Abstract
Objective:
To determine quantitative size thresholds for enlargement of the optic nerve, chiasm, and tract in children with neurofibromatosis type 1 (NF1).
Methods:
Children 0.5–18.6 years of age who underwent high-resolution T1-weighted MRI were eligible for inclusion. This consisted of children with NF1 with or without optic pathway gliomas (OPGs) and a control group who did not have other acquired, systemic, or genetic conditions that could alter their anterior visual pathway (AVP). Maximum and average diameter and volume of AVP structures were calculated from reconstructed MRI images. Values above the 95th percentile from the controls were considered the threshold for defining an abnormally large AVP measure.
Results:
A total of 186 children (controls = 82; NF1noOPG = 54; NF1+OPG = 50) met inclusion criteria. NF1noOPG and NF1+OPG participants demonstrated greater maximum optic nerve diameter and volume, optic chiasm volume, and total brain volume compared to controls (p < 0.05, all comparisons). Total brain volume, rather than age, predicted optic nerve and chiasm volume in controls (p < 0.05). Applying the 95th percentile threshold to all NF1 participants, the maximum optic nerve diameter (3.9 mm) and AVP volumes resulted in few false-positive errors (specificity >80%, all comparisons).
Conclusions:
Quantitative reference values for AVP enlargement will enhance the development of objective diagnostic criteria for OPGs secondary to NF1.
Fifteen to twenty percent of children with neurofibromatosis type 1 (NF1) will develop a low-grade pilocytic astrocytoma involving the anterior visual pathway (AVP; optic nerve, chiasm, or tracts).1,2 These tumors, termed optic pathway gliomas (OPGs), are intrinsic to the AVP and cause vision loss in up to 50% of children with NF1.2
To be considered an OPG, some portion of the AVP must demonstrate enlargement as this is representative of abnormal cellular growth consistent with tumor formation.2,3 It is debatable whether isolated contrast enhancement or T2 hyperintensity without concurrent enlargement should be considered an OPG.
No quantitative MRI criteria exist to define what amount of optic nerve, chiasm, or tract enlargement constitutes an OPG. When rudimentary 1D or 2D manual measures (e.g., diameter) are performed, the lack of an established quantitative definition of enlargement can result in children being inappropriately diagnosed with an OPG, resulting in additional and unnecessary clinical and MRI examinations, and potentially unwarranted therapy. Furthermore, the lack of an operationalized definition of AVP enlargement limits the ability to develop objective diagnostic criteria for OPGs secondary to NF1, compare results across research studies, and importantly, execute an informative clinical trial of OPGs secondary to NF1.
In this study, we performed quantitative analysis of the AVP in control patients as well as patients with NF1 with and without OPGs to establish objective quantitative criteria for AVP enlargement.
METHODS
Participants.
A convenience sample of children from Children's National Health System was retrospectively identified if they had undergone MRI that included high-resolution contrast-enhanced T1-weighted MRI sequences (∼0.4 × 0.4 × 0.6 mm3) between January 2012 and June 2015. In order to ascertain patients with NF1, patients seen in the neurofibromatosis clinic were preferentially identified from this convenience sample as at least one study investigator (R.J.P. or R.A.A.) experienced in caring for children with NF1 had examined the patient and could confirm the diagnosis according to established criteria.1 Eligible patients were divided into 3 separate cohorts: controls, NF1 only without MRI evidence of an OPG (NF1noOPG), and NF1 diagnosed as having an OPG involving, at a minimum, the optic nerves (NF1+OPG). MRI findings suggestive of OPG involvement beyond the optic nerves were permitted, but not required to be classified as a NF1+OPG participant.
Participants for the control cohort were children between age 6 months and 18 years whose MRI was interpreted as normal by a pediatric neuroradiologist. The medical records of the controls were reviewed and participants excluded if they had a current or past history of a systemic (cancer, sickle cell disease, metabolic or rheumatologic condition), congenital (gestational age <37 weeks, congenital heart defect, brain/tissue anomaly, optic nerve hypoplasia, arachnoid cyst, hydrocephalus, intraventricular hemorrhage, nystagmus), genetic (any identified or suspected syndrome), or acquired condition (optic neuritis, multiple sclerosis, neuromyelitis optica, anoxic event, cardiac arrest, encephalomalacia, hypoxia-ischemic encephalopathy, stroke) that could damage or alter their visual pathway or total brain volume.
Participants meeting the NIH established criteria for NF1,1 but without evidence of an OPG on MRI, as determined by the pediatric neuroradiologist, comprised the NF1noOPG cohort. To qualify for the NF1+OPG cohort, the clinical MRI reading had to identify enlargement of at least one of the optic nerves and be interpreted as an OPG. Isolated enlargement of the hypothalamus without optic nerve enlargement was not considered an OPG. Identical to the control cohort, neither the NF1noOPG nor NF1+OPG cohort participants were eligible if they had any of the past or current systemic, congenital, genetic (other than NF1), or acquired conditions that could damage or alter their visual pathway (other than an OPG) or total brain volume.
Standard protocol approvals, registrations, and patient consents.
This retrospective study was approved by the Children's National Health System institutional review board.
MRI segmentation and quantification.
Participants from all cohorts were required to have technically adequate MRI acquisition without patient movement, metallic artifact (e.g., dental hardware), or incomplete visualization of the AVP. T1-weighted MRI sequences were stripped of all identifiers, given a study identification number, reconstructed, and converted into NIFTI format. All structures of the AVP (i.e., left/right optic nerves, chiasm, and left/right tracts) were manually segmented using ITK-Snap (Kitware Inc., Clifton Park, NY) medical image segmentation software. Manual segmentations did not label borders between structures (i.e., nerve to chiasm or chiasm to tract border), as this was performed subsequently (see below). The optic tracts were segmented to the maximum extent distinguishable. Segmentations were first performed by a single trained operator (R.I.) and then edited and verified by the lead clinical investigator (R.A.A.). Quantitative size values for specific structures were then abstracted from the segmentations by another investigator (A.M.) using automated methods. The optic chiasm was separated from the optic nerves and tracts at an angle orthogonal to the chiasm at the optic nerve–chiasm and optic tract–chiasm junctions, respectively (figure).
Figure. Schematic renderings of the anterior visual pathway segmentation.

Segmentation of the optic nerves (red and royal blue), chiasm (green), and optic tracts (yellow and light blue). (A) Control, (B) neurofibromatosis type 1 (NF1) without an optic pathway glioma, (C) NF1 with a glioma isolated to the anterior optic nerve, and (D) NF1 with a glioma throughout the distal optic nerves, chiasm, and optic tracts.
The optic nerve was divided into 4 partitions of equal lengths to permit local quantification of diameters and volumes. For partitioning, the center line of the optic nerve was automatically extracted by using the previously validated fast-marching minimal extraction method available in the Insight Toolkit (National Library of Medicine, Bethesda, MD).4 The diameter along the center line was computed for each partition by calculating the orthogonal distance from the center line to the surface of the optic nerve at every point along the center line. This approach compensates for errors in the estimation of the diameter due to variations in multiplanar reconstructions in the MRI data. The mean and maximum diameters within each partition were then calculated after discarding values outside of a 2 SD band around the mean. This approach eliminates outliers in a fashion similar to the popular interquartile range.5 The first 2 partitions closest to the globe were combined to comprise the first segment and the latter 2 partitions were labeled as the second segment. Volumes were computed by multiplying the volume of a unit voxel by the number of voxels in each partition.
The height, width, and volume of the chiasm were also calculated considering the chiasm to be a single partition. Since the shape of the chiasm approximates that of an ellipsoid, the height and width were estimated as the axes of the bounding box that envelops the chiasm. The chiasm volume was computed as described above.
Given the limited visualization of the optic tracts beyond the optic chiasm, measurements were limited to the first 10 mm from the optic tract–chiasm junction. This limitation ensured that consistent measurements were independent of acquisition parameters, resolution limits, and interobserver variability. A single partition was used for each optic tract. The methods to calculate the mean and maximum diameters as well as volume were identical to those described above for the optic nerves.
Brain volume was calculated from the T1-weighted sequences using a previously established technique.6
Statistical analysis.
Descriptive statistics were reported as mean/median (range) for continuous variables and as percentages for categorical variables. The Shapiro-Wilk test was used to assess normality of all variables. Paired t test was used to compare continuous measures within a cohort whereas unpaired t test compared measures between cohorts. Controls and NF1noOPG participants only contributed the volumetric measures from the right eye given the known between-eye correlation. One or both eyes diagnosed with an OPG could be included in the comparison analysis for NF1+OPG participants. We defined an abnormally large AVP measure using the standard threshold of values above the 95th percentile7 from the controls cohort. Unadjusted and adjusted linear regression models were used to assess the influence of age, sex, optic nerve length, total brain volume, and OPG location (optic nerve only, chiasm ± optic nerve, or optic tract ± chiasm/nerves) on linear and volumetric measures. Independent variables whose level of significance did not reach p < 0.05 during unadjusted analysis were dropped from subsequent adjusted analysis.
RESULTS
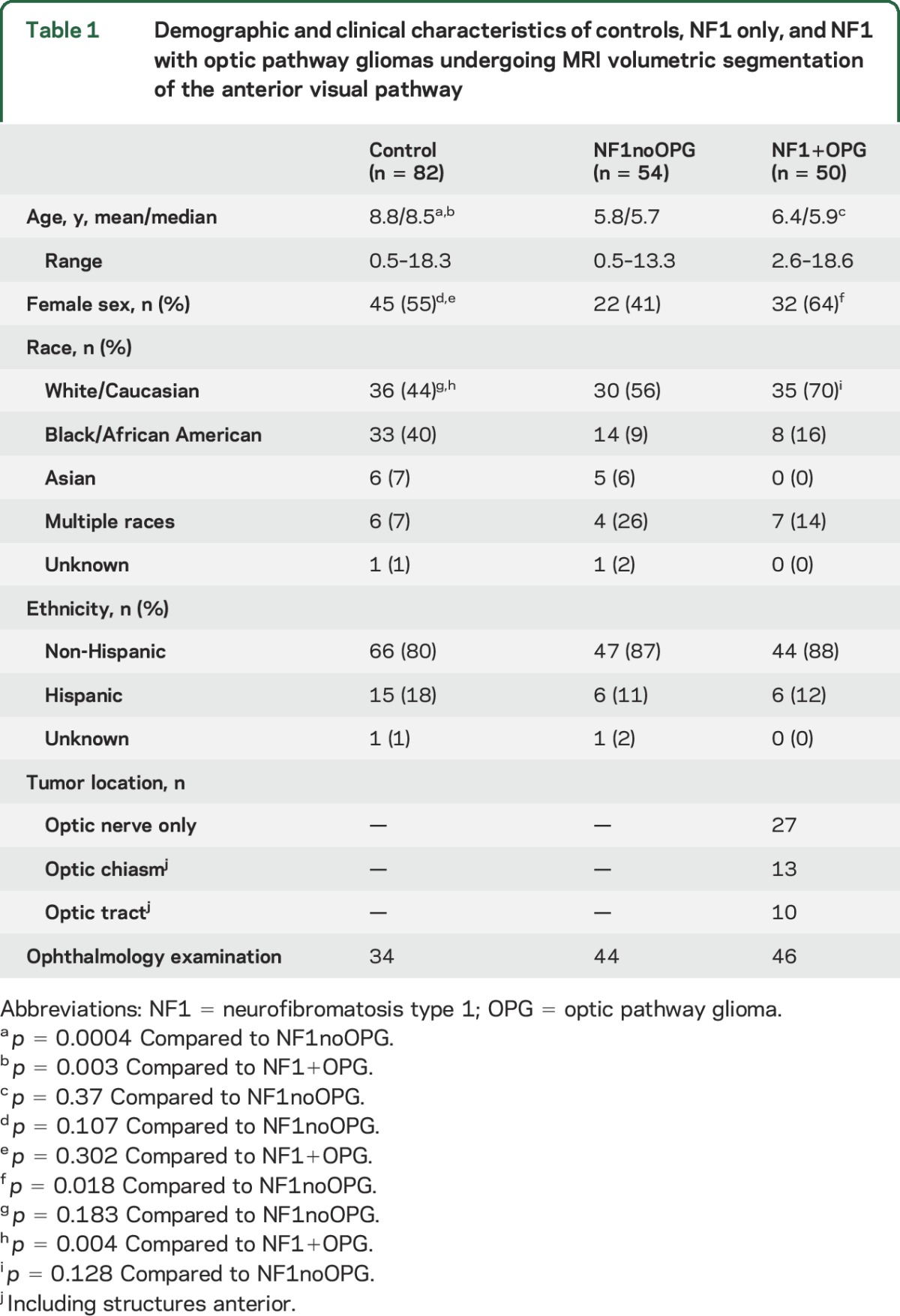
A total of 186 children (controls = 82; NF1noOPG = 54; NF1+OPG = 50) met inclusion criteria (table 1). Controls were slightly older than NF1noOPG and NF1+OPG participants (p < 0.01, both comparisons), although they had comparable absolute numbers in the same age range. NF1+OPG had a greater percentage of female participants compared to NF1noOPG.
Table 1.
Demographic and clinical characteristics of controls, NF1 only, and NF1 with optic pathway gliomas undergoing MRI volumetric segmentation of the anterior visual pathway
Optic nerve.
Table 2 lists the optic nerve diameter, volume, and length measures for all cohorts. The differences in maximum diameters at all locations along optic nerve were relatively small (i.e., typically within 0.3 mm) between controls and NF1noOPG (p < 0.05 for all comparisons). The average diameter along all locations of optic nerve was not different between controls and NF1noOPG (p > 0.05 for all comparisons). Optic nerve length did not differ between these 2 groups (p > 0.05), but optic nerve volume was greater in the NF1noOPG group (0.468 m3) compared to the controls (0.402 m3, p = 0.001). NF1noOPG participants had a larger brain volume (1,376 m3 vs 1,227 m3; p < 0.001) and entire AVP volume compared to controls (p = 0.002). The NF1+OPG cohort had large optic nerve diameter and volume measures compared to controls and NF1noOPG (p < 0.05, all comparisons).
Table 2.
Optic nerve diameter, volume, and length measures for controls and participants with neurofibromatosis type 1 with and without an optic pathway glioma

Linear regression calculated the influence of age, sex, and optic nerve length on the diameter and volume measures both within and between cohorts (table e-1 on the Neurology® Web site at Neurology.org). None of these factors influenced either the maximum or average optic nerve diameter, except for NF1+OPG participants, in which longer optic nerves predicted a greater maximum diameter. As expected, optic nerve length demonstrated a positive relationship with optic nerve volume in all cohorts. In controls, a larger brain volume also predicted a greater optic nerve volume. Extent of tumor burden (isolated optic nerve vs chiasm ± optic nerve involvement vs optic tract ± chiasm or nerves) did not influence any of the optic nerve diameter or volume measures in the NF1+OPG cohort.
Applying the 95th percentile threshold from the controls, relatively few NF1noOPG participants were classified as having abnormally enlarged optic nerve maximum diameter, average diameter, and volume measures, resulting in good specificity (83%, 78%, and 93%, respectively). On the other hand, slightly more than half of the NF1+OPG participants' optic nerve measures were classified as abnormally large (sensitivity 68%, 49%, and 58%, respectively).
Optic chiasm.
The optic chiasm height, width, and volume did not differ between controls and NF1noOPG, but all values from the NF1+OPG group were larger than both cohorts (table 3; p < 0.01, all comparisons). A larger total brain volume predicted a larger optic chiasm width, height, and volume in the control cohort only (table e-2). Greater age predicted a greater chiasm width in controls and NF1noOPG, even when considering brain volume. Chiasm height and total volume were not influenced by age or total brain volume in the NF1noOPG and NF1+OPG cohorts. There were no statistically significant predictors, including tumor extent, of chiasm measures in the NF1+OPG cohort.
Table 3.
Volume and dimensions of the optic chiasm for controls and participants with neurofibromatosis type 1 with and without an optic pathway glioma
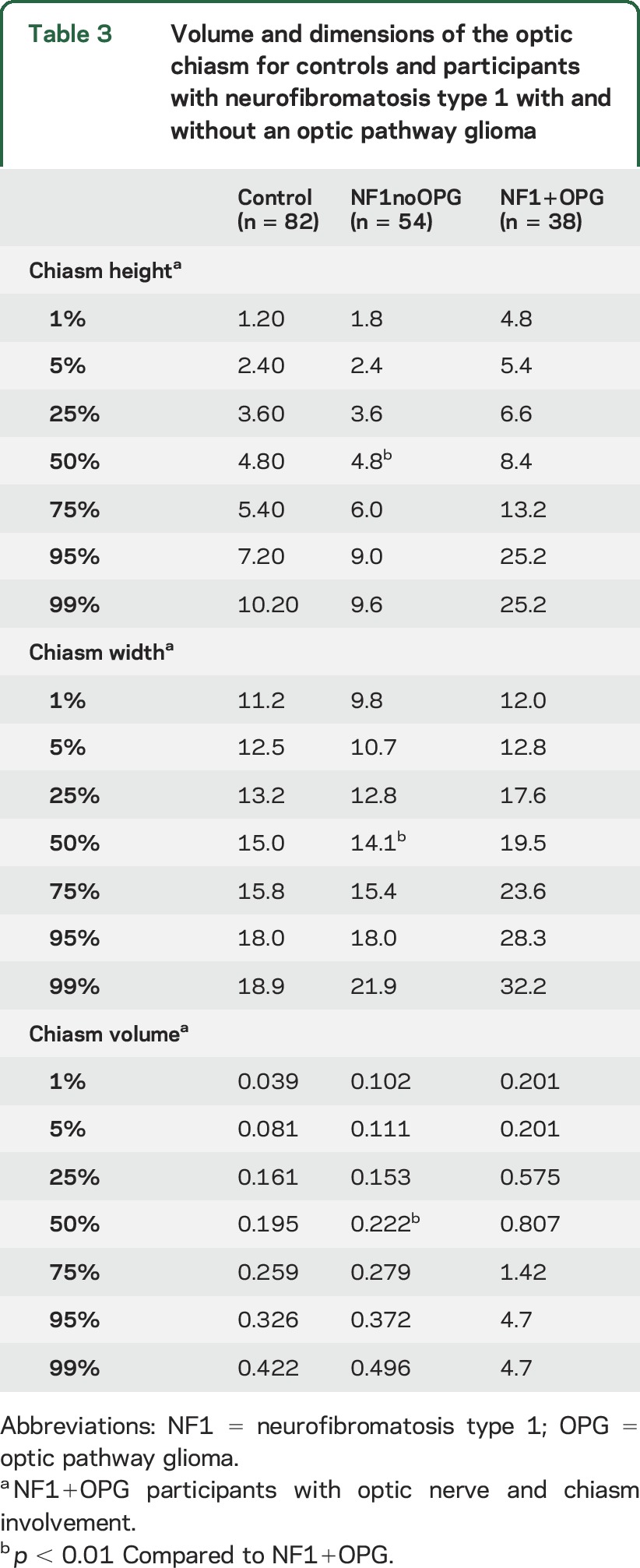
Applying the 95th percentile threshold from the controls, relatively few NF1noOPG participants were classified as having abnormally enlarged optic chiasm height, width, and volume measures, resulting in good specificity (85%, 95%, and 83%, respectively). A majority of the NF1+OPG participants with an OPG involving the chiasm demonstrated measures classified as abnormally large (sensitivity 65%, 79%, and 91%, respectively).
Optic tracts.
The optic tract maximum diameter, average diameter, and volume did not differ between controls and NF1noOPG (table 4). NF1noOPG and NF1+OPG cohorts only differed in optic tract volume (p < 0.05). Controls and NF1+OPG differed by maximum diameter and volume (p < 0.01, all comparisons), but did not differ between average diameter. Age and total brain volume did not significantly predict any of the optic tract measures in the cohorts with the exception of a larger brain volume predicting a larger optic tract diameter only in controls (table e-3).
Table 4.
Optic tract, whole brain, and entire anterior visual pathway volume for controls and participants with neurofibromatosis type 1 with and without an optic pathway glioma

Applying the 95th percentile threshold from the controls, relatively few NF1noOPG participants were classified as having abnormally enlarged optic tract maximum diameter, average diameter, and volume measures, resulting in good specificity (80%, 87%, and 81%, respectively). On the other hand, a relatively small portion of the NF1+OPG participants' optic tract measures were classified as abnormally large (sensitivity 41%, 29%, and 41%, respectively).
DISCUSSION
Numerous diagnostic and clinical challenges exist when caring for children with OPGs secondary to NF1. Without an objective OPG definition, the monitoring of children with NF1 will likely vary significantly across institutions. In this study, we present a reference range and threshold for abnormal enlargement of the AVP that should be one of the metrics used to develop a formal OPG definition.
Controls demonstrated smaller maximum and average diameters all along the AVP when compared to NF1noOPG participants. A larger brain volume, rather than older age, was consistently and more strongly associated with greater AVP measures across cohorts. Despite this association, using an absolute rather than adjusted 95th percentile of the optic nerve maximum diameter (3.9 mm), average diameter (2.3 mm), and optic nerve volume (0.622 mm3) from the control group provides a practical threshold. Greater brain volume also predicted a larger optic chiasm and optic tract volume in controls only, likely due to the high frequency of macrocephaly in children with NF1.1,8 Even when not accounting for brain volume, the above thresholds were useful for discriminating between controls and those NF1 participants with and without an OPG.
Investigators have previously used MRI and human cadavers to perform 2D cross-sectional and mean area measures of the AVP in healthy controls9–12 as well as pathologic conditions such as papilledema,13 optic neuritis,14 optic nerve hypoplasia,15 dominant optic atrophy,16 and NF1.17 Similar to our results, controls demonstrated a greater diameter along the first half of the optic nerve (e.g., starting at the globe) compared to the posterior portion.9–11,13,15,16 By performing manual measures on T2-weighted fast spin-echo MRI of adult controls, similar diameters have been reported of the anterior (3.5 mm), middle (3.1 mm), and posterior portions (3.1 mm) of the optic nerve.16 Other investigators have reported a mean optic nerve thickness of approximately 2.3 mm in children with NF1 using a single cross-sectional measure 2 mm behind the globe.17 Fewer studies have investigated optic chiasm and or optic tract reference ranges, although their measures of chiasm width (14–15 mm) and optic tract diameter (2.8 mm) were similar to our results.12,18
A number of factors likely contribute to the variability of AVP measures reported across studies, including MRI sequence type, MRI slice thickness, orientation of images, interrater variability, frequency of measurements, measurement location, method of measurement, and the consideration of other clinical variables (e.g., age and brain volume). We chose to segment high-resolution T1-weighted MRI sequences because of their close to isotropic voxel size, thus avoiding difficulties arising from slice thickness and variations in the multiplanar reconstruction. We also chose this sequence since it is included in our institution's standard radiology acquisition. As long as high-resolution T1-weighted images are acquired, we do not anticipate significant variability in AVP measures between centers using MRI from different manufacturers. Some investigators prefer T1-weighted images to visualize the optic nerve,12,19 whereas others believe standard or novel T2-weighted sequences better highlight the border between the optic nerve and CSF.9,10,15 While considering a combination of sequences may be preferable, this approach may not always be feasible in pediatric imaging, especially when duration of sedation is considered.
Measuring the AVP of children with NF1 is challenging for a number of reasons. First, they frequently have tortuous optic nerves, so measures at a single location may not be an accurate reflection of the actual diameter or volume.17,20 Next, the amorphous shape of OPGs secondary to NF1 and the need to monitor longitudinal changes in tumor size21 suggest that comprehensive measures along the entire AVP are a more meaningful metric compared to isolated 1D and 2D measures.21 Finally, OPGs can involve single (e.g., optic nerve only) or more frequently multiple structures (e.g., nerve + chiasm) along the AVP. By segmenting the entire AVP and reporting multiple metrics (i.e., maximum diameter, average diameter, volume), our approach provides a more meaningful interpretation than isolated manual measurements.
A number of limitations should be considered when interpreting our study results. The retrospective collection of a convenience sample of participants may have introduced a selection bias, especially since the interpretation of an OPG by the neuroradiologist is subjective and can vary among clinicians. Our study included interpretations from 4 different neuroradiologists within our institution. It is likely that bias in diagnosing OPGs exists both within and between institutions. In order to validate our thresholds, future prospective studies must include blinded data from other institutions.
Some NF1+OPG participants demonstrated diameter and volume measures similar to controls. We can only speculate that the MRI of participants with large OPGs (e.g., isolated to the chiasm) may be interpreted as having enlarged adjacent structures (e.g., optic nerves) that are in fact normal in size. Manual segmentation of the entire AVP is labor intensive and not practical in a busy clinic. Automated segmentation of the AVP, currently being refined in our laboratory, would eliminate the time burden, provide objective and consistent measures, and increase accessibility to other centers using different imaging equipment that were willing to implement standard MRI acquisition protocols.
Defining the radiographic criteria for an OPG secondary to NF1 is beyond the scope of our study. This definition will likely need to consider multiple MRI (e.g., structure size, presence of T2 hyperintensity or contrast enhancement) and clinical features. Instead, we introduce quantitative size thresholds for enlargement of the AVP that will be one factor incorporated into the definition of an OPG secondary to NF1.
Supplementary Material
GLOSSARY
- AVP
anterior visual pathway
- NF1
neurofibromatosis type 1
- OPG
optic pathway glioma
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Avery: drafting and revision of the manuscript, study concept and design, analysis and interpretation of data, acquisition of data, study supervision, obtaining funding. Dr. Mansoor: drafting and revision of the manuscript, study concept and design, analysis and interpretation of data. R. Idrees: drafting and revision of the manuscript, analysis and interpretation of data, acquisition of data. E. Biggs: drafting and revision of the manuscript, analysis and interpretation of data, acquisition of data. M.A. Alsharid: drafting and revision of the manuscript, analysis and interpretation of data. Dr. Packer: drafting and revision of the manuscript, study concept and design, analysis and interpretation of data, obtaining funding. Dr. Linguraru: drafting and revision of the manuscript, study concept and design, analysis and interpretation of data, acquisition of data, study supervision, obtaining funding.
STUDY FUNDING
Supported by the National Eye Institute, Bethesda, MD (K23-EY022673, R.A.A.), the Gilbert Family Neurofibromatosis Institute, Washington, DC (R.A.A., R.J.P., M.G.L.), and a philanthropic gift from the Government of Abu Dhabi to Children's National Health System.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurol Clin 2002;20:841–865. [DOI] [PubMed] [Google Scholar]
- 2.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol 2007;61:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol 2011;31:269–278. [DOI] [PubMed] [Google Scholar]
- 4.Wink O, Niessen WJ, Viergever MA. Fast delineation and visualization of vessels in 3-D angiographic images. IEEE Trans Med Imaging 2000;19:337–346. [DOI] [PubMed] [Google Scholar]
- 5.Upton GJG, Cook I. Understanding Statistics. Oxford: Oxford University Press; 1996. [Google Scholar]
- 6.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004;291:2847–2850. [DOI] [PubMed] [Google Scholar]
- 8.DiMario FJ Jr, Ramsby GR, Burleson JA. Brain morphometric analysis in neurofibromatosis 1. Arch Neurol 1999;56:1343–1346. [DOI] [PubMed] [Google Scholar]
- 9.Yiannakas MC, Wheeler-Kingshott CA, Berry AM, et al. A method for measuring the cross sectional area of the anterior portion of the optic nerve in vivo using a fast 3D MRI sequence. J Magn Reson Imaging 2010;31:1486–1491. [DOI] [PubMed] [Google Scholar]
- 10.Yiannakas MC, Toosy AT, Raftopoulos RE, Kapoor R, Miller DH, Wheeler-Kingshott CA. MRI acquisition and analysis protocol for in vivo intraorbital optic nerve segmentation at 3T. Invest Ophthalmol Vis Sci 2013;54:4235–4240. [DOI] [PubMed] [Google Scholar]
- 11.Karim S, Clark RA, Poukens V, Demer JL. Demonstration of systematic variation in human intraorbital optic nerve size by quantitative magnetic resonance imaging and histology. Invest Ophthalmol Vis Sci 2004;45:1047–1051. [DOI] [PubMed] [Google Scholar]
- 12.Wagner AL, Murtagh FR, Hazlett KS, Arrington JA. Measurement of the normal optic chiasm on coronal MR images. AJNR Am J Neuroradiol 1997;18:723–726. [PMC free article] [PubMed] [Google Scholar]
- 13.Mashima Y, Oshitari K, Imamura Y, Momoshima S, Shiga H, Oguchi Y. High-resolution magnetic resonance imaging of the intraorbital optic nerve and subarachnoid space in patients with papilledema and optic atrophy. Arch Ophthalmol 1996;114:1197–1203. [DOI] [PubMed] [Google Scholar]
- 14.Hickman SJ, Toosy AT, Jones SJ, et al. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain 2004;127:2498–2505. [DOI] [PubMed] [Google Scholar]
- 15.Lenhart PD, Desai NK, Bruce BB, Hutchinson AK, Lambert SR. The role of magnetic resonance imaging in diagnosing optic nerve hypoplasia. Am J Ophthalmol 2014;158:1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Votruba M, Leary S, Losseff N, et al. MRI of the intraorbital optic nerve in patients with autosomal dominant optic atrophy. Neuroradiology 2000;42:180–183. [DOI] [PubMed] [Google Scholar]
- 17.Levin MH, Armstrong GT, Broad JH, et al. Risk of optic pathway glioma in children with neurofibromatosis type 1 and optic nerve tortuosity or nerve sheath thickening. Br J Ophthalmol 2016;100:510–514. [DOI] [PubMed] [Google Scholar]
- 18.Parravano JG, Toledo A, Kucharczyk W. Dimensions of the optic nerves, chiasm, and tracts: MR quantitative comparison between patients with optic atrophy and normals. J Comput Assist Tomogr 1993;17:688–690. [DOI] [PubMed] [Google Scholar]
- 19.Pineles SL, Demer JL. Bilateral abnormalities of optic nerve size and eye shape in unilateral amblyopia. Am J Ophthalmol 2009;148:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong GT, Localio AR, Feygin T, et al. Defining optic nerve tortuosity. AJNR Am J Neuroradiol 2007;28:666–671. [PMC free article] [PubMed] [Google Scholar]
- 21.Warren KE, Poussaint TY, Vezina G, et al. Challenges with defining response to antitumor agents in pediatric neuro-oncology: a report from the response assessment in pediatric neuro-oncology (RAPNO) working group. Pediatr Blood Cancer 2013;60:1397–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


