Abstract
Abnormalities in brain γ-aminobutyric acid (GABA) have been implicated in various neuropsychiatric and neurological disorders. However, in vivo GABA detection by proton magnetic resonance spectroscopy (1H MRS) presents significant challenges arising from low brain concentration, overlap by much stronger resonances, and contamination by mobile macromolecule (MM) signals. This study addresses these impediments to reliable brain GABA detection with the J-editing difference technique on a 3T MR system in healthy human subjects by (a) assessing the sensitivity gains attainable with an 8-channel phased-array head coil, (b) determining the magnitude and anatomic variation of the contamination of GABA by MM, and (c) estimating the test-retest reliability of measuring GABA with this method. Sensitivity gains and test-retest reliability were examined in the dorsolateral prefrontal cortex (DLPFC), while MM levels were compared across three cortical regions: the DLPFC, the medial prefrontal cortex (MPFC) and the occipital cortex (OCC). A 3-fold higher GABA detection sensitivity was attained with the 8-channel head coil compared to the standard single-channel head coil in DLPFC. Despite significant anatomic variation in GABA+MM and MM across the three brain regions (p < 0.05), the contribution of MM to GABA+MM was relatively stable across the three voxels, ranging from 41% to 49%, a non-significant regional variation (p = 0.58). The test-retest reliability of GABA measurement, expressed either as ratios to voxel tissue water (W) or total creatine, was found to be very high for both the single-channel coil and the 8-channel phased-array coil. For the 8-channel coil, for example, Pearson’s correlation coefficient of test vs. retest for GABA/W was 0.98 (R2 = 0.96, p = 0.0007), the percent coefficient of variation (CV) was 1.25%, and the intraclass correlation coefficient (ICC) was 0.98. Similar reliability was also found for the co-edited resonance of combined glutamate and glutamine (Glx) for both coils.
Keywords: GABA, glutamate, Glx, 1H MRS, J-editing, mobile macromolecules, phased-array coil, test-retest reliability
INTRODUCTION
Dysregulations of the inhibitory amino acid neurotransmitter system of γ-aminobutyric acid (GABA) have been implicated in the pathophysiology of most neuropsychiatric and of a number of neurological disorders, including schizophrenia [1], mood [2] and anxiety [3, 4] disorders, and epilepsy [5]. Therefore, there is great interest in studying the function of this neurotransmitter in vivo. However, detection of brain GABA by 1H MRS presents a number of challenges [6–8]. First, the strongly J-coupled GABA multiplets in the 1.9–3.0 ppm range are overlapped by the much stronger resonances of N-acetyl-L-aspartate (NAA), total creatine (tCr), and combined glutamate+glutamine (Glx), rendering the α, β, and γ resonances of GABA undetectable by conventional MR spectroscopic techniques. Second, the estimated brain GABA concentration of 0.5–1.5 mM is at the lower limit of detection by 1H MRS at commonly available static magnetic field strengths (Bo) of 3T or less. And, third, the GABA C4H resonance at 3.0-ppm that is generally targeted for detection overlaps with a resonance due to mobile macromolecules (MM), which has a coupling partner just 0.2 ppm upfield from the 1.9-ppm GABA C3H resonance, and thus co-edits with GABA in nearly all proposed editing approaches. Collectively, these challenges to reliable brain GABA detection can lead to poor estimates of its concentrations, masking of potential pathologic changes and confounding of interpretation.
In recent years, concerted research activity in several laboratories has led to the development of robust approaches for overcoming these impediments to the reliable detection of brain GABA in vivo. These include a variety of spectral editing and post-processing methods for addressing the challenges associated with spectral overlap and MM contamination, and use of phased-array head coils to partially alleviate the low GABA detection sensitivity [9]. Here, we report the results of a study that was designed to (a) evaluate quantitatively the GABA detection sensitivity gains that can be attained with an 8-channel phased-array head coil compared to a standard quadrature single-channel head coil [9] and the tradeoffs that can be made between signal-to-noise ratio (SNR), voxel size and scan time, without sacrificing spectral quality; (b) estimate the magnitude and extent of anatomic variation of the contamination of GABA by the co-edited mobile macromolecule resonance; and (c) evaluate the test-retest reliability of cortical GABA detection for the J-editing spin echo difference technique at 3.0 T. Since the combined glutamate and glutamine (Glx) C2H resonance at 3.71 ppm is co-edited with this method, sensitivity gain and test-retest reliability evaluations of the Glx peak are also reported. While studies evaluating each of these impediments to GABA detection by J-editing have been reported, the differentiating feature of the present study is that it has evaluated all three impediments simultaneously in the same subjects.
METHODS
Study subjects
For each of the three study aims, we recruited 6 subjects from the same pool of 7 young adult subjects (ages 25.9 ± 2.7 years; 4 females), who gave written informed consent to participate. All subjects were medically and psychiatrically healthy graduate or medical students recruited at Columbia University Medical Center to undergo the brain MRS scans at Weill Cornell Medical College, with approval of the Institutional Review Boards of Columbia University, the New York State Psychiatric Institute at Columbia University, and Weill Cornell Medical College.
MRS Data Acquisition Protocol
All the neuroimaging studies were conducted on a research-dedicated GE 3.0 T MR system using manufacturer-supplied quadrature single-channel and 8-channel phased-array head coils. The basic pulse sequence used to acquire the GABA spectra was the J-edited spin echo difference method first described by Rothman et al. [6], for which several variants have been developed [10, 11]. The sequence used in this study was developed by Sailasuta et al. [11] for 3.0T GE scanners. Briefly, a standard PRESS sequence was converted into a volume-selective J-edited spin echo difference technique for GABA detection by adding a pair of frequency-selective cosine-modulated Shinar-LeRoux inversion radiofrequency (rf) pulses [11], flanked by spoiler gradient pulses of opposite signs, before and after the second 180° rf pulse of the double spin echo sequence. Application of this pair of frequency-selective “editing” pulses at the 1.9-ppm resonance frequency of GABA C3H on alternate scans, with TE 68ms, alternately inverts the GABA C4H resonance at 3.0 ppm by allowing or inhibiting its J-modulation. Subtracting the two subspectra thus acquired yields the desired GABA difference spectrum, consisting of the outer lines of the 3.0-ppm C4H triplet, while the much stronger overlapping tCr resonance – a singlet that is not J-modulated – is eliminated. Due to the high structural, magnetic and chemical similarities between GABA, glutamate (Glu) and glutamine (Gln), this pulse sequence also achieves detection of the combined resonance of Glu and Gln, commonly referred to as Glx, at 3.71 ppm, although with much reduced efficiency. Therefore, in this study we also evaluated the 8-channel coil sensitivity gains and test-retest reliability for Glx, in itself of considerable interest since the excitatory amino acid neurotransmitter system of glutamate has been implicated along with the inhibitory GABA system in a variety of brain disorders. A pulse sequence repetition time, TR, of 1500ms was used throughout. Each application of the sequence also included synchronous acquisition of the unsuppressed voxel tissue water (W) resonance, whose integrated area served as an internal intensity reference.
Structural MRI for Voxel Placement
A three-plane, low-resolution, high-speed scout imaging series was obtained, followed by a series of standardized high-resolution axial, coronal and sagittal T1- and T2-weighted scans that were appropriately obliqued to enable optimal prescription of the 1H MRS voxels of interest. In addition, fast Fluid-Attenuated Inversion-Recovery (FLAIR) scans were performed for detection of potentially exclusionary focal brain lesions.
GABA Detection with Single-channel or Eight-channel Head Coil and J-editing
To evaluate the sensitivity gains achieved for GABA and Glx detection with an 8-channel phased-array head coil compared to a standard quadrature single-channel coil, J-edited spectra were obtained with each coil from a single triply-obliqued 1.0 × 2.0 × 4.8-cm3 (9.6-cm3) and a 2.0 × 2.0 × 4.8-cm3 (19.2-cm3) voxel placed using anatomic landmarks in the left dorsolateral prefrontal cortex (DLPFC), with voxel faces oriented parallel to the middle frontal gyrus and to the brain surface (Fig. 1A). An upper limit of 30 min was set as the maximum practical total scan time for acquiring each edited spectrum. To compare the relative SNR values, adjusting for differences in voxel size for data acquired with the single- and/or eight-channel coil, SNR was expressed as the ratio of GABA or Glx peak area divided by the root mean square (rms) of the background noise and by voxel size.
Figure 1.
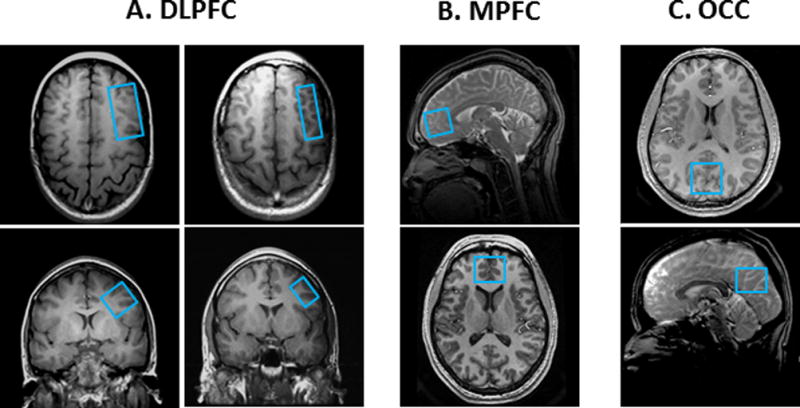
Depiction on high-resolution MR images of the MRS voxels targeted in this study. (A) Dorsolateral prefrontal cortex (DLPFC) voxels: Left, 19.2-cm3 voxel; Right, 9.6-cm3 voxel; (B) 19.2-cm3 medial prefrontal cortex (MPFC) voxel; (C) 19.2-cm3 occipital cortex (OCC) voxel.
Magnitude and Anatomic Variation of MM Contamination of Edited GABA
The short-tau inversion-recovery (STIR) technique with metabolite nulling [12–14] was implemented in this study to measure the levels of mobile macromolecules (MM), which co-edit with the GABA resonance detected by J-editing, in three brain regions: the DLPFC (Fig. 1A), the medial prefrontal cortex (MPFC) (Fig. 1B) including the pregenual anterior cingulate cortex (ACC), and the occipital cortex (OCC) (Fig. 1C). In the present study, we implemented STIR by preceding the J-editing sequence with a single non-selective 180° adiabatic spin inversion pulse, followed by an experimentally determined inversion-recovery delay (TI) of 525ms that allowed the slower-relaxing GABA and other metabolite resonances to reach the zero-crossing point in the rotating frame of reference, before turning on the editing module of the sequence exactly as it would be for GABA detection. A spectrum acquired in this manner would contain only the resonance of the faster-relaxing MM resonance, which would have a net positive magnetization for the value of TI that is optimal for nulling GABA and the other metabolites.
Two sequential MRS acquisitions were performed. First, a J-edited GABA spectrum uncorrected for MM contamination was recorded in 13 min from a 19.2-cm3 voxel localized in the DLPFC (Fig. 1A), MPFC (Fig. 1B), or OCC (Fig. 1C), using the 8-channel phased-array head coil. Second, without moving the subjects, metabolite nulling by STIR was immediately implemented to record the spectra of the MM resonance at 3.0 ppm. The outcome measures from these scans were: (1) total GABA level (GABAT) uncorrected for MM contribution (i.e., GABAT = GABA + MM), (2) the magnitude of MM contribution, and (3) the MM/GABAT ratio, or percent contamination, for each voxel.
Test-Retest Reliability for Brain GABA and Glx Measurement
A triply obliqued 19.2-cm3 voxel was placed in the left DLPFC (Fig. 1A, left) as described. To estimate the test-retest reliability, edited DLPFC spectra were obtained from each of the 6 healthy volunteers, who were removed from the scanner after the first of the duplicate scans (the test) and returned for the second scan (the retest) 1 hour later, with the voxel replaced using the same anatomic landmarks. DLPFC test-retest data were similarly acquired with the voxel size decreased by half to 9.6 cm3 (Fig. 1A, right) to assess the effect of SNR on reliability. Test-retest data were acquired with both the single-channel quadrature head coil and with the 8-channel phased-array head coil to compare the reliability of measuring GABA and Glx with the two different coils.
Eight-Channel GABA 1H MRS Data Processing
The MR signals, Sn(t), recorded with the J-editing technique and the 8-channel phased-array head coil were combined into a single regular time-domain free-induction decay signal, C(t), according to eq. (1) [15]:
| (1) |
where the summation is over all the 8 coil elements, n, of the array; Wn represent the relative coil sensitivities or weighting factors due to physical property differences between coil elements; an are the signal amplitudes which may vary as a function of proximity of the coil elements to the voxel of interest, and were all set to 1 in this study as overall coil dimensions were much greater than voxel size; and ϕn are the relative phases for the MR signal in the different coil elements. The relative phased-array coil sensitivities, Wn, and MR signal phases, ϕn, were obtained, respectively, from the magnitude and phase of the first complex time-domain point in the unsuppressed water signal from each receiver coil (Fig. 2A). These were then used as input into a C-language computer program that performed the complex numbers summation in eq. (1) and subtracted the two resulting edit-on and edit-off free-induction decays in the time domain to yield the difference signal (Fig. 2B), which was Fourier-transformed to yield, automatically, fully phase-corrected and combined GABA and Glx difference spectra (Fig. 2C, difference spectrum).
Figure 2.
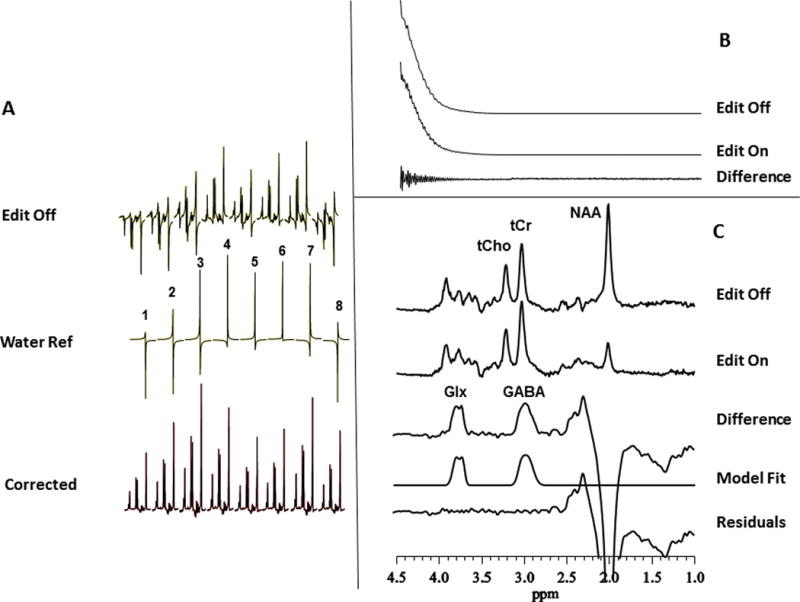
Reconstruction of the recorded J-editing data:
(A) Individual spectra from each of the 8 coil elements of the phased-array used in this study. The top traces illustrate the variations in the amplitudes and phases in the water-suppressed metabolite spectrum with the coils in the array. The middle traces present the amplitude and phase variations as in the top traces but for the unsuppressed water voxel resonance. Note that the variations of the metabolite signal amplitudes and phases (top traces) are identical to those of the unsuppressed water resonance (middle traces), justifying use of the amplitudes and phases of the robust unsuppressed water signal from each receiver coil to combine the data into a single regular 1D signal. The bottom traces show the spectra for each of the 8 coil elements after application of the correction factors from the water signal. Each spectrum shows a pure absorption lineshape, indicating the appropriateness of the phase angles derived from the unsuppressed water resonance for phase correction of the metabolite or edited spectra. The 8 spectra thus reconstructed can simply be summed, after correction for potential frequency shifts, to produce the final single spectrum from the 8-channel coil.
(B) Alternatively, the reconstruction of the phased-array data can be performed entirely in the time-domain. The free-induction decay (FID) signals for the data acquired in each receiver coil with the editing pulse alternately off or on are combined automatically in the time domain also using weighting factors from the corresponding and synchronously acquired unsuppressed voxel water signals. Subtraction of the two FIDs thus derived yields a difference FID that can be processed in the usual manner. Also, note the near complete elimination of the strong water signal after subtraction, which leaves a free-induction decay with a clear interferogram reflecting the chemically shifted GABA and Glx signals; a large residual water signal after subtraction would generally indicate subject head movement during an acquisition.
(C) Frequency-domain illustration of the J-editing method for GABA and Glx detection by 1H MRS in vivo: (top to bottom) single-voxel subspectra acquired in 13 min with the editing pulse on or off and 256 (512 total) interleaved averages; difference between the two subspectra showing the edited brain GABA and Glx resonances; a model fit of the difference spectrum to obtain the GABA and Glx peak areas; and residual of the difference between the experimental and fitted model spectra. GABA, γ -aminobutyric acid; Glx, glutamate + glutamine; NAA, N-acetyl-L-aspartate; tCho, total choline; tCr, total creatine.
Spectral Data Analysis and Quantification
Details of the MRS data quality assessment criteria and procedures used to retain or reject spectra for further processing and analysis are provided in supplementary material online. In this study, all spectra met our data quality assessment criteria and were processed as illustrated in Figure 2C to obtain the area under the GABA and Glx peaks, which are proportional to the concentration of each neurotransmitter in the voxel of interest. Briefly, the GABA and Glx resonances in the J-edited difference spectra were modeled as a linear combination of pseudo-Voigt lineshape functions and then fitted in the frequency domain using a robust and highly optimized public-domain Levenberg–Marquardt nonlinear least-squares minimization routine, MPFIT [16]. The pseudo-Voigt lineshape function enables more precise analysis of lineshapes that consist of mixtures of Lorentzian and Gaussian functions [17], as is often the case for in vivo spectra.
The GABA and Glx peak areas derived with MPFIT were expressed as ratios relative to the synchronously acquired and similarly fitted unsuppressed intravoxel tissue water signal (W), as well as ratios to the fitted area of the total creatine (tCr) resonance in the spectra acquired with the editing pulses off.
Statistical analysis
The outcome measures assessing the magnitude and anatomic variation of MM contamination of the edited GABA resonance in the DLPFC, MPFC and OCC were evaluated and compared using repeated measures ANOVA. Test-retest reliability of the GABA and Glx measurements was assessed with Pearson’s correlation coefficient (R), with the percent coefficient of variation (CV), and with the intraclass correlation coefficient (ICC), the latter being a statistic that compares within- to between-subjects variance such that a maximum ICC of 1.0 indicates identity between test and retest values, and lower values correspond to greater within-subject variability [18].
RESULTS
Eight-channel Sensitivity Gains and Tradeoffs for Scan Time and Voxel Size
Sample edited GABA and Glx spectra obtained with a standard quadrature single-channel head coil and with an 8-channel phased-array head coil are shown and compared in Fig 3. In panels A-C of Fig. 3, single- and 8-channel coil data recorded with a DLPFC voxel size of 19.2 cm3 or 9.6 cm3 (Fig. 1A) are compared. In panels D and E of the same Figure, data obtained with the 8-channel coil are compared for different voxel sizes and scan times.
Figure 3.
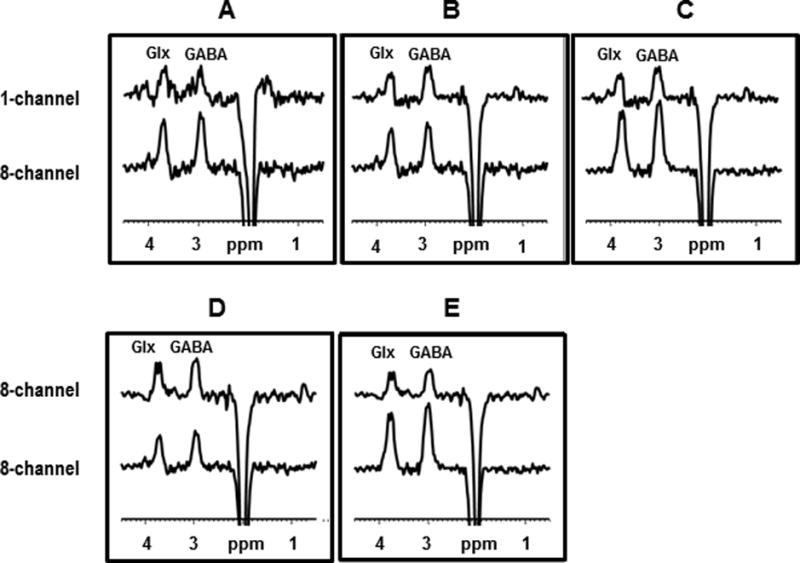
DLPFC spectra contrasting the effects of scan duration, voxel size, and coil type used. The panels show spectra acquired with single-channel (upper panel) and 8-channel (lower panel) coils from (A) a 9.6-cm3 voxel in 26 min; (B) a 19.2-cm3 voxel (upper panel) and a 9.6-cm3 voxel (lower panel), each in 26 min; (C) a 19.2-cm3 voxel in 26 min (both upper and lower panels); and (D) with an 8-channel coil from a 19.2-cm3 voxel in 13 min (upper panel) and from a 9.6-cm3 voxel in 26 min (lower panel); and (E) with 8-channel coil from a 19.2-cm3 voxel in 13 min (upper panel) and in 26 min (lower panel).
Single- vs. Eight-channel Coils Varying Scan Time and Voxel Size
Using a single-channel head coil and signal-averaging for 26 min – the longest scan time used in this study – to record GABA and Glx spectra from a 9.6-cm3 voxel (Fig. 3A, top trace) yielded very poor quality data. By comparison, using the 8-channel head coil with the same voxel size (9.6-cm3) and signal-averaging time (26 min) as for the single-channel acquisition yielded excellent quality GABA and Glx spectra were be obtained (Fig. 3A, bottom trace). Fig. 3B compares a spectrum from a 9.6-cm3 voxel obtained with the 8-channel coil (bottom trace) with that obtained from a 19.2-cm3 voxel – i.e. doubling the voxel size only – with the single-channel coil (top trace) in the same scan time (26 min), and shows the 8-channel coil to yield a slightly higher quality spectrum despite the smaller voxel size. Lastly, comparing spectra obtained from a 19.2-cm3 voxel in 26 min with the single-channel coil (Fig 3C, top trace) and with the 8-channel coil (Fig. 3C, bottom trace) yielded approximately a factor of 3 higher SNR (see Supplementary Table 1S online) for the 8-channel coil for both GABA (3.04 ± 0.38) and Glx (3.13 ± 0.40).
Eight-Channel Coil with Different Voxel Sizes and Scan Times
In Fig. 3D, 8-channel spectra-only obtained in 13 min from a 19.2-cm3 voxel (top trace) and in 26 min from a 9.6-cm3 voxel (bottom trace) – i.e., doubling the scan and halving the voxel size – were compared and found to exhibit nearly identical SNR. Finally, spectra obtained in 13 min (Fig. 3E, top trace) and in 26 min (Fig. 3E, bottom trace) from a 19.2-cm3 voxel – i.e., constant voxel size, different scan time – show the predicted square-root dependence of SNR on scan time.
Overall, acquiring J-edited GABA and Glx spectra under identical conditions for the superficial DLPFC voxel with the 8-channel phased-array head coil achieved a 3-fold higher GABA and Glx detection sensitivities compared to the standard quadrature single-channel head coil (Fig. 3C).
Magnitude and Anatomic Variation of GABA Contamination by MM
Reliable quantification of the contamination of the edited GABA signal by MM using metabolite nulling by STIR is highly dependent on the accuracy of determining the inversion-recovery delay, TI, that yields complete nulling of the metabolite resonances. The results of our STIR experiment to determine the optimal TI value for in vivo metabolite nulling are shown in Fig. 4A. For the shortest values of TI, all the major brain metabolite resonances were fully inverted, then they progressively recovered as TI was incremented, went through zero-crossing or “nulling” point, before relaxing fully at longer TI values. In the human brain, we found complete nulling of the major brain metabolites to occur for a TI value of 525ms (Fig. 4A). Using this optimal TI value, a pair of J-editing experiments, without (Fig. 4B,a) and with (Fig. 4B,b) metabolite nulling were conducted; the second experiment can be seen to yield a spectrum consisting of only the MM resonance. Subtracting the two spectra thus acquired yielded a difference spectrum in which the GABA C4H resonance at 3.0 ppm has been corrected for MM contamination (Fig. 4B,a–b). Note that overlaying the MM spectrum (dotted line in Fig. 4B,a) on the MM-uncorrected spectrum shows a left ‘shoulder’ on the GABA resonance that coincides with the underlying MM resonance, indicating that the clear distortion in the GABA lineshape was due to the underlying MM resonance.
Figure 4.
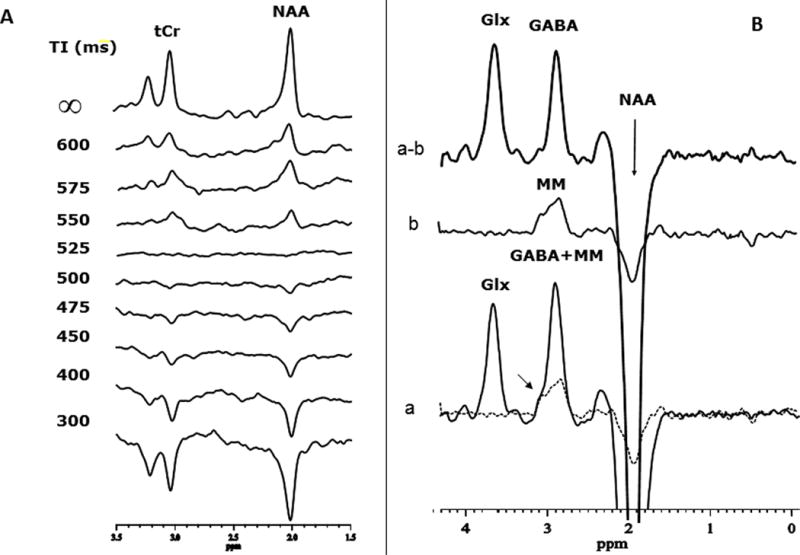
Determination of the contamination of the edited GABA signal by mobile macromolecule (MM) signals using metabolite nulling by STIR: (A) Sample in vivo localized inversion-recovery study to determine the optimal inversion-recovery delay (TI) for nulling the metabolite signals in the STIR experiment, which was about 525ms in this study. (B) J-editing experiment conducted (a) without STIR and (b) with STIR. Note that when metabolites are nulled with STIR, a clear and substantial MM resonance is detected at the GABA chemical shift, although this was likely made possible by the good shim and SNR of these particular spectra; (a–b) shows the edited spectrum with the GABA amplitude corrected for MM contamination
The relative contributions of MM and of “pure” GABA to the total measured GABA (GABAT), all expressed as ratios to the unsuppressed water signal in our three voxels of interest, are shown in Fig. 5. Quantitatively, GABAT showed significant anatomic variability (OCC: [3.11 ± 0.46] ×10−3; MPFC: [2.24 ± 0.57] ×10−3; DLPFC: [3.29 ± 0.67] ×10−3; p = 0.03), as did MM (OCC: [1.36 ± 0.21] ×10−3; MPFC: [1.06 ± 0.21] ×10−3; DLPFC: [1.25 ± 0.18] ×10−3; p = 0.02). However, the ratio of the MM to GABAT (i.e., % contamination) did not differ significantly across brain regions (OCC: 44% ± 10%; MPFC: 49% ± 13%; DLPFC: 41% ± 17%; p = 0.58).
Figure 5.
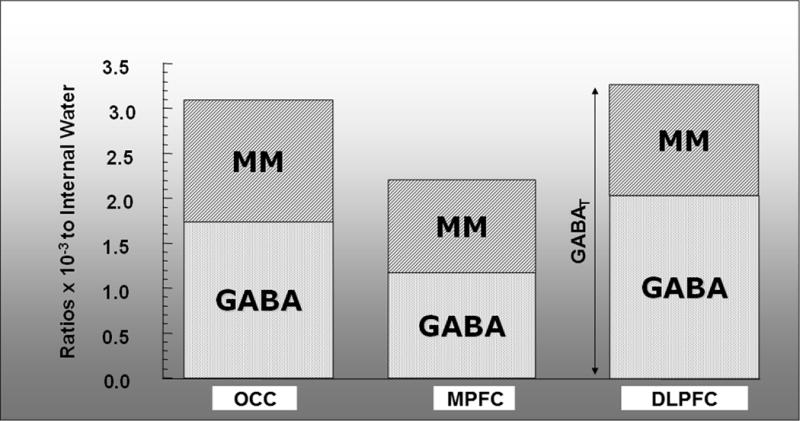
GABAT measurements in each of 3 voxels (OCC, MPFC, DLPFC) showing the contributions in each voxel of GABA and MM. Percent contribution of MM to GABAT ranged from 41% to 49% across these regions.
Test-Retest Reliability of GABA and Glx Measurement
We found the test-retest reliability of GABA and Glx measurement with the J-editing technique to be very high for both the standard quadrature single-channel coil and the 8-channel phased-array coil (Table 1 and Fig. 6). For instance, for the 8-channel coil, Pearson’s correlation coefficient of test vs. retest for GABA/W was 0.98 (R2 = 0.96, p = 0.0007, Fig. 6), the percent coefficient of variation (CV) was 1.25%, and the intraclass correlation coefficient (ICC) was 0.98. Similar reliability was found for Glx to voxel water ratios, as well as for both GABA and Glx ratios to tCr (Table 1; Fig. 6).
TABLE 1.
Test-Retest Results
| a. Single-Channel Coil | ||||
|---|---|---|---|---|
| GABA/H2O | GABA/tCr | Glx/H2O | Glx/tCr | |
| R | 0.93 | 0.90 | 0.90 | 0.94 |
| R2 | 0.86 | 0.81 | 0.81 | 0.88 |
| p (R2) | 0.008 | 0.014 | 0.015 | 0.006 |
| ICC | 0.93 | 0.89 | 0.90 | 0.86 |
| %CV | 3.64 | 6.05 | 3.49 | 4.65 |
| b. Eight-Channel Coil | ||||
|---|---|---|---|---|
| GABA/H2O | GABA/tCr | GIX/H2O | Glx/tCr | |
| R | 0.98 | 0.98 | 0.96 | 0.95 |
| R2 | 0.96 | 0.96 | 0.92 | 0.90 |
| P(R2) | 0.0007 | 0.0006 | 0.0023 | 0.0041 |
| ICC | 0.98 | 0.97 | 0.95 | 0.96 |
| %CV | 1.25 | 1.72 | 2.84 | 2.32 |
Figure 6.
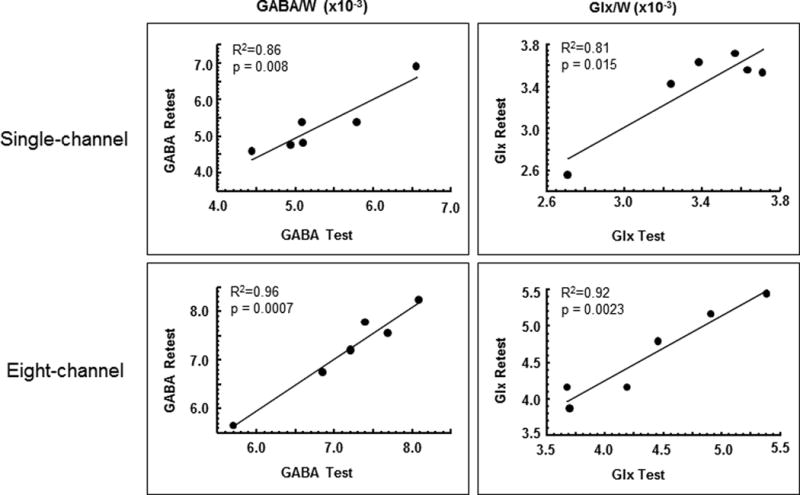
Regression plots of GABA and Glx levels as ratios to the unsuppressed voxel tissue water (W) in the test vs. retest scans for the single-channel and 8-channel coils. All cases showed very high test-retest reliability for the 1-hour duration between test and retest examined in this study.
Discussion
This study has presented the results of a comprehensive assessment (a) of GABA and Glx detection sensitivity gains that can be achieved with an 8-channel phased-array head coil, (b) of the magnitude and anatomic variation of the contamination of GABA by mobile macromolecules, and (c) of the test-retest reliability of measuring GABA and Glx with the J-editing technique at 3T. It should be noted that in the following discussion of items (a) and (c) above, the GABA levels were not corrected for the degree of mobile macromolecule contamination.
Sensitivity Comparisons: 8-channel vs. Quadrature Single-channel Head Coils
Our determination that implementation of the J-editing pulse sequence with an 8-channel phased-array head coil achieves significant gains in GABA and Glx detection sensitivity has confirmed, in general, the SNR advantage of such coils compared to single-channel coils, and, in particular, the results of van der Veen and Shen [9], who first proposed use of phased-array coils with the J-editing technique to enhance GABA detection sensitivity. The 3-fold higher in SNR achieved in this study for the DLPFC was undoubtedly made possible by the proximity of the voxel in this superficial brain region to the individual elements of the phased-array coil. Simulations by Albrecht et al. [19] assessing the spatial variation of SNR for array coils of different dimensions demonstrated that gains achieved with higher dimension array coils for deeper-lying voxels would be considerably less than those for more superficial voxels. For voxels in the DLPFC, MPFC and OCC that are near the elements of a phased-array coil, sensitivity gains can be achieved and traded for either smaller voxel sizes or shorter scan times, by as much as a factor of 3 for an 8-channel coil, without sacrificing spectral quality. In this study, halving the DLPFC voxel size from 19.2 cm3 to 9.6 cm3 (Fig. 1A) while maintaining the scan time at 26 min, allowed sampling of a mostly gray matter DLPFC voxel, which minimized partial volume-averaging with surrounding white matter (Fig. 3B, upper vs. lower traces). In general, the gained detection sensitivity is exchangeable for reductions in voxel sizes or scan times, as illustrated in Fig. 3.
Mobile Macromolecule Contamination of the Edited GABA Resonance
Using metabolite nulling, we have found a significant anatomic variation in the levels of total GABA uncorrected for MM (i.e., GABAT) and those of MM between the DLPFC, MPCF and OCC, with the highest values in both quantities occurring in DLPFC, lowest values in MPFC, and intermediate values in OCC. A prior study examined anatomic variation of MM and reported higher values in cerebellum than in motor cortex, pons, or parietal whiter matter, but did not make a comparison to GABA levels [20]. On the other hand, the ratio of MM to GABAT, i.e. the percent contamination, which ranged between 41% and 49% (mean ± SD: 44% ± 4%) and did not differ across the three brain regions targeted in this study (p=0.58), was in excellent agreement with prior determinations (Table 2) of 44 – 57% [6, 20–24]. This relatively stable contribution of MM to the J-edited GABA signal across different brain regions suggests that it may not be a significant confound if not accounted for in studies of normal human brain. However, the possibility remains that the contribution of MM to the J-edited GABA signal might differ significantly between normal and diseased brain – a limitation that could confound interpretation and thus should be acknowledged in studies comparing GABAT across diagnostic groups.
Table 2.
Mobile Macromolecule Contamination
| Reference | Field strength | Subjects | Method | Brain region(s)/voxel | Results |
|---|---|---|---|---|---|
| Rothman el al. [6] | 2.1T | 2 HCs 2 epilepsy patients |
Symmetric editing | OCC | 44% MM contamination |
| Henry et al. [25] | 3.0T | (in vitro) | Symmetric editing | (in vitro) | elimination of MM contamination |
| Shen et al. [21] | 2.1 T | 7 HCs | Multiple-quantum editing | OCC | Reduced MM contamination |
| Near et al. [22] | 3.0T | 5 HCs | Symmetric editing, “MEGA-SPECIAL” | OCC | 46% MM signal eliminated |
| Edden et al. [40] | 3.0T | 10 HCs | Increased TE (80 ms) and symmetric editing | Medial parietal lobe, (3.5 cm)3 | suppression of coedited MM |
| Aufhaus et al. [23] | 3.0T | 45 HCs | Symmetric editing | dACC | 50% contamination eliminated |
| Rowland et al. [41] | 3.0T | 60 SCH, 77 HCs | Symmetric editing | dACC | uncontaminated GABA lower in older SCH |
| Mikkelsen et al. [24] | 3.0T | 30 HCs | Symmetric editing | dACC, OCC | 52–57% MM contamination |
| This study | 3.0T | 6 HCs | Metabolite nulling | DLPFC, MPFC, OCC | 41–49% MM contamination |
Routine use of metabolite nulling to estimate the magnitude of MM contamination in clinical studies is not practical because it doubles the total scan time. In this respect, implementation of J-editing sequence variants (“symmetric editing”) that can achieve MM correction in a single shot [22, 25] offers a distinct advantage. Symmetric editing has been reported to show test-retest reliability comparable to GABAT from standard GABA editing [24]. Table 2 includes a summary of studies that have implemented this approach in clinical investigation.
Test-Retest Reliability of Brain GABA and Glx Measurement by J-editing
Our assessment of the test-retest reliability for GABA and Glx measurement using J-editing at 3.0 T has yielded high values for either a standard quadrature single-channel head coil or an 8-channel phased-array coil (Table 1). Although we found the reliability for GABA and Glx measurements expressed as ratios to voxel tissue water to be slightly higher than that for ratios to tCr, the reliability for both referencing methods is excellent and both seem appropriate for use in the normal brain. However, because a number of recent studies have reported changes in tCr levels in various pathological conditions [26–28] the previously assumed stability of this resonance has thus been questioned and caution in its use as an intensity reference is warranted.
Several previous studies [24, 29–34] have evaluated the test-retest reliability of GABA under various conditions. All except Wijtenberg et al. [34] reported levels of GABA plus MM, i.e. GABAT, acquired at 3.0T using the J-editing technique that can contain a contribution from macromolecule signals in the range of 41–57% [6, 21–24] (Table 2). With CV values of less than 4% for the single-channel coil and 2% for the 8-channel coil, our reliability values are to date the highest to be reported. The lowest reliability reported had a CV value of about 15%, with most studies reporting CV values that clustered around 6–8% (Table 3).
Table 3.
GABA Test-Retest Reliability
| Reference | Field strength | Subjects | Acquisition sequence | Interval | Analysis | Brain regions/voxels | Results, CV for GABA |
|---|---|---|---|---|---|---|---|
| Evans et al. [29] | 3.0T | 8 HCs | MEGA-PRESS, 8-channel | 2.5 hours, 5 scans per subject | Frequency-domain spectral fitting Unsuppressed water normalization |
OCC, Sensorimotor/27 cm3 | Occipital, 6.5%; Sensorimotor, 8.8% |
| Bogner et al. [30] | 3.0T | 11 HCs | MEGA-PRESS | Variable; at least 1 day; 2–4 scans per subject | AMARES, jMRUI [42] Referencing to both tCr and unsuppressed water |
OCC/22.5 cm3 | GABA/tCR,13.3%; GABA/water, 15.0% |
| O’Gorman et al. [31] | 3.0T | 14 HCs | MEGA-PRESS | 4 scans within-session | LCModel [43], MATLAB, jMRUI; water referencing | DLPFC/30 cm3 | GABA, 7–12% (Glx,6–18%) |
| Geramita et al. [32] | 3.0T | 10 HCs | MEGA-PRESS, single-channel | 28–226 days (131 ± 78 days) | Frequency-domain spectral fitting Referencing to both tCr and unsuppressed water |
dACC, rFWM /18 cm3 | dACC GABA/W, 5.3%; rFWM GABA/W, 8.7%. dACC GABA/tCr, 6.5%; rFWM GABA/tCr, 8.2% |
| Harada et al. [33] | 3.0T | 15 HCs | MEGA-PRESS, single-channel | 1 week | LCModel | Lentiform nuclei, frontal lobe, ACC/ 27 cm3 | 4.6% |
| Wijtenburg et al. [34] | 7.0T | 4 HCs | STEAM, MEGA-PRESS, 32-channel | 8 ± 2 days | STEAM: LCModel MEGA-PRESS: Frequency-domain spectral fitting |
AC, DLPFC/STEAM:27 cm3 MEGA-PRESS: 34.2 cm3 |
AC STEAM, 3.5% DLPFCSTEAM, 16.2% AC MEGA-PRESS, 13.6% DLPFCMEGA-PRESS, 13.4% |
| Near et al. [35] | 3.0T | 17 HCs, male | MEGA-PRESS | 229 ± 42 days | jMRUI; ReferencingtotCr | OCC /27 cm3 | 0.2–12.3%; mean 4.3% |
| Gaetz et al. [36] | 3.0T | 5HCs | MEGA-PRESS | ~5 days, 5 scans per subject | jMRUI; referencing to tCr, NAA, and Glx | Motor cortex/27 cm3 Auditory cortex/24 cm3 Visual cortex:/27 cm3 |
6–14%; average ~10% over regions and referencing methods |
| Mikkelsen et al. [24] | 3.0T | 15 HCs × 2 cohorts | MEGA-PRESS with and without symmetric editing | 15 minutes | Gannet[44] | dACC/ 24 cm3 OCC/27 cm3 |
GABAT: 4.0% OCC, 14.8% dACC; GABA symmetric editing: 8.6% OCC, 12.6% dACC |
| Long et al. [37] | 3.0T | 5HCs, elderly | MEGA-PRESS | 2–28 days | LCModel comparing 2 basis sets | Cerebellum, left and right/ 15.6 cm3 | 4–13% |
| This study | 3.0T | 6 HCs | MEGA-PRESS, single-channel and 8-channel | 1 hour | Frequency-domain spectral fitting Unsuppressed water normalization |
DLPFC/9.5 cm3 | GABA, 3.6% single-channel; 1.3%, 8-channel (Glx, 2.8–3.5%) |
HC=healthy control; SCH=schizophrenia patient; DLPFC=dorsolateral prefrontal cortex; dACC=dorsal anterior cingulate cortex; OCC=occipital cortex; rFWM=right frontal white matter; tCr=total creatine/phosphocreatine; W=unsuppressed water; AC=anterior cingulate.
Evans et al. [29] found occipital and sensorimotor cortex CV values of 6.5% and 8.8%, while Bogner et al. [30] reported occipital lobe CV values ranging from 13.3%–15%, depending on referencing and fitting methods. O’Gorman et al. [31] studied the left DLPFC and reported CV values ranging from 7%–12%, depending on the fitting method used. Geramita et al. [32] assessed the reliability of measuring GABA in the anterior cingulate cortex and right frontal white matter and reported CV values of 5.3% and 8.7%, respectively. Harada et al. [33] used the J-editing technique and a single-channel head coil to assess the reliability of measuring absolute concentrations of GABA in frontal lobe, ACC and lentiform nuclei voxels, and reported average CV and ICC values of 4.6% and 0.7, respectively. Wijtenberg et al. [30] was the only study to report GABA acquired at 7.0T and free of macromolecule signal. They compared STEAM and J-editing acquisitions in the anterior cingulate and DLPFC with different analysis methods, and found comparable CV values, although on the upper side of those obtained at 3.0T (e.g., CV values by J-editing were 13.6% in anterior cingulate and 13.4% in DLPFC). Recent studies have examined reliability over much longer inter-scan intervals [35]; across multiple brain regions [36]; in elderly subjects [37]; and in comparison of GABAT to GABA uncontaminated by MM signal [24] (Table 3).
A potentially significant factor for the smaller CV values in the present study could be the very short (one-hour) inter-scan interval, designed for comparison of studies of pre- and post-interventions at a comparable interval. Regional differences, physiological/metabolic variations during inter-scan delays, and/or systematic instrumental and subject repositioning errors between the scans might account for some of the differences in the test-retest reliability values among the studies. Table 3 provides a summary of these studies with additional methodological details, which in aggregate indicate a relatively high reliability in measuring in vivo brain GABA by 1H MRS.
Study Limitations
The presented results were obtained from a relatively small and homogenous sample of healthy young adult subjects. In addition, only a limited number of brain regions was investigated. Therefore, our results might not be generalizable to other specific in vivo conditions. A clear example is the potential for estimates of anatomic variation in MM contamination to be different under pathological conditions. Furthermore, our relatively short inter-scan delay of 1 h might have minimized within-subject temporal variations in GABA and Glx that may occur over longer inter-scan delays as mental status and experimental conditions vary. Estimates of test-retest reliability across a short interval such as 1 h are appropriate for studies in which a GABA- and/or Glx-altering intervention is presented and levels are determined prior to and immediately following the intervention (e.g., our recent acute ketamine challenge studies [38, 39]).
Conclusion
This study has established under the same conditions, quantitatively, and simultaneously for the first time (a) the sensitivity gains that can be achieved for GABA and Glx detection with the J-editing technique and an 8-channel phased-array head coil, which can be traded for smaller voxels or shorter scan times; (b) the degree and anatomic variation of the contamination of the J-edited GABA signal by co-edited MM signal; and (c) the test-retest reliability of GABA and Glx measured by J-editing. The J-editing technique is a powerful, sensitive, and reliable tool for advancing our understanding of the major inhibitory and excitatory amino acid neurotransmitters in vivo in the normal or diseased brain.
Supplementary Material
Acknowledgments
We are indebted to Napapon Sailasuta, Ph.D., for the initial coding of J-editing to run on GE scanners, and to R. Hurd, Ph.D. (GE) and S. Kohler, Ph.D. (GE) for assistance in porting the editing sequence from the GE ‘LX’ to the ‘EXCITE’ platform. Funding: NIMH 1 R01 MH075895 (DCS), New York State Office of Mental Health, Lieber Center for Schizophrenia Research.
Abbreviations used
- ACC
pregenual anterior cingulate cortex
- ANOVA
analysis of variance
- Bo
static magnetic field strength
- CV
coefficient of variation
- CRLB
Cramer–Rao lower bound
- DLPFC
dorsolateral prefrontal cortex
- FWHM
full width at half-maximum
- GABA
γ-aminobutyric acid
- Glx
combined glutamate and glutamine resonance
- ICC
intraclass correlation coefficient
- MM
mobile macromolecule
- MPFC
medial prefrontal cortex
- NAA
N-acetylaspartate
- ppm
parts per million
- OCC
occipital cortex
- rf
radiofrequency
- ROI
region of interest
- SNR
signal-to-noise ratio
- STIR
short-tau inversion-recovery
- tCr
total creatine
- TI
inversion-recovery time
- W
unsuppressed intravoxel water signal
References
- 1.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology. 2004;174(1):143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 2.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Archives of general psychiatry. 1999;56(11):1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 3.Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, Charney DS, Krystal JH. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Archives of general psychiatry. 2001;58(6):556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- 4.Simpson HB, Shungu DC, Bender J, Jr, Mao X, Xu X, Slifstein M, Kegeles LS. Investigation of cortical glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(12):2684–2692. doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petroff OA, Rothman DL. Measuring human brain GABA in vivo: effects of GABA-transaminase inhibition with vigabatrin. Mol Neurobiol. 1998;16(1):97–121. doi: 10.1007/BF02740605. [DOI] [PubMed] [Google Scholar]
- 6.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Cardiff Symposium on MRSoG. Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014:8643–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Progress in nuclear magnetic resonance spectroscopy. 2012:6029–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Veen JW, Shen J. Multi-channel GABA editing on a clinical 3T scanner. Proc Intl Soc Mag Reson Med. 2005:13214. ( http://cds.ismrm.org/ismrm-2005/Files/00214.pdf)
- 10.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Sailasuta N, LeRoux P, Hurd R, Wang P, Sachs N, Ketter T. Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3 T. Proc Intl Soc Magn Reson Med. 2001:61011. [Google Scholar]
- 12.Behar KL, Ogino T. Characterization of macromolecule resonances in the 1H NMR spectrum of rat brain. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1993;30(1):38–44. doi: 10.1002/mrm.1910300107. [DOI] [PubMed] [Google Scholar]
- 13.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1994;32(3):294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 14.Mader I, Seeger U, Weissert R, Klose U, Naegele T, Melms A, Grodd W. Proton MR spectroscopy with metabolite-nulling reveals elevated macromolecules in acute multiple sclerosis. Brain : a journal of neurology. 2001;124(Pt 5):953–961. doi: 10.1093/brain/124.5.953. [DOI] [PubMed] [Google Scholar]
- 15.Brown MA. Time-domain combination of MR spectroscopy data acquired using phased-array coils. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;52(5):1207–1213. doi: 10.1002/mrm.20244. [DOI] [PubMed] [Google Scholar]
- 16.Markwardt C. Non-linear least squares fitting in IDL with MPFIT. Proceedings of Astronomical Data Analysis Software and Systems XVIII. 2009;(411):251–254. [Google Scholar]
- 17.Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, Seckl J. Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2000;44(4):646–649. doi: 10.1002/1522-2594(200010)44:4<646::aid-mrm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Kirk RE. Experimental design: procedures for the behavioral sciences. California: Brooks/Cole Publishing Company; 1982. [Google Scholar]
- 19.Albrecht J, Burke M, Haegler K, Schopf V, Kleemann AM, Paolini M, Wiesmann M, Linn J. Potential impact of a 32-channel receiving head coil technology on the results of a functional MRI paradigm. Clinical neuroradiology. 2010;20(4):223–229. doi: 10.1007/s00062-010-0029-2. [DOI] [PubMed] [Google Scholar]
- 20.Mader I, Seeger U, Karitzky J, Erb M, Schick F, Klose U. Proton magnetic resonance spectroscopy with metabolite nulling reveals regional differences of macromolecules in normal human brain. Journal of magnetic resonance imaging : JMRI. 2002;16(5):538–546. doi: 10.1002/jmri.10190. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, Rothman DL, Brown P. In vivo GABA editing using a novel doubly selective multiple quantum filter. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2002;47(3):447–454. doi: 10.1002/mrm.10104. [DOI] [PubMed] [Google Scholar]
- 22.Near J, Simpson R, Cowen P, Jezzard P. Efficient gamma-aminobutyric acid editing at 3T without macromolecule contamination: MEGA-SPECIAL. NMR in biomedicine. 2011;24(10):1277–1285. doi: 10.1002/nbm.1688. [DOI] [PubMed] [Google Scholar]
- 23.Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, Meyer-Lindenberg A, Boettcher U, Ende G. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013;69(2):317–320. doi: 10.1002/mrm.24257. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen M, Singh KD, Sumner P, Evans CJ. Comparison of the repeatability of GABA-edited magnetic resonance spectroscopy with and without macromolecule suppression. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2015 doi: 10.1002/mrm.25699. [DOI] [PubMed] [Google Scholar]
- 25.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2001;45(3):517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Deicken RF, Eliaz Y, Feiwell R, Schuff N. Increased thalamic N-acetylaspartate in male patients with familial bipolar I disorder. Psychiatry research. 2001;106(1):35–45. doi: 10.1016/s0925-4927(00)00083-4. [DOI] [PubMed] [Google Scholar]
- 27.Gruber S, Frey R, Mlynarik V, Stadlbauer A, Heiden A, Kasper S, Kemp GJ, Moser E. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Investigative radiology. 2003;38(7):403–408. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- 28.Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry research. 2009;172(1):44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. Journal of magnetic resonance imaging : JMRI. 2010;31(1):204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 30.Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T. In vivo quantification of intracerebral GABA by single-voxel (1)H-MRS-How reproducible are the results? European journal of radiology. 2010;73(3):526–531. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 31.O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. Journal of magnetic resonance imaging : JMRI. 2011;33(5):1262–1267. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geramita M, van der Veen JW, Barnett AS, Savostyanova AA, Shen J, Weinberger DR, Marenco S. Reproducibility of prefrontal gamma-aminobutyric acid measurements with J-edited spectroscopy. NMR in biomedicine. 2011;24(9):1089–1098. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- 33.Harada M, Kubo H, Nose A, Nishitani H, Matsuda T. Measurement of variation in the human cerebral GABA level by in vivo MEGA-editing proton MR spectroscopy using a clinical 3 T instrument and its dependence on brain region and the female menstrual cycle. Human brain mapping. 2011;32(5):828–833. doi: 10.1002/hbm.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijtenburg SA, Rowland LM, Edden RA, Barker PB. Reproducibility of brain spectroscopy at 7T using conventional localization and spectral editing techniques. Journal of magnetic resonance imaging : JMRI. 2013;38(2):460–467. doi: 10.1002/jmri.23997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Near J, Ho YC, Sandberg K, Kumaragamage C, Blicher JU. Long-term reproducibility of GABA magnetic resonance spectroscopy. NeuroImage. 2014;99:191–196. doi: 10.1016/j.neuroimage.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 36.Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TP. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. NeuroImage. 2014:861–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long Z, Dyke JP, Ma R, Huang CC, Louis ED, Dydak U. Reproducibility and effect of tissue composition on cerebellar gamma-aminobutyric acid (GABA) MRS in an elderly population. NMR in biomedicine. 2015;28(10):1315–1323. doi: 10.1002/nbm.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, Mao X, Rodriguez CI, Oquendo MA, Suckow RF, Cooper TB, Keilp JG, Shungu DC, Mann JJ. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Molecular psychiatry. 2016;21(3):320–7. doi: 10.1038/mp.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez CI, Kegeles LS, Levinson A, Ogden RT, Mao X, Milak MS, Vermes D, Xie S, Hunter L, Flood P, Moore H, Shungu DC, Simpson HB. In vivo effects of ketamine on glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder: Proof of concept. Psychiatry research. 2015;233(2):141–147. doi: 10.1016/j.pscychresns.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edden RA, Puts NA, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2012;68(3):657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, Nisonger SJ, Korenic SA, Kochunov P, Hong LE. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. Journal of magnetic resonance. 1997;129(1):35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 43.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 44.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of magnetic resonance imaging : JMRI. 2014;40(6):1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


