Abstract
Integrated electrical activity in the phrenic nerve is commonly used to assess within-animal changes in phrenic motor output. Because of concerns regarding the consistency of nerve recordings, activity is most often expressed as a percent change from baseline values. However, absolute values of nerve activity are necessary to assess the impact of neural injury or disease on phrenic motor output. To date, no systematic evaluations of the repeatability/reliability have been made among animals when phrenic recordings are performed by an experienced investigator using standardized methods. We performed a meta-analysis of studies reporting integrated phrenic nerve activity in many rat groups by the same experienced investigator; comparisons were made during baseline and maximal chemoreceptor stimulation in 14 wild-type Harlan and 14 Taconic Sprague Dawley groups, and in 3 pre-symptomatic and 11 end-stage SOD1G93A Taconic rat groups (an ALS model). Meta-analysis results indicate: 1) consistent measurements of integrated phrenic activity in each sub-strain of wild-type rats; 2) with bilateral nerve recordings, left-to-right integrated phrenic activity ratios are ~1.0; and 3) consistently reduced activity in end-stage SOD1G93A rats. Thus, with appropriate precautions, integrated phrenic nerve activity enables robust, quantitative comparisons among nerves or experimental groups, including differences caused by neuromuscular disease.
Keywords: respiratory motor output, integration, ventilatory control, chemoreflex, ALS, breathing
1. Introduction
Since the 1970s, integrated electrical activity of the cut central end of the phrenic nerve has been routinely used as an indicator of central respiratory drive to the diaphragm (Bartoli et al., 1975; Eldridge, 1971, 1975, 1976; Schmid and Böhmer, 1984). Due to concerns about nerve electrode contact, variations among investigators, and other factors, integrated phrenic output is generally normalized to a standardized value, such as integrated activity during baseline and/or maximal chemoreflex activation. However, such standardization can introduce normalization artifacts not always appreciated by investigators (Fregosi and Mitchell, 1994), and there is often a need to directly compare absolute measures of respiratory motor output, particularly when making bilateral recordings of the same nerve in the same animal, or across animal groups with neural injury and/or disease (Kajana and Goshgarian, 2009; Lee et al., 2010; Nichols et al., 2013a).
To date, there are no thorough, quantitative assessments concerning the validity of comparing absolute values of integrated phrenic nerve activity within or across animals when precautions are taken to standardize nerve-recording conditions. In supplemental data for a previous publication (Nichols et al., 2013a), we presented preliminary evidence that the amplitude of integrated phrenic motor output can be a robust measure of respiratory motor output since simultaneous recordings of integrated left and right phrenic nerve activity yielded the same voltage (i.e. their ratio was ~1.0) in both wild-type and end-stage SOD1G93A rats, a rat model of ALS (Nichols et al., 2013a). These preliminary findings support the idea that integrated phrenic nerve activity can be a consistent and robust indicator of "absolute" phrenic motor output when proper precautions are taken. Here, we extended the preliminary analysis presented as supplemental material in an earlier paper (Nichols et al., 2013a) by performing a meta-analysis of integrated phrenic burst amplitude from many experimental groups using standardized techniques performed by a single, experienced investigator (N.N.). We found that variations among group-means from many different studies of the same rat sub-strains were small in comparison to differences between group means from different experimental conditions (baseline versus maximal chemoreflex activation), rat sub-strains (Harlan versus Taconic Sprague Dawley rats), or after the onset of motor neuron disease (end-stage SOD1G93A Taconic rats). Thus, with suitable precautions, absolute values of integrated phrenic nerve activity can represent a robust and reliable assessment of phrenic motor output, and may be used to detect differences caused by neural injury (e.g. spinal injury) and/or the onset of neuromuscular disease (e.g. ALS).
2. Materials and Methods
2.1 Animals
We did a meta-analysis on 2 to 6.5 month old male Sprague Dawley rats, including rats from multiple colonies and vendors (Harlan 211, Houston, TX; Harlan 217 and 218a, Indianapolis, IN) and Taconic (Taconic Laboratories, Germantown, NY). Taconic rats included SOD1G93A mutant (MT) and age-matched wild-type (WT) littermates at different ages: pre-symptomatic (60–130 days) and end-stage (150–200 days). SOD1G93A mutant (MT) and age-matched wild-type (WT) littermates were bred from transgenic sires overexpressing the human SOD1G93A gene (bred to female WT Taconic rats). Heterozygous SOD1G93A progeny were identified with polymerase chain reaction (PCR) of tail DNA with primers specific for hSOD1. SOD1G93A rats were considered end-stage when they had lost 20% of their peak body mass, as in previous reports (Nichols et al., 2013a; Nichols et al., 2014; Nichols et al., 2015a).
Overall, we report unilateral integrated phrenic nerve activity from 14 Harlan and Taconic WT rat groups, 3 groups of pre-symptomatic Taconic MT rats, and 11 groups of Taconic end-stage MT rats (see below for group details). Lastly, in 6 groups, we compared bilateral integrated phrenic nerve activity (see below for group details). All groups had at least 4 to 14 rats.
Rats were housed under standardized conditions, with a 12-hour light/dark cycle and ad libitum food and water. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the School of Veterinary Medicine, the University of Wisconsin-Madison, and were in agreement with standards set forth in the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals.
2.2 In vivo Neurophysiology
2.2.1 Experimental preparation
Experimental procedures have been described previously in multiple publications (Hoffman et al., 2012; Nichols et al., 2012; Nichols et al., 2013a). Briefly, rats were anesthetized with isoflurane, tracheotomized, paralyzed and ventilated (Rodent Ventilator, model 683; Harvard Apparatus, Holliston, MA, USA; tidal volume ~2.5 mL, frequency ~70–80). Body temperature was assessed with a rectal thermometer (Fisher Scientific, Pittsburgh, PA, USA) and maintained (37.5 ± 1°C) with a heated surgical table. To monitor end-tidal PCO2 (PETCO2), a flow-through carbon dioxide analyzer with sufficient response time to measure PETCO2 in rats was used (Capnogard, Novametrix, Wallingford, CT). PETCO2 was maintained at ~45 mmHg throughout the surgical preparation. Rats were bilaterally vagotomized and a polyethylene catheter (PE50 ID: 0.58mm, OD: 0.965mm; Intramedic, MD, USA) was inserted into the right femoral artery to monitor blood pressure (Gould Pressure Transducer, P23ID, USA) and enable blood gas analysis. The left phrenic nerves (and right for bilateral studies) were isolated (dorsal approach), cut distally, desheathed, and covered with a saline soaked cotton ball until placing the nerves on bipolar silver electrodes (see below). Some rat groups were from studies where a C2 laminectomy had been performed to enable intrathecal drug or vehicle delivery (see below for details of laminectomy).
Isoflurane anesthesia was maintained (3.5% in 50% O2, balance N2) throughout surgical procedures; all rats were then slowly converted to urethane anesthesia over a 15–20 minute period (1.8 g kg−1, i.v.) while concurrently withdrawing isoflurane. After conversion to urethane, an intravenous infusion was initiated to maintain body fluid and acid-base balance; infusions (1.5–6 ml kg−1 hr−1) consisted of a 1:2:0.13 mixture of 6% Hetastarch (in 0.9% sodium chloride), lactated Ringer’s, and 8.4% sodium bicarbonate. Once rats were converted to urethane anesthesia, a minimum of 1 hour was allowed before protocols commenced.
2.2.2 Nerve recordings
The left phrenic nerves (and right for bilateral studies) were submerged in mineral oil and placed on bipolar silver electrodes to record nerve activity. Neural signals were amplified (10,000 X), band-pass filtered (300–10,000 Hz, Model 1800, A-M Systems, Carlsborg, WA, USA), full-wave rectified and integrated (Paynter filter, 50 ms time constant, MA-821, CWE Inc., Ardmore, PA, USA). Integrated nerve bursts were digitized (8 kHz) and analyzed using WINDAQ data acquisition system (DATAQ Instruments, Akron, OH, USA). For animals that received intrathecal drug or vehicle delivery, a small incision was made in the dura and a soft silicone catheter (2 Fr; Access Technologies, Skokie, IL) was inserted caudally 3–4 mm until the tip rested approximately over the C4 segment to deliver pre-treatment of drugs near the phrenic motor nucleus before protocols commenced. The catheter was attached to a 50 µl Hamilton syringe filled with drug or vehicle solutions. Rats were then paralyzed with pancuronium bromide (2.5 mg kg−1, i.v.).
To begin protocols, the apneic CO2 threshold was determined by lowering PETCO2 until nerve activity ceased for approximately one minute. The recruitment threshold was then determined by slowly increasing PETCO2 until nerve activity resumed (Bach and Mitchell, 1996). PETCO2 was raised ~2 mmHg above the recruitment threshold to establish a level of nerve activity that is stable, repeatable and is low enough that it retains substantial capacity to increase, thus minimizing the potential for "ceiling effects, and ~15–20 min were allowed to establish a stable baseline activity. Arterial blood samples were drawn during baseline, and throughout protocols; arterial CO2 (PaCO2) was maintained within ± 1.5 mmHg of baseline levels by adjusting inspired CO2 and/or ventilator rate.
2.3 Experimental Groups
The voltage of respiratory related integrated phrenic nerve activity was assessed at baseline (following intrathecal drugs if given) and the maximum hypercapnic response at the end of the protocol; this was done in all rat groups in a consistent manner (14 groups of Harlan and Taconic WT rats, 3 groups of Taconic pre-symptomatic MT rats, and 11 groups of end-stage MT rats). Data obtained from some of these groups has appeared in previous publications as percent change from baseline to study phrenic motor plasticity after various treatments, such as hypoxia and/or intrathecal drug or vehicle delivery (Devinney et al., unpublished; Hoffman et al., 2012; Nichols et al., 2012; 2013a; 2015a; Nichols et al., unpublished; Strey et al., 2012). However, none of these systemic and/or intrathecal treatments affected absolute phrenic nerve peak amplitude at baseline or during the maximum hypercapnia response.
In six groups, bilateral phrenic nerve recordings were made, including 2 groups of Harlan rats, and 4 groups of Taconic rats (2 WT and 2 MT age-matched groups at disease end-stage). Data from some of these groups have appeared in published studies (all studies conducted by N.N.), and are repeated here to increase the power of our meta-analysis and enable comparisons among rat sub-strains (Nichols et al., 2013a; 2013b; 2015b).
2.4 Data and Statistical analyses
Integrated phrenic nerve burst peak amplitude was averaged over 1 min bins at each time point assessed during baseline and maximum chemoreflex activation. Statistical comparisons among (integrated peak burst amplitude or the right/left ratio) were made via one way ANOVA. When significant ANOVA differences were detected, individual comparisons were made with Fisher’s least significant difference post hoc test (Sigma Plot version 12.0; Systat Software Inc., San Jose, CA, USA). Differences between the groups were considered significant if p < 0.05; all values are expressed as means ± 1 S.E.M.
3. Results
3.1 Integrated phrenic nerve activity is consistent across studies in Harlan rats
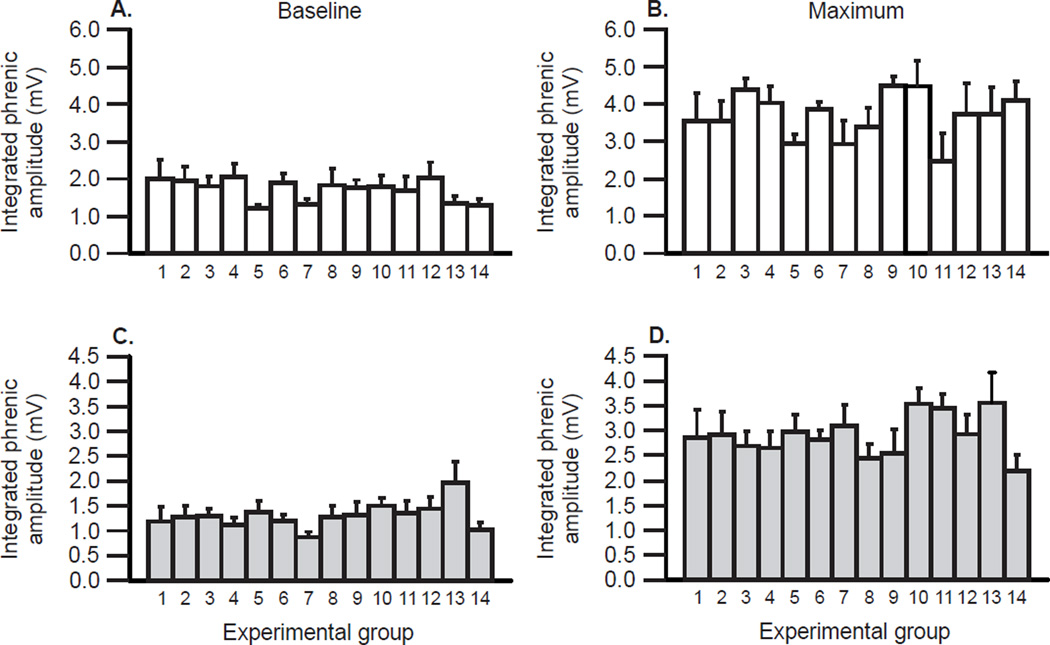
Representative traces of an unprocessed and an integrated phrenic neurogram during baseline and with maximal chemoreflex activation are shown in Figure 1. Mean values of integrated phrenic activity assessed using common methods and equipment in 14 studies on Harlan rats (data obtained from the following studies: Devinney et al., unpublished; Hoffman et al., 2012; Nichols et al., 2012; Strey et al., 2012) during baseline and maximum chemoreflex activation are shown in Figure 2 (A & B). Mean integrated phrenic burst peak amplitude showed no significant variations across studies when comparisons were made during baseline or during maximal chemoreflex activation (p>0.05; Figures 2A & B). In Harlan rats, mean baseline integrated phrenic burst peak amplitude was 1.7 ± 0.09 mV (each group n = 4 or greater; 75 rats total; 95% confidence interval = 1.53 to 1.88) and increased to 3.7 ± 0.15 mV during maximal chemoreflex activation as expected (p<0.05 versus baseline; 95% confidence interval = 3.40 to 4.01). Thus, in Harlan Sprague Dawley rats, measurements of integrated phrenic burst amplitude are repeatable when performed in comparable conditions by an experienced investigator.
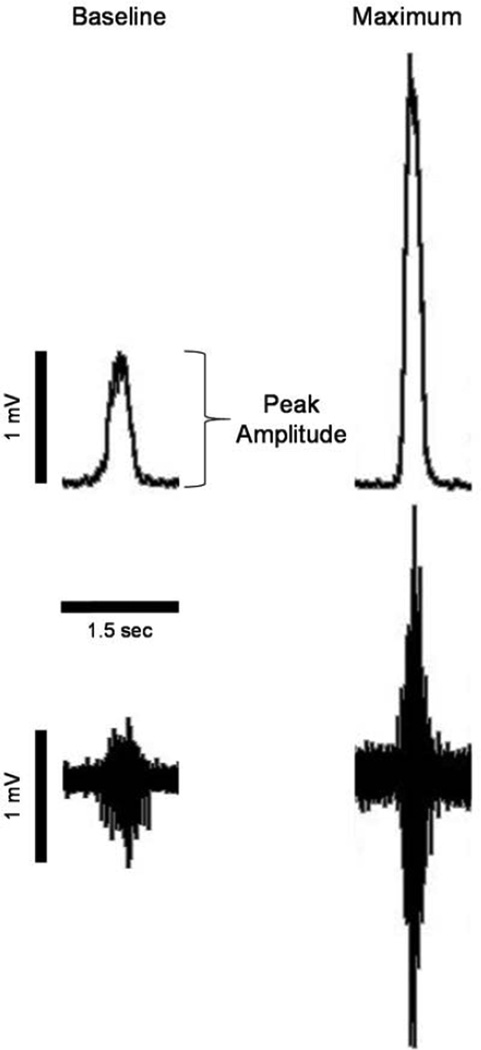
Figure 1.
Representative traces of raw and integrated phrenic nerve activity for single breaths are shown for integrated (top) and raw (bottom) phrenic motor output under baseline conditions (left) and during maximal chemoreflex activation (right).
Figure 2.
Mean integrated phrenic nerve activity is consistent in 14 comparable studies on Harlan Sprague Dawley rats (white bars; A & B) under baseline conditions (A) and with maximal chemoreflex activation (B). There were no significant differences among studies (p>0.05). In the lower panels (C & D), a similar analysis is presented for 14 groups of wild-type Taconic Sprague Dawley rats (light gray bars) under baseline conditions (C) and with maximum chemoreflex activation (D). Although integrated phrenic nerve activity was not significantly different in any study of Taconic rats during baseline or chemoreflex activation (p>0.05), the average activity was significantly lower in Taconic versus Harlan rats, regardless of condition (p<0.05).
3.2 Integrated phrenic nerve activity is lower in Taconic wild-type versus Harlan rats
Mean values of integrated phrenic activity assessed using the same methods in 14 studies on Taconic wild-type rats (data obtained from the following studies: Nichols et al., 2013a; 2015a; Nichols et al., unpublished) during baseline and maximum chemoreflex activation are shown in Figure 2 (C & D). These values were consistently lower in Taconic versus Harlan Sprague Dawley rats during both baseline and chemoreflex activation (p<0.05), indicating that there are systematic differences in integrated phrenic nerve activity among rat sub-strains derived from different colonies/vendors. In these studies on Taconic wild-type rats, mean integrated phrenic burst peak amplitude during baseline conditions was 1.3 ± 0.06 mV (each group n = 5 or greater; 113 rats total; 95% confidence interval = 1.15 to 1.38) and increased to 2.9 ± 0.10 mV during maximal chemoreflex activation (p<0.05 versus baseline; 95% confidence interval = 2.67 to 3.07). Thus, in Taconic Sprague Dawley rats, we confirm that measurements of integrated phrenic burst peak amplitude are repeatable when performed in similar animals under comparable conditions by an experienced investigator, and can reveal subtle differences among groups such as sub-strain variations.
3.3 Integrated phrenic nerve activity is decreased by motor neuron disease
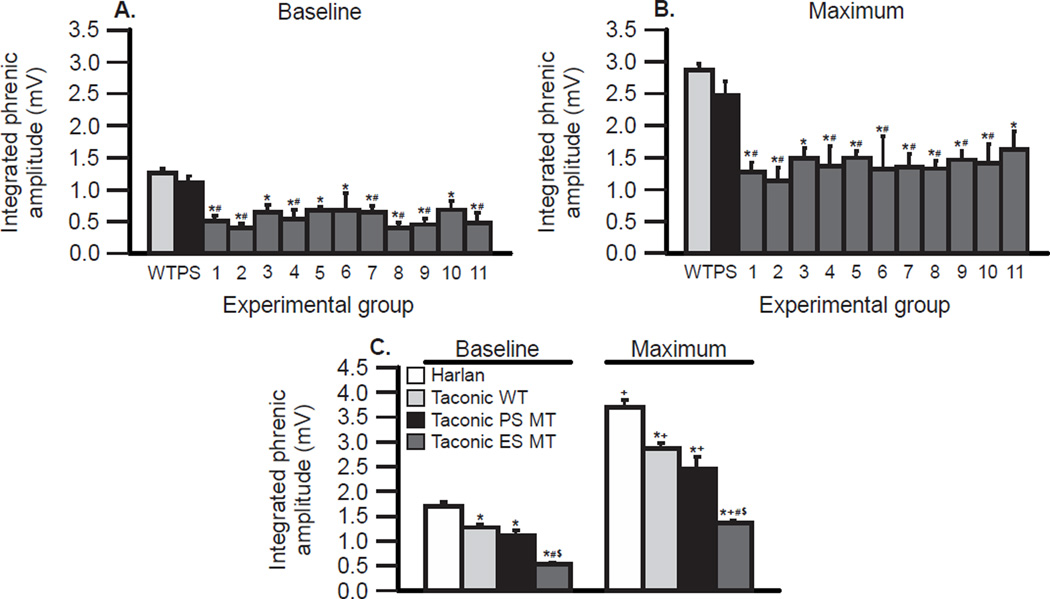
Phrenic burst peak amplitude was also assessed under baseline and maximum conditions in pre-symptomatic and end-stage mutant rats (Taconic Sprague Dawley rats; data obtained from the following studies: Nichols et al., 2013a; 2015a; Nichols et al., unpublished). In 3 studies on pre-symptomatic mutant rats, mean integrated phrenic burst peak amplitude was 1.1 ± 0.11 mV for baseline and 2.5 ± 0.22 mV during chemoreflex activation (each n = 6 or more; 23 rats total; 95% confidence intervals: 0.88 to 1.33 for baseline; 2.01 to 2.93 for maximum). These values in pre-symptomatic rats were not significantly different from wild-type Taconic rats (p>0.05).
In 11 groups of end-stage mutant rats, integrated phrenic burst peak amplitude was assessed during baseline conditions and with maximal chemoreflex activation (Figure 3A & B). Mean integrated phrenic burst peak amplitude was 0.5 ± 0.03 mV during baseline and 1.4 ± 0.06 mV during chemoreflex activation (each n = 4 or more; 71 rats total; 95% confidence intervals: 0.47 to 0.60 for baseline and 1.24 to 1.49 for chemoreflex activation). Although there were minor fluctuations in these 11 group means from end-stage mutant rats in different generations, there were no significant differences among groups at baseline or during chemoreflex activation (p>0.05). On the other hand, all 11 group means were below the 95% confidence interval in either wild-type or pre-symptomatic mutant Taconic rats. Thus, assessment of integrated phrenic burst activity is a robust indicator of diminished phrenic motor output when comparing wild-type or pre-symptomatic mutant rats with mutant rats at disease end-stage.
Figure 3.
Integrated phrenic nerve activity is consistent across 11 groups of end-stage Taconic SOD1G93A (ES MT) rats (dark gray bars; A & B), but is decreased in comparison with wild-type (WT) and pre-symptomatic Taconic rats (PS MT; grand means represented as the light gray and black bars, respectively). In C, grand means are compared between Harlan and Taconic WT rats, and PS MT and ES MT rats during baseline conditions (left) and with maximal chemoreflex activation (right). In A & B, integrated phrenic nerve activity from 11 individual ES MT rat groups (dark gray bars) is compared with grand means from Taconic WT rats in all studies (light gray bars; 113 rats total) and PS MT (black bars; 23 rats total) during baseline (A) and maximum chemoreflex activation (B). There were no significant differences among ES MT groups (p>0.05), but integrated activity in all ES MT groups was lower than WT (*; p<0.05) and PS MT rats (#; p<0.05). In C, integrated grand means of phrenic activity are compared between all rat groups presented in this meta-analysis, including ES MT (dark gray bar; 71 rats total), Harlan (white bar; 75 rats total), Taconic WT (light gray bar; 113 rats), and PS MT rats (black bar; 23 rats) under baseline conditions and with maximum chemoreflex activation. Phrenic activity is significantly greater in Harlan versus all other groups (*; p<0.05). Integrated phrenic activity in ES MT rats is significantly lower versus all other groups (# vs. WT rats, $ vs. PS MT rats; all p<0.05). Maximum phrenic activity was significantly increased versus baseline in all groups (+; p<0.05).
Our results are summarized in Figure 3C, where mean values of peak integrated phrenic burst peak amplitude are compared between Harlan, Taconic wild-type, Taconic pre-symptomatic mutant and Taconic end-stage mutant rats during baseline conditions and maximal chemoreflex activation. Using the methods described here, we are able to distinguish differences in phrenic motor output between baseline and chemoreflex activation (even when paired comparisons are not used), rat sub-strains (Harlan versus Taconic) and with motor neuron disease progression.
3.4 Bilateral phrenic nerve recordings are comparable, regardless of rat group
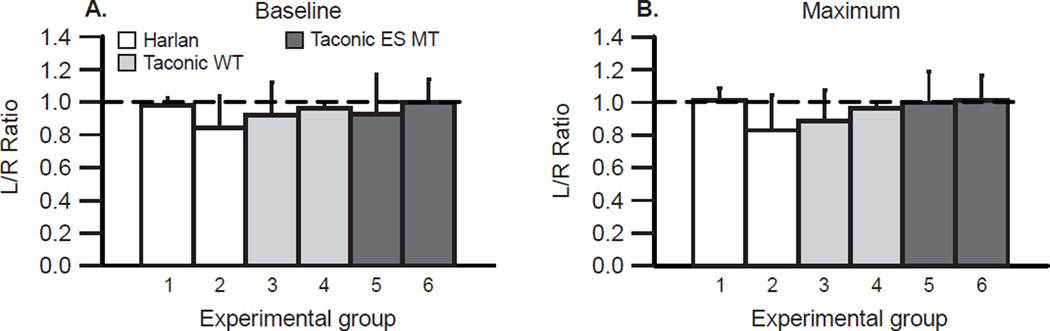
Bilateral phrenic nerve recordings were made at baseline and during chemoreflex activation in multiple groups, including Harlan rats, wild-type Taconic rats, and end-stage Taconic mutant rats (data obtained from the following studies: Nichols et al., 2013a; 2013b; 2015b). In 6 groups, mean values of integrated phrenic nerve burst peak amplitude were not significantly different between the left and right sides in any group at baseline or during chemoreflex activation (all p>0.05). When expressed as a left-to-right ratio (Figures 4A & B), values were not significantly different from 1.0, regardless of group or condition.
Figure 4.
With bilateral phrenic recordings, the mean left-to-right ratio of integrated phrenic activity is 1.0, regardless of animal group. The ratio of integrated phrenic nerve activity (left/right) was assessed in 2 Harlan (white bars), 2 wild-type (WT) Taconic (light gray bars), and 2 Taconic end-stage SOD1G93A mutant (ES MT) rat groups (dark gray bars) under baseline conditions (A) and during maximum chemoreflex activation (B). In all studies, the left/right ratio was not significantly different from 1.0, and did not vary significantly among groups (p>0.05). Bilateral phrenic nerve recordings are the most effective way to assure that recordings are made under comparable conditions. Thus, a ratio of 1.0 demonstrates consistent recording outcomes.
4. Discussion
4.1 Integrated phrenic nerve activity is a robust measure of phrenic motor output
This meta-analysis of studies reporting integrated phrenic nerve burst amplitude using comparable techniques performed by a single experienced investigator demonstrates that integrated phrenic nerve activity can be a robust indicator of phrenic motor output. This finding validates the use of integrated phrenic nerve activity to investigate the impact of neural injury or disease on phrenic motor output since such questions require comparisons across nerves and/or across animals (e.g. Kajana and Goshgarian, 2009; Lee et al., 2010; Nichols et al., 2013a).
The quantitative similarity of mean integrated phrenic nerve activity across experimental groups from the same condition and rat sub-strains exemplifies the repeatability and reliability of this technique to monitor phrenic motor output across animals. For example, during baseline or maximal chemoreflex activation, mean values from 14 different studies are within a tight range in either Harlan (Figures 2A & B) or Taconic wild-type Sprague Dawley rats (Figures 2C & D). The same conclusion is reached when comparing experimental groups with active motor neuron disease (Figures 3A & B), or when comparing bilateral phrenic recordings regardless of the rat sub-strain or animal condition (Figure 4).
However, to achieve such robust, quantitative assessment of phrenic motor output, similar methods must be employed by an experienced investigator, and comparisons must be made under comparable conditions. Relevant methods standardization should include (but is not limited to): 1) similar anesthetic protocols (here, all were isoflurane induction followed by urethane maintenance); 2) similar regulation of fluid and acid-base balance with active monitoring and intervention; 3) similar ventilator settings and endtidal PCO2 regulation during surgical preparations and experimental protocols; 4) active body temperature maintenance; 5) avoidance of seemingly inconsequential stimuli during experimental preparations that may trigger plasticity and render comparisons invalid (e.g. avoid even brief ventilator apneas to trigger nerve activity during surgical/recording preparations); and 6) similar nerve isolation and recording techniques, including electrode placement and signal processing.
4.2 Integrated phrenic nerve activity differs across rat sub-strains
In this meta-analysis, we detected somewhat surprising differences between sub-strains (colonies/vendors) of outbred Sprague Dawley rats (Figure 3C). There are well known differences in ventilatory control among inbred strains of rats (Dwinell et al., 2005; Strohl et al., 1997; Strohl and Thomas, 2001) and mice (Tankersley et al., 1994; 2000), although it is less clear how these differences will be reflected in an outbred strain such as Sprague Dawley rats. On the other hand, there are clear strain (Baker-Herman et al., 2010; Golder et al., 2008; Wilkerson and Mitchell, 2009) and sub-strain (Fuller et al., 2000; 2001; Streeter and Baker-Herman, 2014) differences in the expression of phrenic motor plasticity. How these previous reports of strain and sub-strain differences relate to diminished baseline and maximal chemoreceptor driven phrenic activity is uncertain.
4.3 Integrated phrenic nerve activity decreases with motor neuron disease
Not surprisingly, we detected consistent decreases in integrated phrenic nerve activity under comparable conditions (baseline and chemoreflex activation) in rats with motor neuron disease (Figure 3A & B). This is an important finding since across rat comparisons are at the heart of many studies concerning the impact of spinal cord injury (Alilain et al., 2008; 2011; Doperalski and Fuller, 2006; El-Bohy and Goshgarian, 1999; Fuller et al., 2003; 2006; Golder and Mitchell, 2005; Golder et al., 2003; Kajana and Goshgarian, 2009; Lee et al., 2010; 2013; Liou and Goshgarian, 1994; White et al., 2010) and ALS (Nichols et al., 2013a; Nichols et al., 2015a) on respiratory motor output. With this meta-analysis, we add that the onset of motor neuron disease can be detected since integrated phrenic nerve activity in pre-symptomatic SOD1G93A rats had not yet decreased in comparison with wild-type littermates (Figure 3C).
4.4 Conclusion: Measuring and comparing phrenic motor output
A major key to making comparisons of absolute phrenic nerve activity across multiple studies is to have a highly experienced investigator perform nerve recordings with standardized methods on all rats under investigation. Criteria required to make absolute phrenic nerve activity comparisons across studies are: 1) the same electrodes with a fixed distance between electrodes; 2) consistent signal processing and integration techniques; 3) similar anesthesia; 4) active monitoring and regulation of fluid and acidbase balance, body temperature, respiratory gases, etc.; and 5) avoidance of confounding protocol variations, such as extraneous ventilator apneas. Absolute comparisons of integrated phrenic nerve activity are critical to investigate the impact of neural injury or disease on respiratory motor output.
Highlights.
Reproducibility of integrated phrenic nerve activity was assessed.
Integrated phrenic nerve activity consistently reflects condition across rat groups.
However, phrenic nerve activity varies among rodent sub-strains.
Integrated phrenic nerve activity reveals pathology during motor neuron disease.
Acknowledgements
Supported by NIH K99/R00 HL119606 (NLN), R01 NS057778 (GSM) and R01 HL080209 (GSM). NLN supported by the Francis Families Foundation.
Abbreviations
- O2
Oxygen
- CO2
carbon dioxide
- PETCO2
end-tidal PCO2
- PaO2
partial pressure of arterial O2
- PaCO2
partial pressure of arterial CO2
- mV
millivolts
- pLTF
phrenic long-term facilitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475(7355):196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci. 2008;28(46):11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, MacFarlane PM, Watters JJ, Behan M, Mitchell GS. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir Physiol Neurobiol. 2010;170(3):260–267. doi: 10.1016/j.resp.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli A, Cross BA, Guz A, Huszczuk A, Jeffries R. The effect of varying tidal volume on the associated phrenic motoneurone output: studies of vagal and chemical feedback. Respir Physiol. 1975;25(2):135–155. doi: 10.1016/0034-5687(75)90093-6. [DOI] [PubMed] [Google Scholar]
- Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol. 2006;200(1):74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Dwinell MR, Forster HV, Petersen J, Rider A, Kunert MP, Cowley AW, Jr, Jacob HJ. Genetic determinants on rat chromosome 6 modulate variation in the hypercapnic ventilatory response using consomic strains. J Appl Physiol. 2005;98(5):1630–1638. doi: 10.1152/japplphysiol.01148.2004. [DOI] [PubMed] [Google Scholar]
- El-Bohy AA, Goshgarian HG. The use of single phrenic axon recordings to assess diaphragm recovery after cervical spinal cord injury. Exp Neurol. 1999;156(1):172–199. doi: 10.1006/exnr.1999.7013. [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971;221(2):535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975;39(4):567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Quantification of electrical activity in the phrenic nerve in the study of ventilatory control. Chest. 1976;70(1 Suppl):154–157. doi: 10.1378/chest.70.1_supplement.154. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477(3):469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121(2–3):135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001;4(3):175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100(3):800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23(7):2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23(6):2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25(11):2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine 2A receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol. 2010;588:255–266. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol. 2011;589:1397–1407. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, MacFarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol. 2012;113(8):1184–1193. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Systemic administration of rolipram increases medullary and spinal cAMP and activates a latent respiratory motor pathway after high cervical spinal cord injury. J Spinal Cord Med. 2009;32(2):175–182. doi: 10.1080/10790268.2009.11760769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Dougherty BJ, Sandhu MS, Lane MA, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns following chronic cervical spinal cord injury. Exp Neurol. 2013;249:20–32. doi: 10.1016/j.expneurol.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats. J Appl Physiol. 2010;109(2):377–387. doi: 10.1152/japplphysiol.01429.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou WW, Goshgarian HG. Quantitative assessment of the effect of chronic phrenicotomy on the induction of the crossed phrenic phenomenon. Exp Neurol. 1994;127(1):145–153. doi: 10.1006/exnr.1994.1088. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol. 2012;112(10):1678–1688. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2013a;187(5):535–542. doi: 10.1164/rccm.201206-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Johnson RA, Satriotomo I, Mitchell GS. Neither serotonin nor adenosine-dependent mechanisms preserve ventilatory capacity in ALS rats. Respir Physiol Neurobiol. 2014;197:19–28. doi: 10.1016/j.resp.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Punzo AM, Duncan ID, Mitchell GS, Johnson RA. Cervical spinal demyelination with ethidium bromide impairs respiratory (phrenic) activity and forelimb motor behavior in rats. Neuroscience. 2013b;229:77–87. doi: 10.1016/j.neuroscience.2012.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Satriotomo I, Harrigan DJ, Mitchell GS. Acute intermittent hypoxia induced phrenic long-term facilitation despite increased SOD1 expression in a rat model of ALS. Exp Neurol. 2015a;273:138–150. doi: 10.1016/j.expneurol.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Vinit S, Bauernschmidt L, Mitchell GS. Respiratory function after selective respiratory motor neuron death from intrapleural CTB-saporin injections. Exp Neurol. 2015b;267:18–29. doi: 10.1016/j.expneurol.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Böhmer G. An electronic device for accurate quantification of neuronal mass activity based on a digital integration method. J Neurosci Methods. 1984;11(3):159–167. doi: 10.1016/0165-0270(84)90033-5. [DOI] [PubMed] [Google Scholar]
- Streeter KA, Baker-Herman TL. Spinal NMDA receptor activation constrains inactivity-induced phrenic motor facilitation in Charles River Sprague-Dawley rats. J Appl Physiol. 2014;117(7):682–693. doi: 10.1152/japplphysiol.00342.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey KA, Nichols NL, Baertsch NA, Broytman O, Baker-Herman TL. Spinal atypical protein kinase C activity is necessary to stabilize inactivity-induced phrenic motor facilitation. J Neurosci. 2012;32(46):16510–16520. doi: 10.1523/JNEUROSCI.2631-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Thomas AJ. Ventilatory behavior and metabolism in two strains of obese rats. Respir Physiol. 2001;124(2):85–93. doi: 10.1016/s0034-5687(00)00190-0. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Thomas AJ, St Jean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82(1):317–323. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Elston RC, Schnell AH. Genetic determinants of acute hypoxic ventilation: patterns of inheritance in mice. J Appl Physiol. 2000;88(6):2310–2318. doi: 10.1152/jappl.2000.88.6.2310. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol. 1994;267(5 pt 2):R1371–R1377. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- White TE, Lane MA, Sandhu MS, O’Steen BE, Fuller DD, Reier PJ. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol. 2010;225(1):231–236. doi: 10.1016/j.expneurol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217(1):116–23. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]