Abstract
Objective
To determine the genomic alterations of cancer-related genes in advanced medullary thyroid carcinoma during the course of clinical care.
Methods
Hybrid-capture based comprehensive genomic profiling was performed on 34 consecutive medullary thyroid carcinoma cases to identify all four classes of genomic alterations, and outcome for an index patient was collected.
Results
RET was mutated in 88% (30/34) of cases, with RET M918T being responsible for 70% (21/30) of the RET alterations. The other RET alterations were RET E632_L633del, C634R, C620R, C618G/R/S, V804M, and RET amplification. Two of the four RET wild-type patients harbored mutations in KRAS or HRAS (1/34 each). The next most frequent genomic alterations were amplifications of CCND1, FGF3, FGF19 and alterations in CDKN2A (3/34 each). One case with a RET M918T mutation developed acquired resistance to progressively dose-escalated vandetinib. When the mTOR inhibitor everolimus was added to continued vandetanib treatment, the patient achieved a second, 25% reduction of tumor volume (RECIST 1.1) for 8 months.
Conclusions
Comprehensive genomic profiling identified the full breadth of RET alterations in metastatic medullary thyroid carcinoma and possible co-operating oncogenic driver alterations. This approach may refine the use of targeted therapy for these patients.
Keywords: medullary thyroid carcinoma, RET, genomic profiling, vandetanib, cabozantinib, everolimus, precision medicine
Introduction
Patients with early-stage medullary thyroid carcinoma (MTC), a tumor derived from the neuroendocrine cells of the thyroid, have a favorable prognosis, as surgery is effective in this setting. For patients with metastatic or recurrent disease, however, the 10-year overall survival rate is only 40% regardless of whether the MTC arose in a familial setting or as a sporadic malignancy [1]. Chemotherapy and external beam radiotherapy have shown very little efficacy in patients with advanced MTC [2, 3]. Two targeted therapies are approved in the US and Europe to treat unresectable, locally advanced, or metastatic MTC, but have not been well linked to efficacy in the context of a particular genomic alteration (GA). Vandetanib (Caprelsa® 300 mg once daily) significantly prolonged median progression-free survival (PFS) compared with placebo (30.5 vs. 19.3 months) in a Phase 3 study of patients with advanced MTC [4]. Retrospective subgroup analysis of patients with sporadic MTC suggested a higher objective response rate (ORR) in RET M918T mutation-positive vs. –negative patients (54.5 vs. 30.2%), although RET status was unknown for a high fraction (45%) of patients. The approval of cabozantinib (Cometriq®) is based on a Phase 3 trial of patients with progressive metastatic MTC, in which cabozantinib (140 mg once daily) significantly improved median PFS compared with placebo (11.2 months vs 4.0 months) and achieved an ORR of 28% (0% for placebo) [5]. Responses were observed in patients both with and without any detectable RET mutations (ORR of 32% and 25%, respectively), while sporadic MTC with RET M918T had a numerically lower hazard ratio (HR) than RET M918T-negative cases in this study [5]. Median PFS on cabozantinib was longer for patients with RET mutation than for patients with wild-type RET (60 weeks vs. 25 weeks, p = 0.0001), and RET M918T associated with longer PFS than any other RET mutation (61 weeks vs. 36 weeks, p = 0.009), suggesting that RET mutation, which correlates with worse outcome [6], and RET M918T in particular confer a survival benefit from cabozantinib treatment [7]. The secondary endpoint of this Phase 3 study was improved overall survival (OS) and not met with median OS of 26.6 months for cabozantinib and 21.1 months for placebo (HR = 0.85, p = 0.241); however, patients with RET M918T mutations experienced significantly longer median OS with cabozantinib (44.3 months vs. 18.9 months with placebo, HR = 0.60, p = 0.026) [8].
Examining the results of these trials, the heterogeneity of responses suggests the possibility of de novo resistance to vandetanib or cabozantinib. The gatekeeper RET V804M/L mutation, present in 1-2% of MTC, has been characterized as resistant to vandetanib and less sensitive to cabozantinib [9, 11, 12]. Moreover, a Phase 2 study of vandetanib in MEN2B-associated MTC reported 3 patients who experienced disease progression after an initial decrease in tumor size, indicative of acquired resistance, despite high prevalence of the RET M918T driver mutation (>95%) in this population [13].
In this study, comprehensive genomic profiling (CGP) was performed in the course of clinical care on 34 advanced MTC cases to assess opportunities for benefit from targeted therapy. An index case (patient #1) with acquired resistance to vandetanib monotherapy derived clinical benefit from the addition of everolimus to the regimen.
Patients and Methods
CGP was performed in a CLIA-certified, CAP-accredited, NYS regulated reference laboratory (Foundation Medicine, Inc.). At least 50 ng of DNA per specimen was extracted from 34 clinical formalin-fixed paraffin-embedded consecutively submitted MTC tumor samples and next-generation sequencing was performed on hybridization-captured, adaptor ligation based libraries to high, uniform coverage (>500×) for all coding exons of 315 cancer-related genes and 28 genes commonly rearranged in cancer (n = 22), 236 cancer-related genes plus 19 genes frequently rearranged in cancer (n = 8), or 182 genes and 14 genes frequently rearranged in cancer (n = 4), with the different number of sequenced genes representing different generations of FoundationOne®.
Base substitutions, short insertions and deletions (indels), focal gene amplifications, homozygous deletions and select rearrangements, were determined and reported for each patient sample. To maximize mutation detection sensitivity in heterogeneous tumor biopsies and resections, the test was validated to detect base substitutions at ≥10% mutant allele frequency with ≥99% sensitivity and indels at ≥20% mutant allele frequency with ≥95% sensitivity, with a false discovery rate of <1% [14]. Actionable alterations are defined as those whose effect is targetable using anticancer drugs currently on the market or in registered clinical trials. Local site permissions to use clinical samples were obtained for this study.
A computational method was used to predict somatic versus germline RET variant status for each of the RET-mutant specimens without a matched normal control as previously described [15].
We reviewed the medical records of a patient (case #1) with sporadic MTC who presented to the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center after failing standard of care therapy. Treatment and consent on investigational trial (NCT01582191) as well as data collection were conducted in accordance with the guidelines of the University of Texas MD Anderson Cancer Center Institutional Review Board (IRB). Tumor response was determined using RECIST (version 1.1) by CT scans obtained about every six to eight weeks. Clinical evaluation and assessments were performed per protocol.
Results
Thirty four consecutively submitted MTC cases had CGP performed in the course of clinical care (Table 1). The median age of the patients was 53 years (range 21-85); 71% (23/34) patients were male and 29% (10/34) were female. MTC generally shows a slight female preponderance [1, 16], and we do not have an explanation for the relatively high frequency of male cases in this unselected dataset. At the time of profiling, 33 MTC were Stage IV and one MTC was Stage II. CGP was performed on tumor specimens from the primary tumor in 47% (16/34) cases, from lymph node metastases in 21% (7/34) or other metastatic sites in 32% (11/34) of cases.
Table 1. Genomic alterations (GA) identified in 34 cases of medullary thyroid carcinoma.
| Case | Age | Gender | Specimen | RET GA | Predicted RET GA status | GAs in non-RET genes |
|---|---|---|---|---|---|---|
| 1 | 42 | M | thyroid | RET M918T | ambiguous | ATM L804fs*4, S978fs*12 |
| 2 | 50 | M | metastasis | RET M918T | somatic | |
| 3 | 45 | M | thyroid | HRAS Q61R, CDKN2C G38* | ||
| 4 | 85 | F | thyroid | RET C634R | somatic | |
| 5 | 37 | F | thyroid | RET M918T | ambiguous | TP53 R283C |
| 6 | 31 | F | metastasis | RET M918T | somatic | DDR2 R806Q |
| 7 | 50 | M | metastasis | RET E632_L633del | somatic | |
| 8 | 35 | F | thyroid | RET M918T | somatic | |
| 9 | 21 | M | thyroid | RET M918T | ambiguous | |
| 10 | 41 | F | thyroid | KRAS amp, CCND1 amp, CCND2 amp, CDKN2A p16INK4a D108H and p14ARF R122P, FGF19 amp, FGF23 amp, FGF3 amp, FGF4 amp, FGF6 amp, SMARCA4 K1219*, TP53 E294* | ||
| 11 | 46 | M | metastasis | RET M918T | somatic | |
| 12 | 58 | M | metastasis | RET C618G | germline | |
| 13 | 49 | M | thyroid | RET E632_L633del | somatic | |
| 14 | 44 | M | thyroid | RET M918T | somatic | |
| 15 | 60 | M | metastasis | BCOR K454fs*2 | ||
| 16 | 57 | M | metastasis | RET C634R | somatic | |
| 17 | 62 | M | metastasis | RET M918T | somatic | VHL L198fs*4 |
| 18 | 45 | M | thyroid | RET M918T | somatic | CDKN2A splice site 151-2A>T, RAD50 R1077*, VHL V130F |
| 19 | 67 | F | thyroid | RET C620R | somatic | EPHA5 E279K |
| 20 | 67 | M | metastasis | RET M918T | somatic | DNMT3A R736C |
| 21 | 62 | M | thyroid | RET M918T | germline | |
| 22 | 74 | F | thyroid | RET M918T | somatic | GRIN2A T1223S |
| 23 | 71 | F | metastasis | KRAS Q61H, LRP1B N2330fs*2, MYC amp, RAD50 R193W, RBM10 E451* | ||
| 24 | 78 | M | metastasis | RET V804M | germline | ARID1A splice site 5125-1G>A, CDKN2A/B loss |
| 25 | 50 | M | metastasis | RET M918T | germline | SF3B1 K700E |
| 26 | 45 | M | thyroid | RET M918T | somatic | LZTR1 K89fs*12 |
| 27 | 57 | M | metastasis | RET M918T | somatic | CCND1 amp, CDK4 amp, EMSY amp, ERBB3 amp, ERBB4 amp, FGF19 amp, FGF3 amp, GLI1 amp, MDM2 amp, RBM10 F175fs*88 |
| 28 | 46 | M | thyroid | RET M918T | somatic | |
| 29 | 82 | F | thyroid | RET amp, M918T | ambiguous | CCND1 amp, FGF19 amp, FGF3 amp |
| 30 | 73 | M | metastasis | RET C618S | somatic | |
| 31 | 66 | F | metastasis | RET M918T | somatic | PTEN V16fs*14, CDK6 amp |
| 32 | 79 | M | metastasis | RET M918T | somatic | MAP3K1 E1242*, MUTYH Y165C |
| 33 | 73 | M | metastasis | RET C618R | somatic | |
| 34 | 39 | M | metastasis | RET M918T | somatic | NOTCH2 R1410H |
M, male; F, female; amp, amplification; RET GA status (somatic, germline, or ambiguous) was predicted with a computational method [15].
All cases harbored at least one genomic alteration (GA), with 83 total GA identified for an average of 2.4 GA per tumor (range 1-11). There were 44 base substitutions and indels, 23 gene amplifications, 15 truncations, 1 homozygous deletion, and no rearrangements. 67% (56/83) of the genomic alterations were considered clinically relevant GA (CRGA) and could potentially guide decisions regarding targeted therapy, with an average of 1.6 CRGA per tumor (range 1-7).
RET was the most frequently altered gene, mutated in 88% (30/34) of cases (Table 1). RET M918T was the most common RET alteration, observed in 62% (21/34) of patients [17-19]. RET C618G/R/S was detected in three cases (9%), RET C634R and E632_L633del each in two cases (6%); RET V804M or C620R was each present in one tumor (3%) [9, 20-25]. One case harbored both a RET amplification and RET M918T [26], and no other cases had more than one RET alteration. A computational method was used to determine the RET variant status without a matched normal control [15] and predicted the RET GA to be somatic in the majority of the RET-positive cases (73%), consistent with the pathology reports that did not indicate any MEN2A or MEN2B cases. Although the two patients with predicted germline RET M918T (cases #21 and #25) could be MEN2B, their ages are more consistent with sporadic MTC and their pathology reports did not mention any known clinical history of MEN2B or pheochromocytoma.
Other clinically relevant alterations occurred in CCND1 (9%), CDKN2A (9%), FGF19 (9%), KRAS (6%), VHL (6%), and for one case each (3%) in ATM, CCND2, CDK4, CDK6, CDKN2B, CDKN2C, DNMT3A, ERBB3, ERBB4, GLI1, HRAS, MDM2, MYC, NOTCH2, and PTEN.
A 42-year old male (case #1) presented with a neck mass, and metabolic imaging suggested a neoplastic thyroid lesion. The patient underwent total thyroidectomy with lymph node dissection. Surgical pathology revealed multifocal MTC with the largest lesion of 2.2 cm in the right lobe. Metastases were detected in 8 out of 8 central nodes and multiple foci indicated extranodal disease. Hot-spot testing identified the RET M918T mutation. Five months later, right thoracotomy was performed and mediastinal metastases were resected. Abdominal CT imaging indicated hepatic metastases and the patient received external beam radiation to treat right choroidal orbital metastases 8 months after the thyroidectomy.
CGP of the thyroidectomy specimen demonstrated the tumor harbored RET M918T (32% mutant allele frequency, MAF) and revealed the ATM truncations L804fs*4 (30% MAF) and S978fs*12 (28% MAF). The patient was treated with vandetanib monotherapy, which was dosed progressively (100 mg once daily, 3 weeks; 200 mg once daily, 8.5 months; 300 mg once daily, 2 months). Electrocardiographic QTc interval prolongation from 405 milliseconds at baseline to 442 milliseconds was the main side effect. Circulating calcitonin eventually increased, however, and vandetanib was discontinued due to disease progression after one year. The patient presented in the Phase 1 clinic at MD Anderson and was enrolled on the clinical trial (NCT01582191) combining vandetanib (300 mg once daily) and everolimus (10 mg once daily) at the defined maximum tolerated dose. After 2 cycles of therapy, the patient had a 25 % reduction in greatest unidimensional tumor measurement per RECIST 1.1 (Figure 2), designated as stable disease, while just under the partial response threshold. In addition, the patient reported that the nodes in the neck were less congested. The main toxicities included grade 2 diarrhea, likely from vandetanib, which was controlled by diphenoxylate and atropine, and grade 2 cough. However, the hepatic metastases in the liver were progressing after an initial mixed response. The patient received particulate trans-arterial embolization for the hepatic metastases and continued on systemic therapy. The stable disease in the neck area lasted for at least 8 months, but the patient was taken off the protocol because of progressive disease in the liver.
Figure 2.
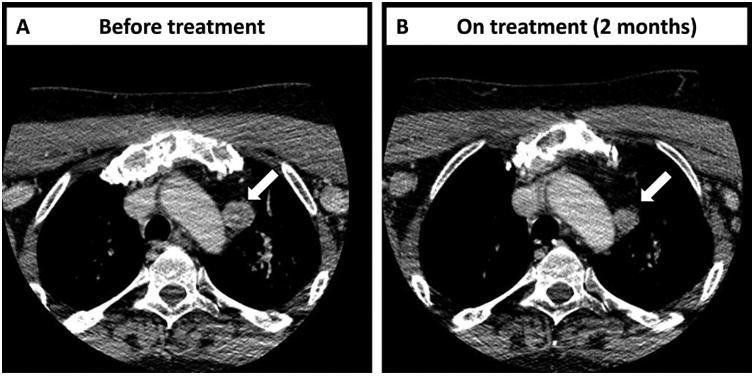
(A) Computed tomography (CT) scans of the index patient (case #1) before treatment shows a right mass measuring 2.2 × 1.9 cm. (B) CT scan of the same patient was taken 2 months after beginning of treatment with vandetanib plus everolimus and shows a mass of 1.5 × 1.5 cm. White arrows point to the target lesion.
The patient underwent hepatic radiotherapy with trans-arterial Y90 SIR-Spheres®, which lead to decrease in the volume of the liver disease, and a subsequent bilateral adrenalectomy for refractory ACTH-induced Cushing syndrome. He started on cabozantinib (100 mg once daily) and experienced painful keratotic rash on feet and intermittent diarrhea, which was managed with dose interruption and reduction. Given the symptomatic progressive disease, the patient underwent extended and complicated right parotidectomy with preservation of the seventh cranial nerve, right neck dissection, wide excision of the entire cervical skin, and reconstruction of the neck and upper chest. Pathology demonstrated MTC, and local hot-spot testing identified only RET M918T mutation. Cabozantinib was increased to 120 mg daily and then to 140 mg daily. The patient experienced transient symptomatic benefit but had a declining performance status and passed away 20 months after initiation of treatment with vandetanib plus everolimus.
Discussion
Medullary thyroid carcinoma (MTC) is an aggressive tumor of neuroendocrine origin arising from the calcitonin-producing parafollicular C cells of the thyroid gland and accounts for approximately 5% of thyroid cancers, which translates to an incidence of nearly 3000 new cases in the US. 75% of MTC cases are dubbed sporadic, and the remainder are part of the inherited autosomal dominant syndromes multiple endocrine neoplasia 2A (MEN2A), MEN2B, or familial. Germline mutations in RET are found in almost all patients with inherited MTC [17, 20, 21, 27] and RET somatic mutations have been observed in 40-65% of patients with sporadic MTC [6, 28]. The activating RET M918T mutation is the most frequent somatic and MEN2B germline alteration. Mutations in the RAS pathway have been identified in patients with RET wild-type sporadic MTC [29-31]. Retrospective next-generation sequencing studies of the exomes or select cancer genes in 17 and 20 sporadic MTC cases, respectively, confirmed mutant RET, HRAS, and KRAS as principal drivers. These studies reported few additional somatic alterations and recurrent mutations only in the gene MDC1, which was not profiled in this study [32, 33].
The recent clinical trials for MTC with targeted therapy demonstrated clinical benefit for only some MTC cases, suggesting the other cases may be de novo resistant to targeted therapy. Several observations from the genomic profiles in this series may explain de novo resistance. We identified a range of RET mutations that occurred mutually exclusively to RET M918T, specifically RET C618G/S/R, C634R, E632_L633del, C620R, and RET V804M. Various RET mutations may be differentially sensitive to targeted therapy, as preclinical data suggests for RET V804M, but this remains a hypothesis for now as clinical evidence linking specific RET alterations other than RET M918T to therapeutic efficacy is lacking. The potential value of RET mutations as predictive markers of therapy response hinges on the ability of the deployed assay to identify RET alterations with high sensitivity. Both large Phase 3 studies of MTC with targeted therapy reported unknown RET mutation status for 30-40% of cases [4, 5], increasing the sample size required to correlate RET mutation status with treatment outcome, although many cases with unknown RET status in the cabozantinib trial had no tumor sample available at the time of analysis [5]. Furthermore, both vandetanib and cabozantinib are anti-angiogenic and inhibit other tyrosine kinases in addition to RET, with vandetanib also targeting VEGFR2 and EGFR and cabozantinib also targeting VEGFR2 and MET [4, 5]. Predicting responses to these therapies solely on the basis of RET status thus likely misses other mechanisms of benefit and may be improved by correlating responses with comprehensive genomic profiles of MTC.
De novo resistance may also be linked to alterations in genes other than RET. Activated RAS acts downstream of RET and is associated with resistance to therapies targeting other receptor protein tyrosine kinases; however, in the cabozantinib Phase 3 trial, patients with RAS mutation experienced similar response rate and PFS as patients with RET mutation (31% vs. 32% and 47 weeks vs. 60 weeks, respectively)[7]. Alterations in CCND1, CCND2, CDK4, CDK6, CDKN2A/B or CDKN2C occurred in 21% of specimens and may lead to hyperactivation of the cyclin D-dependent kinases CDK4 and CDK6. Future clinical studies need to address whether CCND1 or CDK4 amplification may associate with sensitivity to CDK4/6 inhibitors, as preliminary clinical data suggest for other tumor types [34, 35]. CDKN2C has previously been observed to be inactivated in MTC and CDKN2C loss cooperated with oncogenic RET to promote MTC development in mice [36, 37]. Alterations in cell cycle genes co-existent with RET mutations may therefore contribute to resistance to RET targeting. The current study sensitively assesses alterations throughout the entire RET and RAS genes as well as most known cancer genes, and thus is a better foundation for illuminating potential mechanisms of resistance to the approved therapies.
Everolimus demonstrated modest activity against metastatic MTC, as a Phase 2 study of everolimus reported durable stable disease (at least 24 weeks) for 7 out of 9 cases, with 5 patients experiencing tumor shrinkage [38]. Case studies describe one patient who achieved stable disease for at least one year on everolimus [39] and two patients with biochemical responses on everolimus plus octreotide [40]. These clinical data are supported by preclinical studies that validate mTOR signaling as potential target in MTC [41, 42]. However, as the efficacy of single-agent everolimus in MTC has been limited and may result in even less clinical benefit in the second-line setting [43], mTOR inhibitors may be most useful in targeted therapy combinations to overcome resistance to RET targeting. A case report describes systemic activity of vandetanib plus everolimus against RET-rearranged lung cancer [44]. Addition of everolimus to HER2-targeted therapy has shown efficacy against trastuzumab-resistant breast cancer [45], and persistent mTOR activation correlates with resistance to other targeted therapies [46]. Although a specimen of the progressive vandetanib-resistant disease was not available for CGP in the case described here, CGP can potentially identify genomic alterations in the mTOR pathway and point to benefit from added everolimus [45]. Vandetanib combined with everolimus may hold merit in the second-line setting for metastatic MTC, and could be most rationally deployed with the use of genomic profiling.
In conclusion, CGP of MTC has the potential to better delineate potential markers of response to approved targeted therapies, while suggesting routes to overcome resistance to these therapies. Genomic profiles of MTC need to be further investigated in the course of clinical care for patients with advanced MTC to inform the optimal management of these patients.
Figure 1.
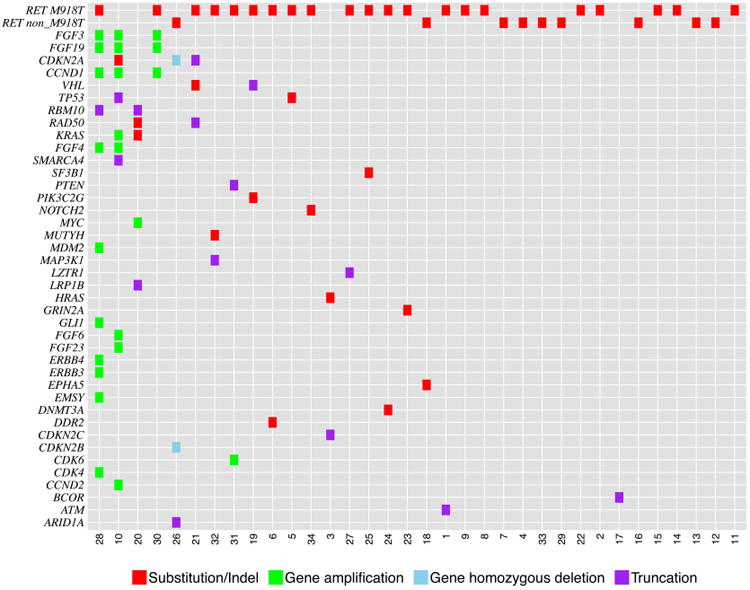
Tile plot of genomic alterations identified by genomic profiling in 34 cases of advanced medullary thyroid carcinoma.
Acknowledgments
We thank Dr. Jon Chung and Dr. Jennifer Spangle for helpful discussions.
Funding: The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health through Cancer Center Support Grant CA016672. VS would like to thank the Dennis Lawrence and Joyce Lawrence research funds.
Footnotes
Declaration of interest: AMH, JXS, JAE, JC, JV, RLE, DL, JSR, VAM, SMA, PJS are employed by and have equity interests in Foundation Medicine. KW and RY are former employees of Foundation Medicine. SIS has a consultant/advisory role for Eisai. NLB has a consulting/advisory relationship with Eisai and Bayer and she has received research funding from Bayer. MHS has received research funding from Bayer, Eisai, and Exelixis.
References
- 1.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–2142. doi: 10.1002/cncr.22244. [DOI] [PubMed] [Google Scholar]
- 2.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–547. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 3.Schlumberger M, Bastholt L, Dralle H, et al. 2012 european thyroid association guidelines for metastatic medullary thyroid cancer. Eur Thyroid J. 2012;1:5–14. doi: 10.1159/000336977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase iii trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic ret oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 7.Sherman SI, Cohen EE, Schoffski P, et al. Efficacy of cabozantinib (cabo) in medullary thyroid cancer (mtc) patients with ras or ret mutations: Results from a phase iii study. J Clin Oncol. 2013;31(suppl; abstr 6000) [Google Scholar]
- 8.Schlumberger M, Elisei R, Muller SP, et al. Final overall survival analysis of exam, an international, double-blind, randomized, placebo-controlled phase iii trial of cabozantinib (cabo) in medullary thyroid carcinoma (mtc) patients with documented recist progression at baseline. J Clin Oncol. 2015;(33)(suppl; abstr 6012) [Google Scholar]
- 9.Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the ret receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004;23:6056–6063. doi: 10.1038/sj.onc.1207810. [DOI] [PubMed] [Google Scholar]
- 10.Bentzien F, Zuzow M, Heald N, et al. In vitro and in vivo activity of cabozantinib (xl184), an inhibitor of ret, met, and vegfr2, in a model of medullary thyroid cancer. Thyroid. 2013;23:1569–1577. doi: 10.1089/thy.2013.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodama T, Tsukaguchi T, Satoh Y, et al. Alectinib shows potent antitumor activity against ret-rearranged non-small cell lung cancer. Mol Cancer Ther. 2014;13:2910–2918. doi: 10.1158/1535-7163.MCT-14-0274. [DOI] [PubMed] [Google Scholar]
- 12.Vitagliano D, De Falco V, Tamburrino A, et al. The tyrosine kinase inhibitor zd6474 blocks proliferation of ret mutant medullary thyroid carcinoma cells. Endocr Relat Cancer. 2011;18:1–11. doi: 10.1677/ERC-09-0292. [DOI] [PubMed] [Google Scholar]
- 13.Fox E, Widemann BC, Chuk MK, et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2b associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19:4239–4248. doi: 10.1158/1078-0432.CCR-13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Frampton GM, Wang K, et al. A computationalmethod for somatic versus germline variant status determination fromtargeted next-generation sequencing of clinical cancer specimens without a matched normal control. AACR Annual Meeting; San Diego, CA. 2014. [Google Scholar]
- 16.Roy M, Chen H, Sippel RS. Current understanding and management of medullary thyroid cancer. Oncologist. 2013;18:1093–1100. doi: 10.1634/theoncologist.2013-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson KM, Dou S, Chi D, et al. Single missense mutation in the tyrosine kinase catalytic domain of the ret protooncogene is associated with multiple endocrine neoplasia type 2b. Proc Natl Acad Sci U S A. 1994;91:1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eng C, Smith DP, Mulligan LM, et al. Point mutation within the tyrosine kinase domain of the ret proto-oncogene in multiple endocrine neoplasia type 2b and related sporadic tumours. Hum Mol Genet. 1994;3:237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- 19.Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the ret proto-oncogene associated with multiple endocrine neoplasia type 2b and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- 20.Donis-Keller H, Dou S, Chi D, et al. Mutations in the ret proto-oncogene are associated with men 2a and fmtc. Hum Mol Genet. 1993;2:851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan LM, Kwok JB, Healey CS, et al. Germ-line mutations of the ret proto-oncogene in multiple endocrine neoplasia type 2a. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 22.Santoro M, Carlomagno F, Romano A, et al. Activation of ret as a dominant transforming gene by germline mutations of men2a and men2b. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 23.Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific ret proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International ret mutation consortium analysis Jama. 1996;276:1575–1579. [PubMed] [Google Scholar]
- 24.Ito S, Iwashita T, Asai N, et al. Biological properties of ret with cysteine mutations correlate with multiple endocrine neoplasia type 2a, familial medullary thyroid carcinoma, and hirschsprung's disease phenotype. Cancer Res. 1997;57:2870–2872. [PubMed] [Google Scholar]
- 25.Bongarzone I, Vigano E, Alberti L, et al. The glu632-leu633 deletion in cysteine rich domain of ret induces constitutive dimerization and alters the processing of the receptor protein. Oncogene. 1999;18:4833–4838. doi: 10.1038/sj.onc.1202848. [DOI] [PubMed] [Google Scholar]
- 26.Ciampi R, Romei C, Cosci B, et al. Chromosome 10 and ret gene copy number alterations in hereditary and sporadic medullary thyroid carcinoma. Mol Cell Endocrinol. 2012;348:176–182. doi: 10.1016/j.mce.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Kouvaraki MA, Shapiro SE, Perrier ND, et al. Ret proto-oncogene: A review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid. 2005;15:531–544. doi: 10.1089/thy.2005.15.531. [DOI] [PubMed] [Google Scholar]
- 28.Marsh DJ, Andrew SD, Eng C, et al. Germline and somatic mutations in an oncogene: Ret mutations in inherited medullary thyroid carcinoma. Cancer Res. 1996;56:1241–1243. [PubMed] [Google Scholar]
- 29.Moura MM, Cavaco BM, Leite V. Ras proto-oncogene in medullary thyroid carcinoma. Endocr Relat Cancer. 2015;22:R235–252. doi: 10.1530/ERC-15-0070. [DOI] [PubMed] [Google Scholar]
- 30.Moura MM, Cavaco BM, Pinto AE, et al. High prevalence of ras mutations in ret-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011;96:E863–868. doi: 10.1210/jc.2010-1921. [DOI] [PubMed] [Google Scholar]
- 31.Schlumberger MJ, Elisei R, Bastholt L, et al. Phase ii study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal N, Jiao Y, Sausen M, et al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in ret and ras. J Clin Endocrinol Metab. 2013;98:E364–369. doi: 10.1210/jc.2012-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simbolo M, Mian C, Barollo S, et al. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch. 2014;465:73–78. doi: 10.1007/s00428-014-1589-3. [DOI] [PubMed] [Google Scholar]
- 34.Dickson MA, Tap WD, Keohan ML, et al. Phase ii trial of the cdk4 inhibitor pd0332991 in patients with advanced cdk4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–2028. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infante JR, Shapiro G, Witteveen P, et al. A phase i study of the single-agent cdk4/6 inhibitor lee011 in pts with advanced solid tumors and lymphomas. J Clin Oncol. 2014;32(suppl; abstr 2528) [Google Scholar]
- 36.van Veelen W, Klompmaker R, Gloerich M, et al. P18 is a tumor suppressor gene involved in human medullary thyroid carcinoma and pheochromocytoma development. Int J Cancer. 2009;124:339–345. doi: 10.1002/ijc.23977. [DOI] [PubMed] [Google Scholar]
- 37.van Veelen W, van Gasteren CJ, Acton DS, et al. Synergistic effect of oncogenic ret and loss of p18 on medullary thyroid carcinoma development. Cancer Res. 2008;68:1329–1337. doi: 10.1158/0008-5472.CAN-07-5754. [DOI] [PubMed] [Google Scholar]
- 38.Lim SM, Chang H, Yoon MJ, et al. A multicenter, phase ii trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:3089–3094. doi: 10.1093/annonc/mdt379. [DOI] [PubMed] [Google Scholar]
- 39.Druce M, Chung TT, Grozinsky-Glasberg S, et al. Preliminary report of the use of everolimus in a patient with progressive medullary thyroid carcinoma. Clin Endocrinol (Oxf) 2012;77:154–155. doi: 10.1111/j.1365-2265.2011.04296.x. [DOI] [PubMed] [Google Scholar]
- 40.Faggiano A, Ramundo V, Dicitore A, et al. Everolimus is an active agent in medullary thyroid cancer: A clinical and in vitro study. Journal of cellular and molecular medicine. 2012;16:1563–1572. doi: 10.1111/j.1582-4934.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapa I, Saggiorato E, Giachino D, et al. Mammalian target of rapamycin pathway activation is associated to ret mutation status in medullary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:2146–2153. doi: 10.1210/jc.2010-2655. [DOI] [PubMed] [Google Scholar]
- 42.Tamburrino A, Molinolo AA, Salerno P, et al. Activation of the mtor pathway in primary medullary thyroid carcinoma and lymph node metastases. Clin Cancer Res. 2012;18:3532–3540. doi: 10.1158/1078-0432.CCR-11-2700. [DOI] [PubMed] [Google Scholar]
- 43.Owonikoko TK, Chowdry RP, Chen Z, et al. Clinical efficacy of targeted biologic agents as second-line therapy of advanced thyroid cancer. Oncologist. 2013;18:1262–1269. doi: 10.1634/theoncologist.2013-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subbiah V, Berry J, Roxas M, et al. Systemic and cns activity of the ret inhibitor vandetanib combined with the mtor inhibitor everolimus in kif5b-ret re-arranged non-small cell lung cancer with brain metastases. Lung Cancer. 2015;89:76–79. doi: 10.1016/j.lungcan.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andre F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, her2-positive, advanced breast cancer (bolero-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 46.Corcoran RB, Rothenberg SM, Hata AN, et al. Torc1 suppression predicts responsiveness to raf and mek inhibition in braf-mutant melanoma. Sci Transl Med. 2013;5:196r. doi: 10.1126/scitranslmed.3005753. a198. [DOI] [PMC free article] [PubMed] [Google Scholar]


