant was collected for protein analysis using the Bradford assay. The remaining sample was lyophilized and stored at −80° C until further use. For the RIA, lyophilized samples were reconstituted in assay buffer consisting of 300 µL phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, and 1.8 mM KH2PO4 in dH20; pH 7.4), 0.1% (w/v) gelatin and 0.1% (w/v) Triton X-100. Nunc-Immunoplates (ISI Bio-Express; Kaysville, UT) were prepared for the assay by incubating 50 µL protein G solution (50 ng/100 mL in 0.1 mol/L NaHCO3; Invitrogen; Carlsbad, CA) per well overnight at 4 °C followed by three washes with wash buffer (150 mM K2HPO4, 20 mM Na2HPO4, 0.2 mM ascorbic acid, 0.2% (v/v) Tween-20 and 0.1% (w/v) sodium azide in dH20; pH 7.4). Neurotensin (25 µL; 1:20000 dilution) and substance P (25 µL) antisera were diluted in assay buffer [wash buffer containing 0.1% (w/v) gelatin], incubated in plate wells for 2 h at 25° C to attach antibody to protein G surface, and then washed three times with wash buffer. Twenty-five µL of samples or standards were added to wells and incubated for 3 h at 25 °C. Radiolabeled neurotensin or substance P ([125I]neurotensin or [125I]substance P; 6500 dpm per 25 µL diluted in assay buffer) was then added to each well and incubated for 2 h at 25 °C. Following incubation, wells were washed with wash buffer and protein G was removed from wells, placed in polypropylene tubes and radioactivity was counted in a five-channel Packard Cobra II Auto-Gamma counter (Packard Instrument Co.; Meriden, CT). NTLI and SPLI concentrations were determined by comparing bound to free [125I]neurotensin or [125I]substance P in each sample to standard curves ranging from 1 to 125 pg protein per assay tube.
Dependent Measures
Lever-pressing was the primary dependent measure during the self-administration phase of the experiment. To show inactive lever responding relative to active lever responding, a discrimination index was calculated using the following formula: Discrimination Index = [Active Lever Presses/(Active Lever Presses + Inactive Lever Presses)]. A Discrimination Index value of 0.5 indicates equal responding on the active and inactive lever (i.e., no discrimination between levers); a value >0.5 indicates more pressing on the active lever. Total nicotine intake controlling for body weight was calculated by the equation: nicotine intake = (group average weight (g) used to calculate dose/rat’s weight (g) on each self-administration day * 0.03 mg * infusions earned). This calculation allowed us to keep the dose of nicotine per infusion constant and still properly assess possible differences in total nicotine intake. NTLI and SPLI levels were measured in all of the brain regions.
Data Analyses
Analyses of active lever-pressing in the SA phase used two-factor ANOVAs with Sex (females vs. males) as a between-subjects factor and Sessions as a within-subjects factor. The nicotine groups were analyzed separate from the saline-yoked groups. An independent samples t-test was used to analyze differences in total nicotine intake between females and males. NTLI and SPLI levels for each brain region were analyzed using separate two-factor ANOVAs with Sex (females vs. males) and Drug (nicotine vs. saline) as between-subjects factors. For all analyses, significant main effects or interactions were followed by post-hoc pairwise comparisons. Pairwise tests were conducted on the following comparisons: nicotine-males vs. nicotine-females; saline-males vs. saline-females; nicotine-males vs. saline-males; nicotine-females vs. saline-females. Bonferroni correction was used to control family-wise error rate with significance set at p<0.05. Corrected p values are reported for all significant post-hoc tests. Outliers were identified and excluded from analysis using Grubs’ test for outliers.
Results
Self-administration
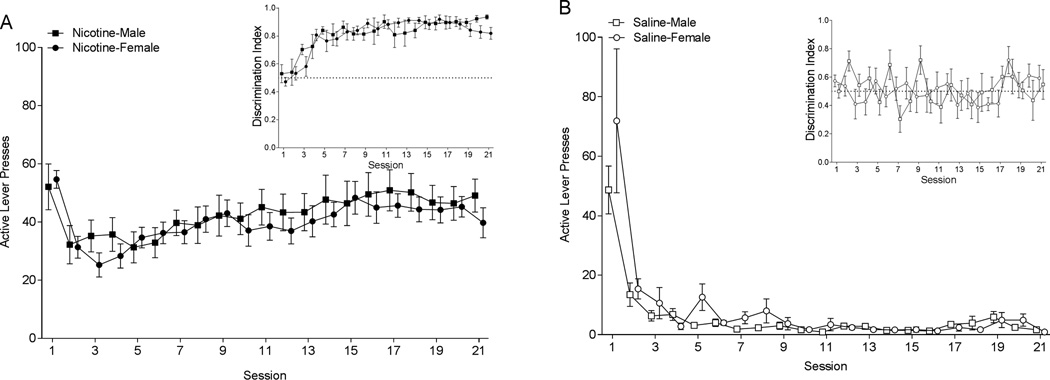
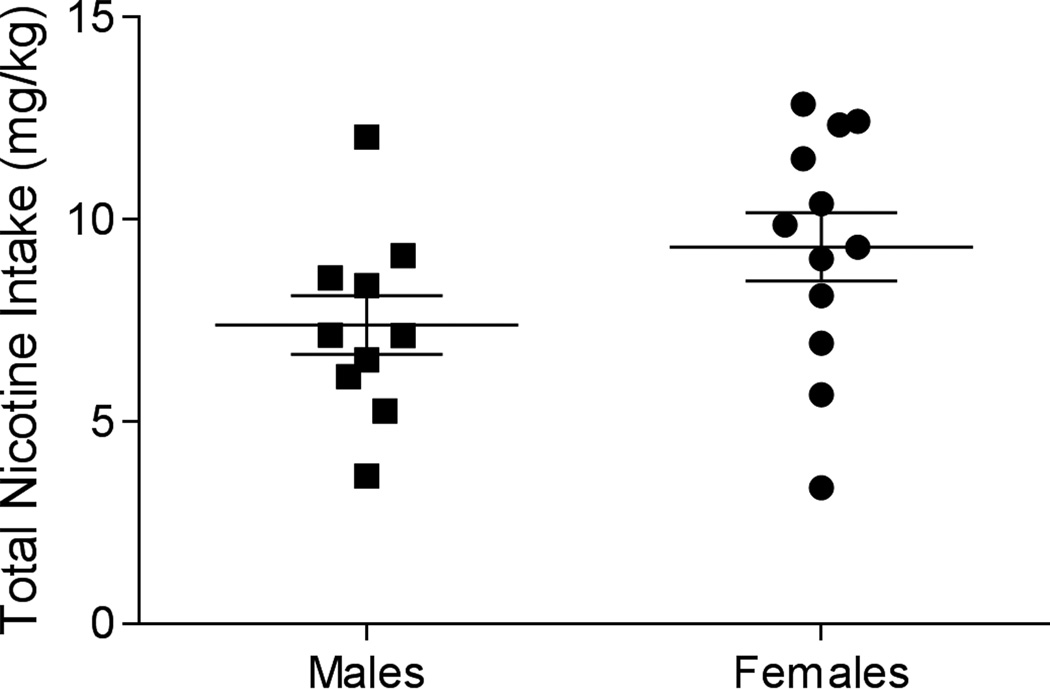
Rats in the nicotine groups demonstrated robust active lever-pressing (Figure 1A). Analysis of active lever-pressing in nicotine groups revealed a significant main effect of Session [F(20,400)=5.413; p<0.001]. Males and females responded similarly as neither the main effect of Sex (F<1; p=0.597), nor the Sex × Session interaction (F<1; p=0.9310) was significant. Responding in the saline-yoked groups quickly decreased following preliminary training (Figure 1B). The saline-yoked groups, analysis revealed a main effect of Session [F(20,340)=16.57; p<0.001]. The main effect of Sex (F<1; p=0.996) and the Sex × Session interaction (F<1; p=0.833) were not significant. Males and females that had nicotine reinforcing lever pressing displayed clear discrimination between the active and inactive lever (Inset Figure 1A; discrimination ratio well above 0.5), whereas rats in the saline-yoked groups did not discriminate between the levers (Inset Figure 1B; discrimination ratio around 0.5). This latter finding was expected as the saline-yoked rats essentially had two inactive levers. Total nicotine intake during the SA phase did not differ between male (7.39 mg/kg ± 0.739) and female (9.32 mg/kg ± 0.842) rats [t(20)=1.69, p=0.106; Figure 2].
Figure 1.
Panel A shows active lever presses for the males (closed square) and females (closed circle) responding for nicotine infusions during self-administration sessions. Rats responded on a VR3. The inset graph shows discrimination between the active and inactive lever over self-administration sessions. Panel B shows active lever presses for males (open square) and females (open circle) in the yoked-saline group. Responding was recorded, but yoked rats did not control the saline infusions. The inset graph shows discrimination between the active and inactive lever during self-administration sessions.
Figure 2.
Total nicotine intake throughout self-administration is displayed for male (closed square) and female (closed circle) rats.
Neurotensin
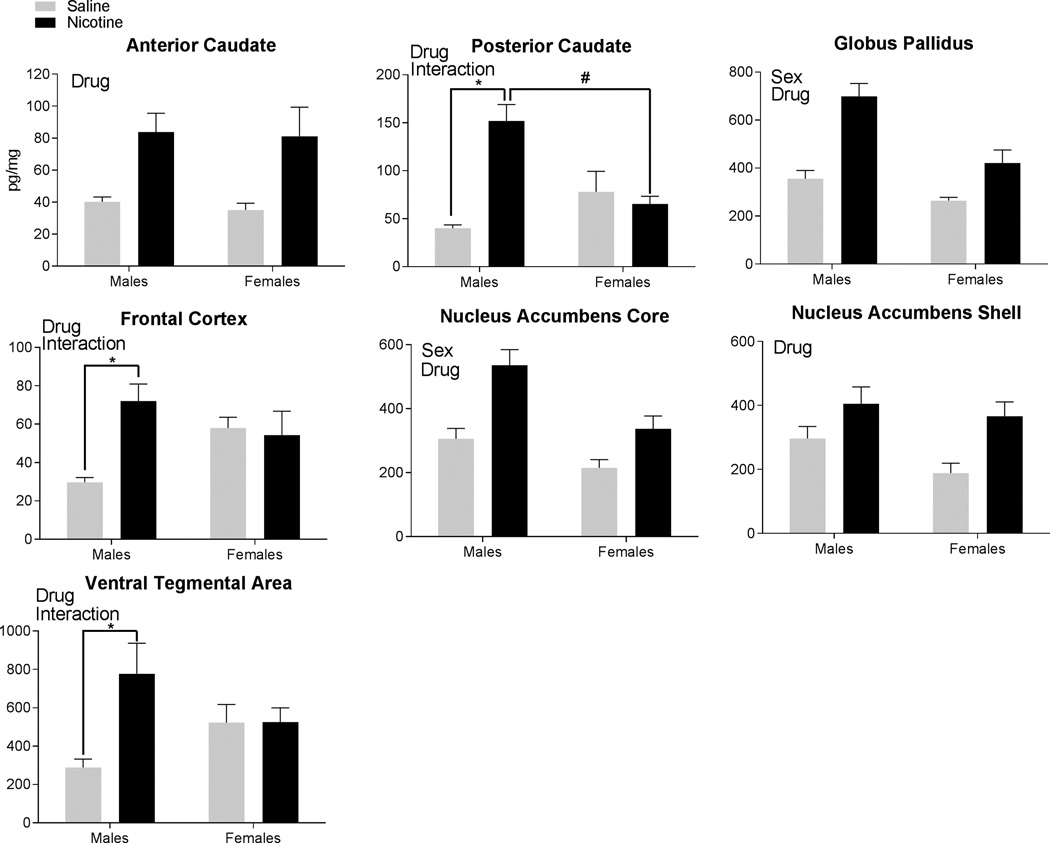
Tissue levels of NTLI were significantly higher in rats self-administering nicotine compared to the saline-yoked rats throughout the examined brain areas (Figure 3). The main effect of Drug was significant in the anterior caudate [F(1,35)=13.25, p<0.001], posterior caudate [F(1,31)=17.22, p<0.001], globus pallidus [F(1,33)=28.06, p<0.001], frontal cortex [F(1,35)=4.61, p<0.05], nucleus accumbens core [F(1,33)=19.17, p<0.001], nucleus accumbens shell [F(1,34)=10.61, p<0.01], and the ventral tegmental area [F(1,32)=5.51, p<0.05]. All brain areas had higher NTLI levels in the nicotine rats compared to the saline-yoked rats. The main effect of Sex was significant in the globus pallidus [F(1,33)=15.32, p<0.001] and the nucleus accumbens core [F(1,33)=12.90, p<0.01]. In these areas, higher tissue levels of NTLI were detected in males compared to females.
Figure 3.
Neurotensin-like immunoreactivity found in the brain regions following self-administration is displayed. Each panel uses the term “Sex” to signify regions with a significant main effect of Sex, the term “Drug” to signify regions with a significant main effect of Drug, and the term “Interaction” to denote regions with significant Sex × Drug interactions. A significant interaction was followed by post-hoc tests. * signifies significant differences within each sex identified in post-hoc testing following nicotine vs. saline-yoked self-administration. # signifies significant differences within each drug condition identified in post-hoc testing between the females and males.
The Sex × Drug interaction was significant in 3 brain regions. For the posterior caudate [F(1,31)=17.22, p<0.001], post-hoc tests revealed that the nicotine-male rats were significantly higher than the saline-male rats (p<0.001) and higher than the nicotine-female rats (p<0.001). No other comparisons were significant. There was also a significant Sex × Drug interaction in the frontal cortex [F(1,35)=6.57, p<0.05]. Post-hoc tests revealed that the nicotine-males were higher than the saline-males; no other comparisons differed significantly. The Sex × Drug interaction was also significant in the ventral tegmental area [F(1,32)=5.51, p<0.05]. Post-hoc tests indicated that male-nicotine rats were significantly higher than the male-saline rats; no other comparisons were significant.
Substance P
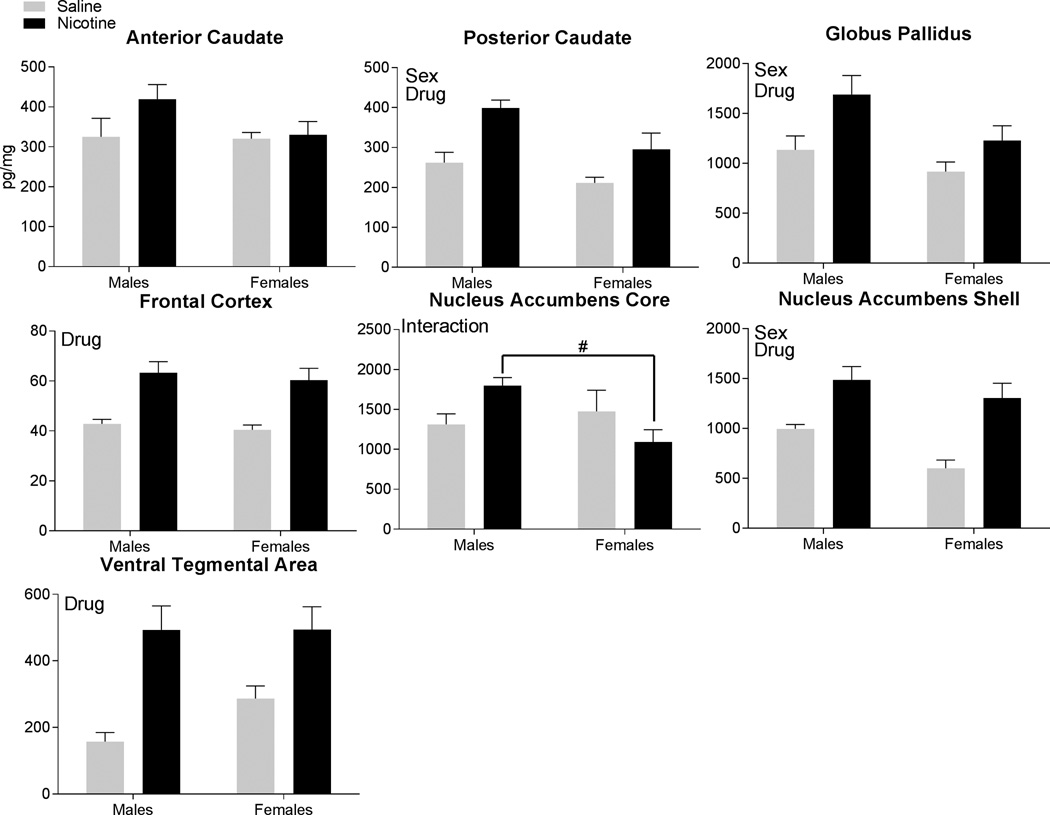
The main effect of Drug was significant for the analysis of SPLI tissue levels (Figure 4) in the posterior caudate [F(1,35)=14.91, p<0.001], globus pallidus [F(1,32)=7.93, p<0.01], frontal cortex [F(1,35)=28.66, p<0.001], nucleus accumbens shell [F(1,33)=22.95, p<0.001], and the ventral tegmental area [F(1,31)=23.06, p<0.001]. In all of these brain regions, the nicotine rats had higher tissue levels of SPLI compared to the saline-yoked rats. The main effect of Sex was significant in the posterior caudate [F(1,35)=7.30, p<0.05], globus pallidus [F(1,32)=4.87, p<0.05], and nucleus accumbens shell [F(1,33)=5.36, p<0.05]. In these areas, males had higher tissue levels of SPLI than females. The Sex × Drug interaction was significant only in the nucleus accumbens core [F(1,35)=13.25, p<0.001]. Post-hoc tests revealed that nicotine-males had significantly higher levels of substance P than the nicotine-females; no other comparisons were significant.
Figure 4.
Substance P-like immunoreactivity found in the brain regions following self-administration is displayed. Each panel uses the term “Sex” to signify regions with a significant main effect of Sex, and the term “Drug” to signify regions with a significant main effect of Drug, and the term “Interaction” to denote regions with significant Sex × Drug interactions. A significant interaction was followed by post-hoc tests. * signifies significant differences within each sex identified in post-hoc testing following nicotine vs. saline-yoked self-administration. # signifies significant differences within each drug condition identified in post-hoc testing between the females and males.
Discussion
Consistent with previous studies, male and female rats in the present report readily self-administered nicotine (Chaudhri et al, 2005; Chen et al, 2007; Donny et al, 2000; Feltenstein et al, 2012; Grebenstein et al, 2013; Johnson et al, 2012; Levin et al, 2011b; Li et al, 2014; Lynch, 2009; Park et al, 2007; Rezvani et al, 2008; Swalve et al, 2015; Wang et al, 2014). Further, nicotine intake was not significantly different between male and female rats, similar to several previous studies (Chen et al, 2007; Feltenstein et al, 2012; Li et al, 2014; Maehler et al, 2000; Nesil et al, 2011; Swalve et al, 2015). This finding is not universal, however, as studies have reported higher intake in females than males (Chaudhri et al, 2005; Donny et al, 2000; Li et al, 2014; Lynch, 2009; Rezvani et al, 2008 Wang et al, 2014), and there is at least one study reporting greater intake by males than by females (Johnson et al, 2012). The inconsistencies across studies likely reflects methodological differences. Inter-laboratory variations are prevalent in the route of nicotine administration (i.e., IV vs. oral), schedule of reinforcement (e.g., FR1 vs. FR5 vs. VR3), nature and use of signaled timeouts, dose of nicotine (0.01–0.15 mg/kg/inf), age of the rats (e.g., adolescent vs. adult), and training procedures (e.g., presence vs. absence of preliminary lever training; escalating vs. fixed reinforcement schedule). Elucidation of how these factors alter sex differences in nicotine intake is important, but largely outside of the scope of this report. From our perspective, the finding that nicotine intake was not different between the sexes allows us to conclude that possible sex differences in LR NTLI and SPLI levels following nicotine self-administration does not reflect quantitative differences in nicotine exposure.
Tissue levels of NTLI were significantly augmented by nicotine SA in several LR brain regions. Tissue levels of NTLI in all 7 brain regions investigated were increased following nicotine SA in the male rats. This consistent increase in males induced by nicotine SA contrasts with findings obtained from studies where the nicotine was acutely administered by the investigator. For example, Alburges et al (2007) found that IP injections of 4.0 mg/kg (5 injections of 0.8 mg/kg; injections separated by 2 hours) actually decreased NTLI in tissue of the ventral tegmental area, prefrontal cortex, and anterior striatum after drug treatment, with no effect in the nucleus accumbens core and shell areas. Alburges and colleagues (2007) suggested that the transiently reduced NTLI levels in these regions reflected enhanced neurotensin release, turnover, and depletion (Merchant et al, 1989; Wagstaff et al, 1994; Wagstaff et al, 1996). Albeit speculative, the increased NTLI tissue levels reported herein after long-term exposure to self-administered nicotine, may be a compensatory response and reflect a reduced decrease in neurotensin release as previously reported for the SA of methamphetamine (Frankel et al 2010), another stimulant. That is, the chronic effects on these neurotensin systems induced by repeated, long-term nicotine intake may evoke increased synthesis and/or decreased release via opponent-process counter-adaptations. Contrary neurobiological effects following acute vs. chronic drug administration are not uncommon (for a review see, Koob and Le Moal, 2001). Future studies characterizing the precise mechanisms by which nicotine SA increases tissue levels of neurotensin will be of interest. It is also noteworthy that the reduction in NTLI levels caused by the acute investigator-induced nicotine treatments was mediated by D2 and not D1 receptors. This conclusion is supported by findings that D2 agonists alone reduce NTLI tissue levels in basal ganglia and limbic regions, whereas D1 agonists do the opposite and elevate NTLI levels in these brain regions (Merchant et al, 1989). Thus, the current observations suggest that the responses of neurotensin systems to acute nicotine noncontingent exposures are D2-mediated (i.e., NTLI levels decrease), but D1 receptors become critical when exposure to nicotine is done contingently for extended periods (i.e., NTLI levels increase).
Given the importance of dopaminergic action to nicotine addiction (Benwell et al, 1992; Benwell et al, 1994; Calabresi et al, 1989; Corrigall et al, 1994; Corrigall et al, 1992; Damsma et al, 1989; Grenhoff et al, 1986; Imperato et al, 1986; Marshall et al, 1997; Mifsud et al, 1989; Nisell et al, 1997), neurotensin modulation of dopamine in LR brain regions suggests elucidation of the effects of nicotine on this neuropeptide system is vital to understanding nicotine-taking behavior. Previous work has found that neurotensin agonists block nicotine-induced sensitization (Fredrickson et al, 2003a, 2003b), attenuates nicotine, as well as methamphetamine, SA via indirect inhibitory modulation of dopamine neurotransmission (Boules et al, 2011; Frankel et al 2010), and restores dopamine levels following nicotine SA relative to saline controls (Boules et al, 2011; Liang et al, 2008). These findings support the possibility that nicotine SA decreases the release of neurotensin from limbic-related brain regions, prohibiting neurotensin mitigation of dopaminergic neurotransmission, thereby enhancing the response by dopaminergic pathways to nicotine exposure and enhancing the tenacity of nicotine addiction.
In female rats, tissue NTLI levels were also increased in the anterior caudate, globus pallidus, nucleus accumbens core, nucleus accumbens shell, and ventral tegmental area following SA, but were unaffected by nicotine exposure in the posterior caudate, frontal cortex, and ventral tegmental area. The differences between neurotensin in males (i.e., NTLI was increased in all 7 brain regions examined) and females were significant. Main effects of Sex were found in the globus pallidus and nucleus accumbens core; male rats had higher tissue levels of NTLI than the females, suggesting a possible sex-related role by neurotensin systems in these regions. The finding that neurotensin sex differences are region-dependent parallel the results of previous studies (Bello et al, 2004; Herbison and Theodosis, 1992; Rugarn et al, 1999). Relevant to our study, Rugarn et al (1999) similarly did not detect sex differences in the frontal cortex. Contrary to our report, however, they did not detect any sex differences in the striatum, whereas we do see differences in the nucleus accumbens core. These differences may be a result of the striatum being investigated as a whole vs. separated further into sub-regions, or may be a consequence of methodological differences such as the age of the animal and the time spent exploring and interacting with the operant chamber. In addition to sex differences in basal tissue levels of neurotensin, nicotine SA differentially altered neurotensin levels in males and females. In the posterior caudate, frontal cortex, and ventral tegmental area, nicotine intake increased tissue levels of NTLI in the males, but did not alter levels in the females. Increases in tissue levels found exclusively in the males may represent elevated synthesis of NTLI, however, as mentioned earlier it may reflect inhibited neurotensin release and turnover in the males, but not in the females. The functional outcome of these sex differences in neurotensin is of great interest. While we did not see behavioral differences in nicotine self-administration between the sexes in this study, the possibility remains that these differences in neurotensin may play a role in behavioral differences in nicotine sensitization (Harrod et al, 2004; Kanyt et al, 1999), nicotine withdrawal (Gentile et al, 2011; Slotkin et al, 2014; Torres et al, 2015), and/or nicotine reinstatement (Wang et al, 2012). Future studies will be needed to examine and possibly manipulate neuropeptide levels in limbic-related brain regions to determine their role in sex differences in these behavioral paradigms.
For comparison, we also examined the effect of nicotine on substance P systems, which increase the activity of dopamine pathways. Nicotine SA in this study elevated SPLI levels in the posterior caudate, globus pallidus, frontal cortex, nucleus accumbens shell, and ventral tegmental area in male and female rats. Similar to our neurotensin results, the increased SPLI tissue levels following 21 days of nicotine SA differ from findings following a short-term non-contingent nicotine “binge” treatment (Alburges et al, 2009; Alburges et al, 2007; Naftchi et al, 1988). Thus, after investigator-administered nicotine, tissue levels of SPLI were lower in the ventral tegmental area, prefrontal cortex, nucleus accumbens shell, and very posterior caudate in male rats (Alburges et al, 2009; Alburges et al, 2007, Naftchi et al, 1988). As with the neurotensin, these decreases in SPLI levels likely reflected increased substance P release and turnover (Alburges et al, 2009). Thus, as discussed with the effects in the neurotensin systems, the difference in SPLI levels after acute non-contingent binge treatment vs. long-term SA of nicotine may be explained by compensatory responses and a shift in dopamine receptor mechanisms. That is, increased release and turnover following nicotine treatment may trigger counter-adaptations in dopamine systems when nicotine is administered chronically, inducing the decreased substance P-release pattern (i.e., increased SPLI tissue levels) found herein.
Several limitations of this study warrant discussion. First, female rats were allowed to freely cycle and we did not assess stages of the estrous cycle throughout the experiment. While work has found that phase of estrous does not predict nicotine self-administration in adult rats (Donny et al. 2000; Feltenstein et al, 2012; Rezvani et al, 2008), others have found that nicotine responding is negatively associated with progesterone and positively correlated with estradiol to progesterone ratio in adolescent rats (Lynch, 2009). We also did not determine how variance in sex hormones may have affected neuropeptide levels at the time of sacrifice. Previous work has shown that estrogen treatment in ovariectomized rats does alter dopaminergic function, including increasing dopamine transporter levels and reducing D2 receptor density in the nucleus accumbens and caudate nucleus (Chavez et al, 2010). Estrogen also alters nicotine-evoked dopamine release (Dluzen and Anderson; 1997), increasing dopamine levels in ovariectomized females treated with nicotine. Additionally, neuropeptides can be regulated by estrogen exposure (Alexander et al, 1989a, 1991; Alexander and Leeman, 1994; Szot and Dorsa, 1994; Waters and Dorsa, 1998; for a review see Rostene and Alexander, 1997). Examining how fluctuations in hormones throughout the estrous cycle may alter nicotine-evoked neuropeptide levels and these neuropeptides subsequent interaction with dopamine will be important to understanding the possible biological mechanisms underlying sex differences in nicotine dependence. A second limitation of this study was that the antisera used in the RIA assays recognize the 5 amino acid carboxyl terminal for neurotensin and substance P. Consequently as long as the same carboxyl terminal is present, the antiserum cannot distinguish between the peptide itself, a precursor, or a metabolite fragment. However, because the carboxyl terminal is thought to be principally responsible for the peptides’ physiological activities, the RIA determinations are indirect measures of peptide effects (Letter et al. 1987).
In summary, based on assessments of limbic and basal ganglia dopamine-linked neuropeptides, we have determined that the interactions between dopamine and neurotensin/substance P systems are differentially influenced by nicotine exposure according to the duration and the contingent vs. noncontingent nature of the treatment. We also observed that some of the neuropeptide responses to nicotine SA appear to be sex-dependent. Consequently our findings suggest elucidation of the dopaminergic mechanism underlying our neuropeptide responses to nicotine SA will help to better understand the process of nicotine SA and the role of gender in the development and maintenance of nicotine dependence.
Acknowledgments
This research was supported by NIH research grant DA034389 and DA031883.
References
- Alburges ME, Frankel PS, Hoonakker AJ, Hanson GR. Responses of limbic and extrapyramidal substance P systems to nicotine treatment. Psychopharmacology (Berl) 2009;201(4):517–527. doi: 10.1007/s00213-008-1316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alburges ME, Hoonakker AJ, Hanson GR. Nicotinic and dopamine D2 receptors mediate nicotine-induced changes in ventral tegmental area neurotensin system. Eur J Pharmacol. 2007;573(1–3):124–132. doi: 10.1016/j.ejphar.2007.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alburges ME, Hoonakker AJ, Horner KA, Fleckenstein AE, Hanson GR. Methylphenidate alters basal ganglia neurotensin systems through dopaminergic mechanisms: a comparison with cocaine treatment. J Neurochem. 2011;117:470–478. doi: 10.1111/j.1471-4159.2011.07215.x. [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Leeman SE. Estrogen-inducible neurotensin immunoreactivity in the preoptic area of the female rat. J Comp Neurol. 1994;345:496–509. doi: 10.1002/cne.903450403. [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Dobner PR, Miller MA, Bullock BP, Dorsa DM, Leeman SE. Estrogen induces neurotensin/neuromedin N messenger ribonucleic acid in a preoptic nucleus essential for the preovulatory surge of luteinizing hormone in the rat. Endocrinology. 1989a;125:2111–2117. doi: 10.1210/endo-125-4-2111. [DOI] [PubMed] [Google Scholar]
- Alexander MJ, Kiraly ZJ, Leeman SE. Sexually dimorphic distribution of neurotensin/neuromedin N mRNA in the rat preoptic area. J Comp Neurol. 1991;311:84–96. doi: 10.1002/cne.903110107. [DOI] [PubMed] [Google Scholar]
- Bello AR, Reyes R, Hernández G, Negrín I, González M, Tramu G, et al. Developmental expression of neurotensin in thyrotropes and gonadotropes of male and female rats. Neuroendocrinology. 2004;79(2):90–99. doi: 10.1159/000076632. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine Addiction. N Engl J Med. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105(4):849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Khadra LF. Studies on the influence of nicotine infusions on mesolimbic dopamine and locomotor responses to nicotine. Clin Investig. 1994;72(3):233–239. doi: 10.1007/BF00189320. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001;53(4):453–486. [PubMed] [Google Scholar]
- Boules M, Oliveros A, Liang Y, Williams K, Shaw A, Robinson J, et al. A neurotensin analog, NT69L, attenuates intravenous nicotine self-administration in rats. Neuropeptides. 2011;45(1):9–16. doi: 10.1016/j.npep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98(1):135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quitting Smoking Among Adults—United States, 2001–2010. Morbidity and Mortality. Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults—United States, 2005–2013. Morbidity and Mortality Weekly Report. 2014;63(47):1108–1112. [PMC free article] [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72(4):712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, et al. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology. 2013;75C:138–144. doi: 10.1016/j.neuropharm.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180(2):258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–59. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Commons KG. Neuronal pathways linking substance P to drug addiction and stress. Brain Res. 2010;1314:175–182. doi: 10.1016/j.brainres.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653(1–2):278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107(2–3):285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168(3):363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230(2):140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther. 2010;336(3):809–815. doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson P, Boules M, Yerbury S, Richelson E. Blockade of nicotine-induced locomotor sensitization by a novel neurotensin analog in rats. Eur J Pharmacol. 2003a;458(1–2):111–118. doi: 10.1016/s0014-2999(02)02689-4. [DOI] [PubMed] [Google Scholar]
- Fredrickson P, Boules M, Yerbury S, Richelson E. Novel neurotensin analog blocks the initiation and expression of nicotine-induced locomotor sensitization. Brain Res. 2003b;979(1–2):245–248. doi: 10.1016/s0006-8993(03)02895-6. [DOI] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull. 2011;85(3–4):145–152. doi: 10.1016/j.brainresbull.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114–115:70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128(3):351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, et al. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol Biochem Behav. 2004;78(3):581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50(2):283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132(2–3):337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, et al. Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology (Berl) 2012;222(2):269–276. doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanýt L1, Stolerman IP, Chandler CJ, Saigusa T, Pöğün S. Influence of sex and female hormones on nicotine-induced changes in locomotor activity in rats. Pharmacol Biochem Behav. 1999;62(1):179–187. doi: 10.1016/s0091-3057(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Letter AA, Merchant K, Gibb JW, Hanson GR. Effect of methamphetamine on neurotensin concentrations in rat brain regions. J Pharm Exp Ther. 1987;241:443–447. [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, et al. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther. 2011a;338(3):890–896. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, et al. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behav Brain Res. 2011b;225(2):473–481. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, Lê AD. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict Biol. 2014;19(2):156–164. doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- Liang Y, Boules M, Shaw AM, Williams K, Fredrickson P, Richelson E. Effect of a novel neurotensin analog, NT69L, on nicotine-induced alterations in monoamine levels in rat brain. Brain Res. 2008;1231:6–15. doi: 10.1016/j.brainres.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehler R, Dadmarz M, Vogel WH. Determinants of the voluntary consumption of nicotine by rats. Neuropsychobiology. 2000;41(4):200–204. doi: 10.1159/000026660. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem. 1997;68(4):1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Gibb JW, Hanson GR. Role of dopamine D-1 and D-2 receptors in the regulation of neurotensin systems of the neostriatum and the nucleus accumbens. Eur J Pharmacol. 1989;160(3):409–412. doi: 10.1016/0014-2999(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Mifsud JC, Hernandez L, Hoebel BG. Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res. 1989;478(2):365–367. doi: 10.1016/0006-8993(89)91518-7. [DOI] [PubMed] [Google Scholar]
- Naftchi NE, Maker H, Lapin E, Sleis J, Lajtha A, Leeman S. Acute reduction of brain substance P induced by nicotine. Neurochem Res. 1988;13(4):305–309. doi: 10.1007/BF00972478. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61(1–2):189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Marcus M, Nomikos GG, Svensson TH. Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J Neural Transm. 1997;104(1):1–10. doi: 10.1007/BF01271290. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2014;76(Pt B):566–580. doi: 10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockene Jk. Smoking among women across the life span: Prevalence, interventions, and implications for cessation research. Annals of Behavioral Medicine. 1993;15(2–3):135–148. [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 2007;195(2):235–243. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han SH, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86(2):297–305. doi: 10.1016/j.pbb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1986. [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebr Symp Motiv. 2009;55:143–169. doi: 10.1007/978-0-387-78748-0_9. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10(7):1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Peters SA, Huxley RR, Woodward M. Do smoking habits differ between women and men in contemporary Western populations? Evidence from half a million people in the UK Biobank study. BMJ Open. 2014;4(12):e005663. doi: 10.1136/bmjopen-2014-005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogun S. Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning. Int J Psychophysiol. 2001;42(2):195–208. doi: 10.1016/s0167-8760(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89(3):463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, et al. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154(3):885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostene WH, Alexander MJ. Neurotensin and neuroendocrine regulation. Front Neuroendocrinol. 1997;18(2):115–173. doi: 10.1006/frne.1996.0146. [DOI] [PubMed] [Google Scholar]
- Rugarn O, Hammar M, Theodorsson A, Theodorsson E, Stenfors C. Sex differences in neuropeptide distribution in the rat brain. Peptides. 1999;20(1):81–86. doi: 10.1016/s0196-9781(98)00139-9. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Nicotine administration in adolescence reprograms the subsequent response to nicotine treatment and withdrawal I adulthood: sex-selective effects on cerebrocortical serotonergic function. Brain Res Bull. 2014;102:1–8. doi: 10.1016/j.brainresbull.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME. Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology. 2015:1–9. doi: 10.1007/s00213-015-4183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Pipkin JA, Ferree P, Carcoba LM, O’Dell LE. Nicotine withdrawal increases stress associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine Tob Res. 2015;17(4):420–430. doi: 10.1093/ntr/ntu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, O'Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall CD, Ginsberg D, Hall SM. Quitting smoking. Int J Addict. 1985;20(6–7):1089–1112. doi: 10.3109/10826088509047766. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000;93(1–3):125–136. doi: 10.1016/s0167-0115(00)00183-x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. A Report of the Surgeon General. Atlanta. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking—50 Years of Progress. [Google Scholar]
- Wang T, Han W, Wang B, Jiang Q, Solberg-Woods LC, Palmer AA, Chen H. Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genens Brain Behav. 2014;13(2):202–212. doi: 10.1111/gbb.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff JD, Bush LG, Gibb JW, Hanson GR. Endogenous neurotensin antagonizes methamphetamine-enhanced dopaminergic activity. Brain Res. 1994;665(2):237–244. doi: 10.1016/0006-8993(94)91343-9. [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Gibb JW, Hanson GR. Dopamine D2-receptors regulate neurotensin release from nucleus accumbens and striatum as measured by in vivo microdialysis. Brain Res. 1996;721(1–2):196–203. doi: 10.1016/0006-8993(96)00132-1. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Dorsa DM. Transcriptional Effects of Estrogen on Neuronal Neurotensin Gene Expression Involve cAMP/Protein Kinase A-Dependent Signaling Mechanisms. J Neurosci. 1998;18(17):6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67(4):555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]