Abstract
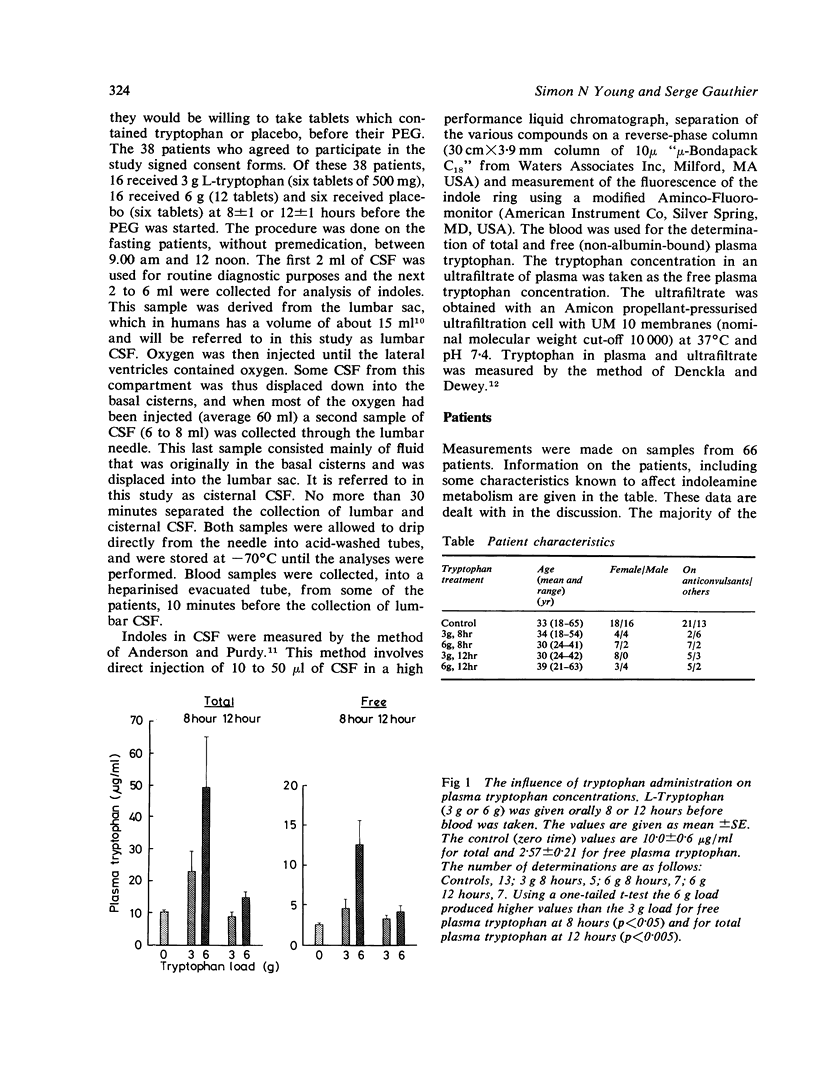
Tryptophan 5-hydroxyindoleacetic acid and indoleacetic acid were measured in cerebrospinal fluid taken during pneumoencephalography from patients, some of whom took a 3 g or 6 g tryptophan load at various times before. Measurements were made on both lumbar and cisternal cerebrospinal fluid and the results showed similarities between indoleamine metabolism in human brain and spinal cord. Our data suggested that (1) the blood-brain barrier active transport system for tryptophan is not far from saturation with tryptophan and the rate-limiting enzyme in 5-hydroxytryptamine (5HT) synthesis, tryptophan hydroxylase, is about half saturated. Therefore, both 3 g and 6 g tryptophan loads produced the same maximum rise in 5HT synthesis of just under 100%, (2) tryptamine differs from 5HT in two respects. It is more sensitive to changes in tryptophan availability than 5HT and the 6 g load increased brain tryptamine metabolism more than the 3 g load; also some of the tryptamine in brain is derived from peripheral sources and diffuses from blood to brain, (3) although the brain tryptamine content is much lower than that of 5HT, its rate of metabolism as indicated by CSF metabolite levels is not. In controls the rate of tryptamine metabolism is 15% of the rate of 5HT metabolism and this can increase to 40% after a 6 g tryptophan load.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. M., Purdy W. C. Liquid chromatographic-fluorometric system for the determination of indoles in physiological samples. Anal Chem. 1979 Feb;51(2):283–286. doi: 10.1021/ac50038a030. [DOI] [PubMed] [Google Scholar]
- Ashcroft G. W., Crawford T. B., Cundall R. L., Davidson D. L., Dobson J., Dow R. C., Eccleston D., Loose R. W., Pullar I. A. 5-Hydroxytryptamine metabolism in affective illness: the effect of tryptophan administration. Psychol Med. 1973 Aug;3(3):326–332. doi: 10.1017/s0033291700049618. [DOI] [PubMed] [Google Scholar]
- Chouinard G., Young S. N., Annable L., Sourkes T. L. Tryptophan-nicotinamide, imipramine and their combination in depression. A controlled study. Acta Psychiatr Scand. 1979 Apr;59(4):395–414. doi: 10.1111/j.1600-0447.1979.tb04482.x. [DOI] [PubMed] [Google Scholar]
- Curzon G., Kantamaneni B. D., Bartlett J. R., Bridges P. K. Transmitter precursors and metabolites in human ventricular cerebrospinal fluid. J Neurochem. 1976 Mar;26(3):613–615. doi: 10.1111/j.1471-4159.1976.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Denckla W. D., Dewey H. K. The determination of tryptophan in plasma, liver, and urine. J Lab Clin Med. 1967 Jan;69(1):160–169. [PubMed] [Google Scholar]
- Eccleston D., Ashcroft G. W., Crawford T. B. Effect of tryptophan administration on 5HIAA in cerebrospinal fluid in man. J Neurol Neurosurg Psychiatry. 1970 Apr;33(2):269–272. doi: 10.1136/jnnp.33.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne P., Young S. M., Sourkes T. L. Inhibition by albumin of tryptophan uptake by rat brain. Nature. 1976 Jul 8;262(5564):144–145. doi: 10.1038/262144a0. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Kappelman A. H., Kaufman S. Partial purification and characterization of tryptophan hydroxylase from rabbit hindbrain. J Biol Chem. 1972 Jul 10;247(13):4165–4173. [PubMed] [Google Scholar]
- Garelis E., Young S. N., Lal S., Sourkes T. L. Monoamine metabolites in lumbar CSF: the question of their origin in relation to clinical studies. Brain Res. 1974 Oct 11;79(1):1–8. doi: 10.1016/0006-8993(74)90562-9. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971 Jun;18(6):1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Jakupcević M., Lacković Z., Stefoski D., Bulat M. Nonhomogeneous distribution of 5-hydroxyindoleacetic acid and homovanillic acid in the lumbar cerebrospinal fluid of man. J Neurol Sci. 1977 Mar;31(2):165–171. doi: 10.1016/0022-510x(77)90103-4. [DOI] [PubMed] [Google Scholar]
- Marsden C. A., Curzon G. The contribution of tryptamine to the behavioural effects of l-Tryptophan in tranylcypromine-treated rats. Psychopharmacology (Berl) 1978 Apr 14;57(1):71–76. doi: 10.1007/BF00426960. [DOI] [PubMed] [Google Scholar]
- Vapalahti M., Hyyppä M. T., Nieminen V., Rinne U. K. Brain monoamine metabolites and tryptophan in ventricular CSF of patients with spasm after aneurysm surgery. J Neurosurg. 1978 Jan;48(1):58–63. doi: 10.3171/jns.1978.48.1.0058. [DOI] [PubMed] [Google Scholar]
- Young S. N., Anderson G. M., Gauthier S., Purdy W. C. The origin of indoleacetic acid and indolepropionic acid in rat and human cerebrospinal fluid. J Neurochem. 1980 May;34(5):1087–1092. doi: 10.1111/j.1471-4159.1980.tb09944.x. [DOI] [PubMed] [Google Scholar]
- Young S. N., Anderson G. M., Purdy W. C. Indoleamine metabolism in rat brain studied through measurements of tryptophan, 5-hydroxyindoleacetic acid, and indoleacetic acid in cerebrospinal fluid. J Neurochem. 1980 Feb;34(2):309–315. doi: 10.1111/j.1471-4159.1980.tb06598.x. [DOI] [PubMed] [Google Scholar]
- Young S. N., Etienne P., Sourkes T. L. Relationship between rat brain and cisternal CSF tryptophan concentrations. J Neurol Neurosurg Psychiatry. 1976 Mar;39(3):239–243. doi: 10.1136/jnnp.39.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. N., Gauthier S., Anderson G. M., Purdy W. C. Tryptophan, 5-hydroxyindoleacetic acid and indoleacetic acid in human cerebrospinal fluid: interrelationships and the influence of age, sex, epilepsy and anticonvulsant drugs. J Neurol Neurosurg Psychiatry. 1980 May;43(5):438–445. doi: 10.1136/jnnp.43.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. N., Lal S. CNS tryptamine metabolism in hepatic coma. J Neural Transm. 1980;47(3):153–161. doi: 10.1007/BF01250597. [DOI] [PubMed] [Google Scholar]



