Abstract
NAD+ and NADH play key roles in cellular respiration. Intracellular redox state defined by the NAD+/NADH ratio (RX) reflects the cellular metabolic and physiopathological status. By taking the advantage of high/ultrahigh magnetic field strengths, we have recently established a novel in vivo 31P MRS based NAD assay for noninvasive and quantitative measurements of intracellular NAD concentrations and redox state in animal and human brains at 16.4 T, 9.4 T and 7 T, respectively. To explore its potential for clinical application, in this study, we investigated the feasibility of assessing the NAD metabolism and redox state in human brain at a relatively lower field of 4 T by incorporating 1H-decoupling technique with the in vivo 31P NAD assay. The use of 1H-decoupling significantly narrowed the linewidths of NAD and α-ATP resonances, resulting in higher sensitivity and better spectral resolution as compared to the 1H-coupled 31P spectrum. These improvements made it possible for reliably quantifying cerebral NAD concentrations and RX, which are consistent with previously reported results obtained from similar aged human subjects at 7 T. In summary, this work demonstrates the capability and utility of the 1H-decoupled 31P MRS based NAD assay at lower field strength; thus, it opens new opportunities for studying intracellular NAD metabolism and redox state in human brain at clinical setting. This conclusion is supported by the simulation results, indicating similar performance and reliability can be achieved at 3 T and 4 T with the same signal to noise ratio.
Keywords: in vivo31P MRS, intracellular redox state, NAD+ and NADH, human brain, 1H-decoupling
INTRODUCTION
Nicotinamide adenine dinucleotide (NAD), functioning as an important coenzyme and co substrate, has been tightly linked to energy production and metabolic regulation in all living cells (1,2). Through chemical conversion between its reduced form (NADH) and oxidized form (NAD+), NAD participates in various redox reactions as electron donating and accepting coenzymes to facilitate the synthesis of adenosine triphosphate (ATP), which can release energy via hydrolysis for supporting all cellular activities and physiological functions. In addition, NAD also involves in many biological processes (3,4) including signal transduction, calcium homeostasis (5,6), and aging (7) or cell death (2,8). The concentration ratio of NAD+ and NADH (RX=[NAD+]/[NADH]) is defined as intracellular NAD redox state, which reflects the fundamental balance of cellular oxidative-reductive reactions including glycolysis and oxidative phosphorylation (9). Moreover, accumulating evidence has also suggested a correlation between the changes of intracellular redox state and the alterations in energy metabolism during normal aging processing (10) and under many diseased conditions such as diabetes, stroke (11), cancer (12) and epilepsy (3,13). Recently, NAD+ metabolism and NAD+-dependent enzymes have been considered as promising therapeutic targets for neurological diseases (14).
Although the critical roles of NAD+, NADH and their ratio (RX, or redox state) in cellular metabolism and regulation highlights the urgent need for in vivo measurement of NAD contents and its redox state noninvasively, direct assessment of NAD concentrations and NAD+/NADH redox state in vivo is still challenging. To date, there have been limited options available for the assessment of intracellular NAD+ and NADH. Biochemical analysis uses HPLC (15), capillary electrophoresis (16), or enzymatic cycling assays (12) to measure the NAD+ and NADH concentrations ex vivo. Since this method requires tissue biopsy and extraction, it is invasive and not suitable for longitudinal studies, especially when human tissues or organs are targeted, and it is certainly prohibited in human brain applications. The autofluorescence approach utilizes weak endogenous fluorescence signals to monitor the intracellular NADH level (17,18). Although less invasive, it is unable to detect the NAD+ signal and also suffers from artifacts, limited tissue penetration, invasive surgery and potential cell injury.
By taking the advantage of high/ultrahigh magnetic field strengths, we have recently established an in vivo 31P MRS method for noninvasive measurement of intracellular NAD concentrations and NAD+/NADH redox state in animal and human brains (19). By least-square fitting the partially overlapped NAD+, NADH and α-ATP resonances in a 31P spectrum using the quantification model developed in our lab, the separation of NAD+ and NADH signals can be achieved and the individual concentrations of NAD+ and NADH can also be determined. This approach has been applied and validated in normal cat brains at 16.4 Tesla (T) and 9.4 T (19); in normal, ischemic (20) and hypoxic (21) rat brains at 16.4 T; and in healthy (10,22) and Parkinson’s (23) human brains at 7 T. Although it prefers higher signal-to-noise ratio (SNR) and better spectral resolution offered by high/ultrahigh field (19), this in vivo NAD assay has the potential to be extended to lower fields and clinical scanners as long as a good spectral quality with narrow resonance linewidth and adequate SNR is achieved.
Comparing with higher field strength, the major challenges of in vivo 31P MRS application at low field are relatively poor spectral resolution and detection sensitivity (24). While optimized B0 homogeneity with high-order shim may improve the linewidth to a certain degree, the spectral resolution still suffers from the presence of extensive J-coupling between the phosphorus (31P) and hydrogen (1H) nuclei, which results in complicated spectra with overlapping and broadening resonances and leads to further reduction in signal sensitivity. The unresolved heteronuclear coupling exists in multiple phosphorous metabolites including phosphocreatine (PCr), ATP, phosphodiester (GPE: glycerophosphoethanolamine; GPC: glycerophosphocholine) and phosphomonoester (PE: phosphoethanolamine; PC: phosphocholine). To achieve better-resolved signals and reliable quantification, in vivo 31P MRS applications at low field could benefit substantially from 1H-decoupling (25). To explore the potential of the in vivo 31P NAD assay for clinical translation, we incorporate the 1H-decoupling technique to investigate the feasibility of assessing the cellular NAD metabolites and redox state in human brain at a relatively lower field of 4 T. Both 1H-coupled and 1H-decoupled spectra were acquired in the same brain to demonstrate the effect of the 1H-decoupling.
METHODS
In Vivo 31P MRS
Seven healthy volunteers (Age: 23.4±4.2 years old, 4 Male/3 Female) participated in this study. The experimental procedures were approved by the Institutional Review Board of the University of Minnesota with written informed consent obtained from all subjects prior to study. All measurements were conducted on the 4 T/90 cm bore human magnet (Oxford) interfaced with Varian console (Agilent, CA). One passively decoupled dual-coil RF probe, consisting of a linear butterfly 1H surface coil and a 5 cm-diameter single-loop 31P surface coil, was tuned to 4 T operation frequencies (1H: 170 MHz; 31P: 69 MHz), and used in this study for B0 shimming and anatomic imaging and for collecting in vivo 31P spectra from the human visual cortex, respectively. A single-pulse-acquisition sequence with the option of on/off broadband 1H-decoupling (WALTZ-16: 35 μs for the 90° composite pulse width, and 149 Hz B1 decoupling strength) during the FID acquisition was applied to obtain 31P spectra with a nominal 90° RF excitation pulse and following parameters: 2.5 kHz spectral width, 800 number of points for each FID, 3 s repetition time (TR) and 320 (N=1) or 640 (N=6) total scan number. The radio-frequency (RF) power of broadband 1H-decoupling for in vivo 31P MRS acquisition was experimentally calibrated and set below the SAR limit according to the FDA guideline. The raw FID of the 31P MRS data were zero-filled and processed by exponential filtering with a line broadening of 2 Hz to enhance apparent SNR prior to Fourier transformation. The SNR was calculated by dividing the peak height of PCr or α-ATP resonance by the peak-to-peak spectral noise, and multiplying it by 2.5 (24). The resonance signals of phosphorous metabolites were assigned in the in vivo 31P MR spectrum based on their chemical shifts with PCr resonance set at −2.5 ppm as a reference. The spectrum region containing NAD+, NADH and α-ATP resonances (−9 to −11.5 ppm) was processed manually for spectral corrections of phase and baseline before the NAD quantification.
NAD Quantification and Model Simulation
31P magnetic resonances of NAD+, NADH and α-ATP at 4 T follow their typical patterns as previously described at higher field strength (19). Briefly, in addition to a large α-ATP doublet centered at −10.07 ppm, NADH resonates at −10.63 ppm as a singlet while NAD+ has four resonance peaks (quartet). The chemical shifts and relative peak intensity ratios of the NAD+ quartet are highly dependent on the field strength, and were predicted for 4 T application based on the second-order coupling effect and high-resolution NMR results obtained at 11.7 T (see details in Fig. 1 of reference (19)). The recently developed NAD quantification model (19) capable of simulating and/or fitting NAD signals at any given field strength was then applied to 4 T spectra. For convenience, the half linewidth (HLW) of each singlet in the α-ATP doublet was employed in fitting and quantification, and the HLW values of NAD+ and NADH were set to equal to the α-ATP HLW minus 1.5 Hz in the fitting model for acheiving best fitting outcomes (19). Through regressions of the overlapped α-ATP and NAD resonances with the model functions, well-resolved signal intensities and linewidths of NAD+, NADH and α-ATP were determined (19). Individual integrals of NAD+ and NADH calculated from the model-fitted spectrum were then compared with that of α-ATP, which corresponds to 2.8 mM in healthy brain tissue (19,26) and serves as an internal standard, thus, the concentrations of intracellular NAD+, NADH, total NAD ([NAD]total=[NAD+]+[NADH]) and RX can be calculated.
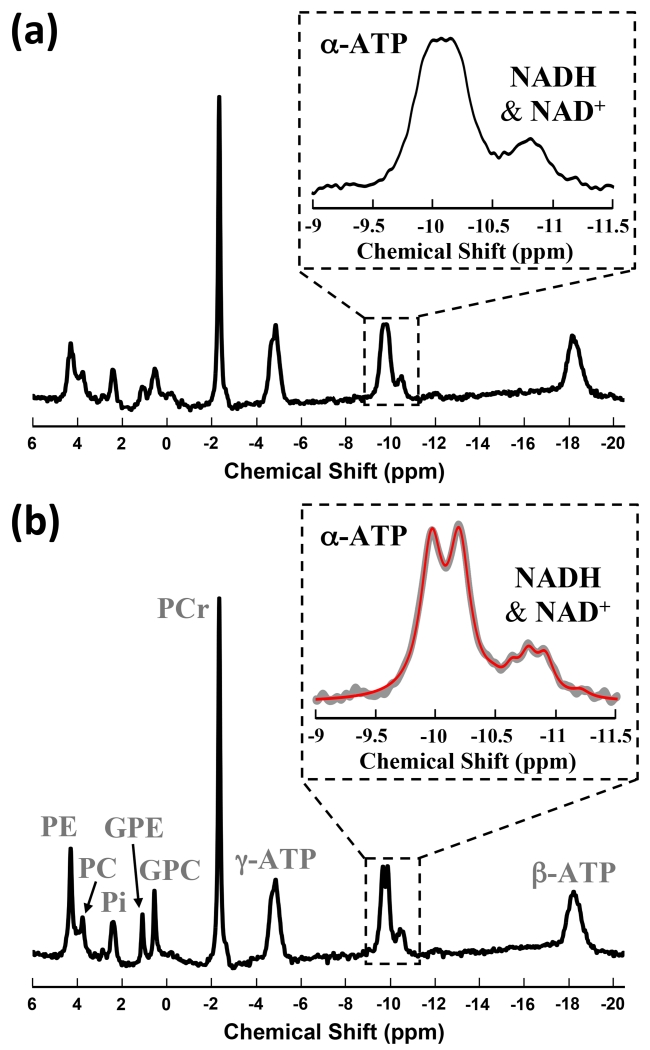
Figure 1.
1H-coupled (a) and 1H-decoupled (b) in vivo 31P MR spectra of human visual cortex from a representative subject (320 scans). Inserts are the expanded spectra in the chemical shift range of −9 to −11.5 ppm. In the insert of (b), original signals (gray trace) and model fitted total signals of α-ATP and NADs (red trace) are displayed.
To examine the reliability of the NAD quantification method and its SNR dependence at lower field, eight different levels of randomly generated white Gaussian noise were added to a simulated 4 T 31P spectra with predetermined half linewidth (HLW) and RX values that were similar to those of human brains measured in this study using the 1H-decoupling method. Monte Carlo simulations of 100 trials per noise level were performed with the following parameters: RX=5.21, [NAD+]=0.276 mM, [NADH]=0.053 mM and HLW=8 Hz. By fitting the 31P spectra with eight SNR levels of α-ATP (=5, 10, 15, 20, 40, 60, 80 and 100), the model determined values were compared with their true values to evaluate the fitting accuracy (=%abs(mean-real)/real; real: predetermined value) and error (=%standard deviation/mean) of the NAD quantifications. In addition, similar simulation was also conducted for the in vivo 31P spectra obtained with the same simulation parameters except using a broader α-ATP HLW (=10.5 Hz) to mimic the 1H coupling condition.
The model simulation was also performed for 3 T application using the similar predetermined parameters as employed in 4 T simulation and 7 Hz HLW based on its field dependence (24) at 3 T with 1H-decoupling.
All results were presented as mean ± standard deviation in this study.
RESULTS
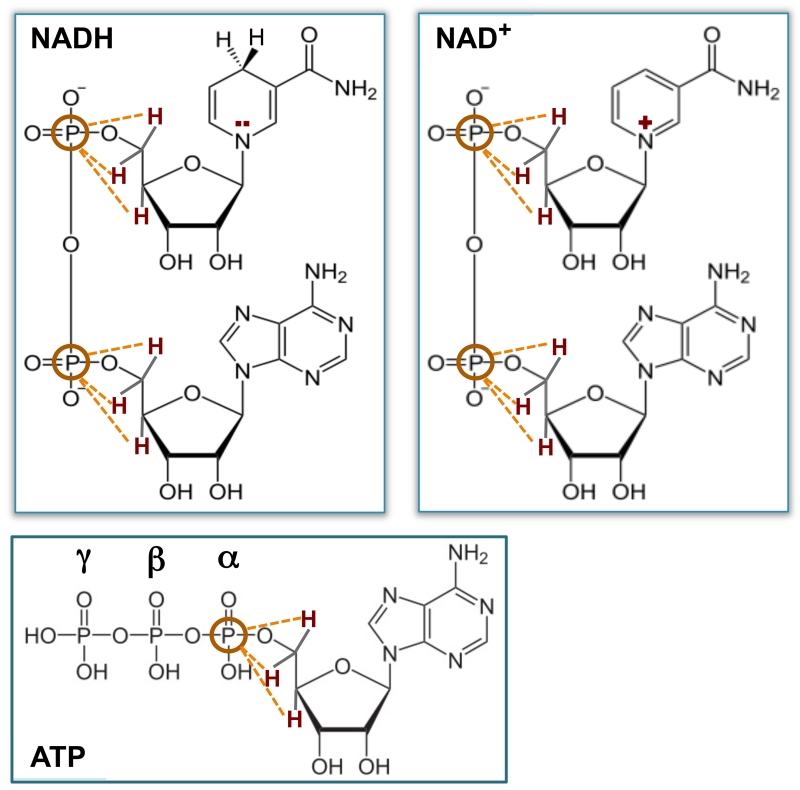
In vivo 31P MR spectra of human visual cortex acquired from a representative subject were displayed in Fig. 1. While both spectra illustrate good spectral quality and high SNR, the 1H-decoupled spectrum (Fig. 1b) demonstrates significantly improved spectral quality when compared with the 1H-coupled one (Fig. 1a). Specifically, narrower resonance linewidths and higher signal intensities, thus, improved SNRs due to the same spectral noise level, were observed in phosphorus metabolites including NADs, α-ATP, PCr, PE, PC, GPE, GPC and inorganic phosphate (Pi) in the spectrum acquired with 1H-decoupling. Most importantly, as shown in the insert of Fig. 1b, 1H-decoupling not only revealed the intrinsic split of α-ATP resonance doublets caused by 31P-31P J-coupling, but also resulted in much better separation in NAD signals (i.e., three peaks were observed) than that of 1H-coupled spectrum (insert of Fig. 1a). The excellent SNR of the 1H-decoupled 31P spectra (254±60 for PCr; 71±10 for α-ATP; N=6 with 2 Hz line broadening) and the improved peak separation in the spectral region with overlapped NAD resonances allow reliable spectral fitting to decompose the NAD+ and NADH signals and consequently provide accurate NAD quantifications. The 1H-decoupling effect significantly improved the detectability of α-ATP, NAD+ and NADH resonances, for instance, resulting in substantially narrowed linewidth (26±3%, p<0.001, N=6) and enhanced signal intensity or SNR gain (27±3%, p<0.001, N=6) for α-ATP with unchanged resonance integral (p=0.3) (Table 1). In contrast, such decoupling effect on γ- and β-ATP resonances was not significant (p≥0.2). This is due to the J-coupling effect between the 1H spins on the ribose C5’and the α-phosphorus spin, which is the closest one among the three phosphate groups to the adenosine; and the extensive J-coupling effect also exists for the phosphorus spins of NAD+ and NADH owing to their similar spin environment to α-ATP as shown in Fig. 2.
Table 1.
Signal intensity and linewidth improvements of α-ATP resulting from 1H-decoupling
| Subjects # | 1 | 2 | 3 | 4 | 5 | 7 | Mean±Std | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | ||
|
Peak
Intensity (×10−3 a.u.) |
3.2 | 4.1 | 3.2 | 4.0 | 3.2 | 4.0 | 2.7 | 3.4 | 3.2 | 4.2 | 3.4 | 4.2 | +(27.0±2.8) % |
| +28.5% | +26.9% | +24.7% | +25.3% | +31.8% | +24.8% | ||||||||
| HLW (Hz) | 10.0 | 7.3 | 10.4 | 7.9 | 10.5 | 8.2 | 10.0 | 7.8 | 10.5 | 7.4 | 11.4 | 8.3 | −(25.5±3.0) % |
| −26.8% | −24.7% | −22.2% | −22.2% | −29.5% | −27.4% | ||||||||
|
Integrals
(×10−3 a.u.) |
2.7 | 2.6 | 2.8 | 2.7 | 2.8 | 2.8 | 2.3 | 2.2 | 2.8 | 2.7 | 3.1 | 3.0 | - |
‘N’ or ‘Y’ indicates the 1H-decoupling ‘off’ and ‘on’, respectively.
Subject #6 is not included in this Table for comparison because the 1H-coupled spectrum was not available in this subject.
Figure 2.
Schematic of molecular structures, showing strong heteronuclear J-coupling effects between the 31P spin(s) and closest 1H spins (red) for NADH, NAD+ and α-ATP, respectively.
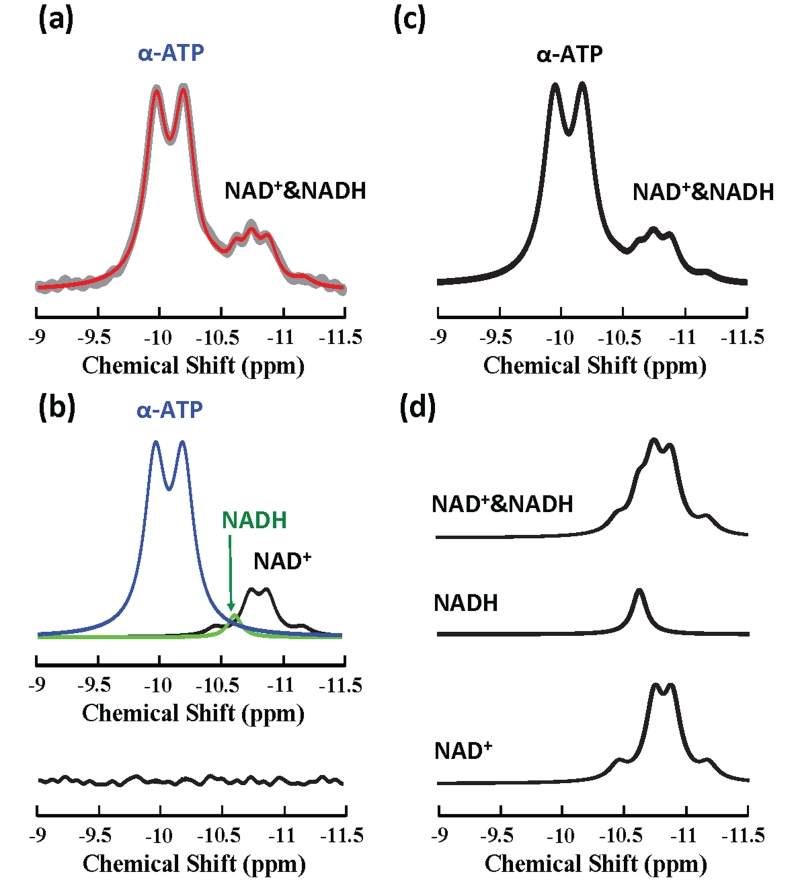
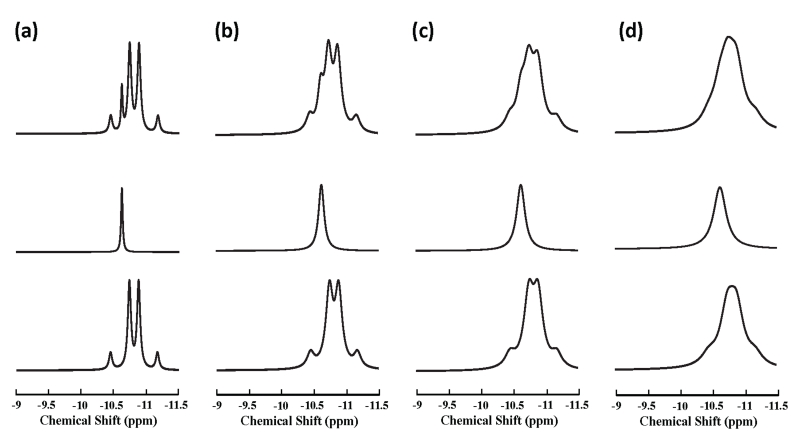
In vivo measured (gray trace) and model-fitted (red trace) 1H-decoupled 31P spectra obtained from another human subject at 4 T are displayed in Fig. 3a with the chemical shift range covering NAD+, NADH and α-ATP resonances. The selected region of spectrum was satisfactorily fitted by the linear summation of the model-decomposed signals of NAD+, NADH and α-ATP (top in Fig. 3b), which was evident from the small residue (within the noise level) between the original spectrum and model fittings (bottom in Fig. 3b). Figure 4 demonstrates the linewidth dependence of the NAD resonance pattern at 4 T based on model simulations with a fixed NAD+/NADH ratio (=4.8). When linewidths (i.e., 2×HLW) increasing from 7 Hz to 15.6 Hz, which mimics the situations from phantom solution to in vivo brain tissue under 1H-decoupling condition (e.g., in the current study), the combined resonances of NAD+ and NADH characterized by the five sharp peaks (top in Fig. 4a) evolves to a broader spectrum with weakened separations among apparent resonance peaks (top in Fig. 4c); further linewidth broadening as in the case of 1H-coupling diminishes such separation (see Fig. 4d). Figure 3d illustrates another example of simulated individual NAD+, NADH and their combined spectra using the same linewidth and RX values obtained from the in vivo spectrum shown in Fig. 3a. By including α-ATP doublet, Fig. 3c displays a simulated spectrum that is highly comparable to the 1H-decoupled in vivo 31P spectrum measured in the human brain at 4 T (gray trace in Fig. 3a).
Figure 3.
In vivo and simulated 31P spectra of human brain at 4 T: (a) Expanded in vivo 1H-decoupled 31P MR spectrum (from −9 to −11.5 ppm) of human visual cortex from another representative subject (640 scans) (gray trace) with its model fittings (red trace). (b) Model decomposed individual component of α-ATP (blue), NAD+ (black) and NADH (green) with small fitting residue shown in the bottom. (c)&(d) Model simulated spectra of NAD+ quartet, NADH singlet, total NAD and combined α-ATP with NAD signals using the linewidth of 14.3 Hz and NAD+/NADH redox (RX) ratio of 4.8, which are the same as those measured from the spectrum displayed in (a).
Figure 4.
Linewidth dependence of the NAD resonance pattern at 4 T. Model simulations of NAD+ quartet (bottom), NADH singlet (middle) and combined NAD resonance (top) with predetermined NAD+/NADH redox ratio (RX) of 4.8 are displayed as the α-ATP HLW increasing from 3.5 Hz (a), 6 Hz (b), 7.8 Hz (c) for mimicking in vivo 1H-decoupled 31P spectrum, to 10.5 Hz (d) for mimicking in vivo 1H-coupled 31P spectrum.
Table 2 summarizes the results of intracellular concentrations of NAD+ (0.31±0.02 mM, or 0.28±0.02 μmol/g after an unit conversion using a brain tissue density of 1.1 g/ml), NADH (0.058±0.004 mM, or 0.053±0.004 μmol/g), total NAD ([NAD]total=0.37±0.02 mM, or 0.34±0.02 μmol/g) and NAD+/NADH redox ratio (RX=5.3±0.4) obtained from all seven healthy human subjects scanned at 4 T. To evaluate the reliability of the NAD quantification method, model simulation results under different SNR levels of α-ATP are summarized in Table 3a. As shown in Tables 3b and 3c, excellent fitting accuracy (less than 1%; lower value means more accurate) and small fitting error (≈5% or lower) could be achieved as long as the SNR of α-ATP reaches the level of 60 or higher. Considering the superior 31P spectral quality and SNR demonstrated in this study (SNR>70 for α-ATP; with 2 Hz line broadening), the reliability of the 1H-decoupled in vivo NAD approach in human brain at 4 T was confirmed.
Table 2.
Results of NAD concentrations and NAD+/NADH redox state (RX) measured in healthy human brain at 4 T using in vivo 31P MRS with 1H decoupling
| Subjects # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean±Std |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| [NAD+] (mM) | 0.32 | 0.34 | 0.30 | 0.32 | 0.32 | 0.30 | 0.27 | 0.31±0.02 |
| [NADH] (mM) | 0.060 | 0.056 | 0.052 | 0.062 | 0.057 | 0.061 | 0.055 | 0.058±0.004 |
| RX | 5.3 | 6.0 | 5.7 | 5.2 | 5.6 | 4.9 | 4.9 | 5.3±0.4 |
| [NAD]total (mM) | 0.38 | 0.40 | 0.35 | 0.39 | 0.38 | 0.36 | 0.33 | 0.37±0.02 |
Table 3.
Model simulation results at 4 T (100 Trials per SNR level)
| (a) Fitting Results | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SNR (α-ATP) | 20 | 40 | 60 | 80 | 100 | W/O Noise | |
|
| |||||||
|
4T Fitted
Values |
HLW | 8.01±0.09 | 8.00±0.04 | 7.99±0.03 | 8.00±0.02 | 8.00±0.02 | 8.00 |
| RX | 5.22±0.77 | 5.27±0.44 | 5.23±0.28 | 5.20±0.19 | 5.21±0.14 | 5.21 | |
| [NAD*] | 0.275±0.009 | 0.277±0.005 | 0.276±0.003 | 0.276±0.002 | 0.276±0.002 | 0.276 | |
| [NADH] | 0.054±0.006 | 0.053±0.004 | 0.053±0.002 | 0.053±0.002 | 0.053±0.001 | 0.053 | |
| [NAD]total | 0.329±0.007 | 0.329±0.004 | 0.329±0.002 | 0.329±0.002 | 0.329±0.001 | 0.329 | |
| (b) Fitting Accuracy (%) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SNR (α-ATP) | 20 | 40 | 60 | 80 | 100 | W/O Noise | |
|
| |||||||
|
4T Fitted
Values |
HLW | 0.13 | 0 | 0.13 | 0 | 0 | - |
| RX | 0.19 | 1.15 | 0.38 | 0.19 | 0 | - | |
| [NAD*] | 0.36 | 0.36 | 0 | 0 | 0 | - | |
| [NADH] | 1.89 | 0 | 0 | 0 | 0 | - | |
| [NAD]total | 0 | 0 | 0 | 0 | 0 | - | |
| (c) Fitting Error (%) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SNR (α-ATP) | 20 | 40 | 60 | 80 | 100 | W/O Noise | |
|
| |||||||
|
4T Fitted Values |
HLW | 1.12 | 0.50 | 0.38 | 0.25 | 0.25 | - |
| RX | 14.75 | 8.35 | 5.35 | 3.65 | 2.69 | - | |
| [NAD*] | 3.27 | 1.81 | 1.09 | 0.72 | 0.72 | - | |
| [NADH] | 11.11 | 7.55 | 3.77 | 3.77 | 1.89 | - | |
| [NAD]total | 2.13 | 1.22 | 0.61 | 0.61 | 0.30 | - | |
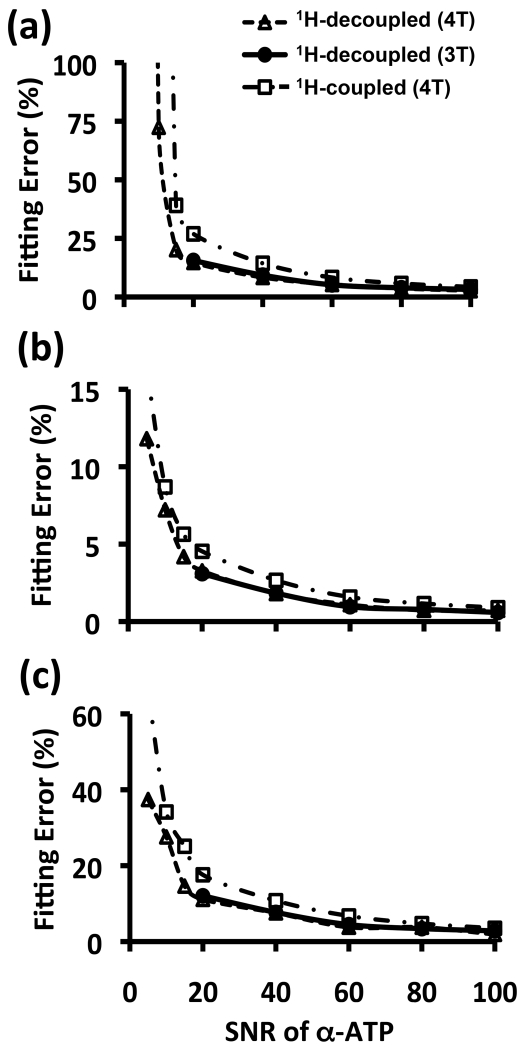
Figure 5 shows the comparison results of fitting errors between 3 T and 4 T model simulations and their dependence on SNR. Due to the unique spectral pattern of NAD+ quartet resonances and slightly narrow linewidth at 3 T, the fitting errors are almost the same for these two fields at a given SNR. Figure 5 also reveals that the errors for fitting the 1H-decoupled 31P spectra were approximately two times smaller than that of 31P spectra without 1H-decoupling, owing to 26% linewidth narrowening and 27% SNR gain by 1H-decoupling (Table 1). Due to the larger fitting error, similar regression of 1H-coupled 31P spectra in most subjects failed to provide consistent NAD and RX results when the α-ATP SNR was below 40 with average linewidth or shimming quality in this study. In contrast, 1H-decoupled 31P spectra with a significantly lower α-ATP SNR of ~20 provided tolerable fitting error (see Fig. 5). These results suggest that both spectal resoluton and SNR are critical for reliable NAD quantification, which is especially challenging for 1H-coupled 31P spectra; nevertheless, using the 1H-decoupling method could alleviate this technical limitation.
Figure 5.
Comparison results of fitting error between 4 T and 3 T, and between 1H-decoupled and coupled in vivo 31P spectra, and their dependence on α-ATP SNR for (a) RX, (b) NAD+ and (c) NADH.
DISCUSSION AND CONCLUSION
In this study, the feasibility of the 31P MR-based in vivo NAD assay for assessing the cellular [NAD+], [NADH] and NAD+/NADH redox state in healthy human brains was demonstrated and validated at 4 T with 1H-decoupling. Performing 1H-decoupling during 31P acquisition provides adequate spectral quality with improved SNR, narrow linewidth and good spectral resolution, which could be achieved on clinical scanners operating at lower field strength. The successful extension of the capability for noninvasively quantifying NAD concentrations from high/ultrahigh (7 T, 9.4 T and 16.4 T) to lower field strengths indicates a potential opportunity for studying NAD metabolism in human brains at clinical setting.
Based on the results of this study, to ensure accurate determination of the low concentration NAD metabolites from their overlapped 31P signals, 1H-decoupling technique is critical. As shown in the insert of Fig. 1b and Fig. 3a, the three separated peaks observed on the right shoulder of α-ATP resonance are identified to be the singlet of NADH and the two central peaks of the NAD+ quartet, respectively. The other two smaller peaks on the edge of NAD+ quartet are hardly visualized on the in vivo 31P spectrum at 4 T due to their low sensitivity and/or overlapping with the dominant α-ATP doublet (Fig. 3), nevertheless, their signal contributions were accounted in the model simulation and spectral fitting.
Interestingly, at higher field strength (such as 7 T, 9.4 T and 16.4 T), the in vivo NAD 31P spectrum demonstrates two apparent peaks consisting of: 1) the NADH singlet overlapping with the left-side part of the NAD+ resonance; and 2) the right-side part of the NAD+ resonance (10,19,22). In contrast, the three apparent NAD peaks observed at 4 T in this study can be explained by the field dependence of the NAD+ spectral pattern with various chemical shifts and intensity ratios between the NAD+ quartet resonances with a fixed and field independent center chemical shift: the two central (stronger) peaks of the NAD+ quartet move towards each other when the field strength decreases and become separated from the NADH singlet; meanwhile, the two outside (weaker) peaks depart from each other along with an increasing intensity ratio of the stronger to weaker peaks that further enhance the signals of the central quartet (19). Therefore, for in vivo spectra with broad resonance linewidth, mixed NADH singlet with NAD+ quartet results in two apparent spectral peaks at higher field strength, whereas the unique pattern of 1H-decoupled NAD+ spectra at lower field (e.g., at 4 T in this work) improves the separation between the NADH and NAD signals. Therefore, the three-peak-separation of NAD resonances observed in the 1H-decoupled 31P spectrum at 4 T clearly distinguishes the NADH singlet from the NAD+ quartet in the human brain, which confirms the chemical shift assignment of NADH singlet at −10.63 ppm and subsequently conveys the reliability of the in vivo NAD quantifications at lower field.
To reduce the scan time, in vivo 31P MRS experiments usually are not performed under fully relaxed condition. Although the T1 relaxation times of NAD+ and NADH in human brain at 4 T are unknown, they are expected to be similar to each other at the same field strength and to increase at lower field strength (19,24). Therefore, the T1 value of NAD+ or NADH at 4 T is expected to be longer than that of cat brain measured at 9.4 T (1.5-1.6 s) (19,27) and human brain at 7 T. Due to partially saturated acquisition condition in this study (TR=3 s), the saturation effect would lead to an underestimation of the NAD concentrations, i.e., the actual brain [NAD+], [NADH] and [NAD]total should be slightly higher than those reported in this study after correcting the saturation effects once the T1 values become available. This is evident from the comparison, showing the slightly higher values of [NAD+]=0.35±0.01 mM, [NADH]=0.06±0.01 mM, and [NAD]total=0.41±0.02 mM obtained from the age-matched young human subject group (N=7, age: 23.4±4.2 years old) at 7 T in our previous work (10). Nevertheless, the correction of saturation effect is not necessary for RX quantification, since similar T1 values of NAD+ and NADH at the same field would result in the same saturation effects. The RX value (5.3±0.4, N=7, age: 23.4±4.2 years old) measured at 4 T in this study (Table 2) is in an excellent agreement with those 7 T results (5.4±0.8, N=7, age: 22.7±2.3) from our previous work (10), suggesting excellent reliability and accuracy of 1H-decoupled 31P MRS-based NAD assay for assessing the human brain NADs and redox state at 4 T. Moreover, the comparison of 3 T model simulation results with that of 4 T (Fig. 5) clearly indicate that similar performance is achievable for clinical scanners at 3 T with the same SNR.
Reducing the magnetic field inhomogeneity by B0 shimming is crucial for improving 31P spectral resolution and SNR, in particular, if the 1H-decoupling method was not used for assessment of brain NADs and RX. Nevertheless, the shimming was highly robust in this study, showing <15% variation in the measured α-ATP HLWs among inter subjects (Table 1). The averaged α-ATP HLW without 1H-decoupling was 10.5 Hz (N=6). This value can be used to calculate the apparent full linewidth of α-ATP including two doublets to be 34.5 Hz (=2×10.5Hz + Jα-ATP – 2Hz line-broadening employed in the spectral processing in this study; where Jα-ATP is the homonuclear J coupling constant of α-ATP and equals to 15.5 Hz (19)). This result is identical to the predicted value at 4T based on Eq. 2b of an independent study (24). Therefore, obtaining reasonable B0 shimming should not be a major technical hurdle for realibe NAD and RX quantification using 1H-decoupled 31P MRS. This notion is supported by small inter-subject variations of NAD concentrations and RX shown in Table 2.
Beside the benefits of detecting α-ATP, NADs and redox state in human brain at 4 T, the use of 1H-decoupling also significantly improves the signal intensity and SNR for other phosphorus metabolites such as PCr (17±2%, p<0.01, N=6), PE (66±15%, p<0.001), PC (27±13%, p<0.05), GPE (138±45%, p<0.001) and GPC (81±23%, p<0.001). Since the 1H-decoupling does not change the signal integral as illustrated in Table 1, there is no complication for spectral quantification based on the integral analysis but with huge SNR gain for some phosphorus metabolites, for instance, >100% for GPE. In contrast, the nuclear Overhauser effect (NOE) generated by employing a low-power and continuous wave at the water proton Larmor frequency prior to the RF excitation pulse could also enhance the signals of phosphorus metabolites without linewidth change. However, the NOE induced SNR gains rely on the increase of resonance integral (28), thus, a careful calibration of the integral enhancement is required for proper quantification of metabolites concentration.
Recently, an alternative approach based on in vivo 1H MRS using a frequency-selective RF pulse excitation for detecting the brain NAD+ resonances around 9 ppm has been reported (29). This 1H method could avoid the issue of overlapped resonances from other metabolites, however, it is incapable of detecting intracellular NADH, thus, NAD redox ratio (RX) in the brain. In addition, it also loses the useful signals of other metabolites commonly detected by conventional 1H MRS approaches owing to a narrow band width of the selective RF pulse (29).
In conclusion, this work supports the feasibility and reliability of the new in vivo NAD assay based on the 1H-decoupled 31P MRS approach for direct and quantitative assessment of intracellular NAD metabolites and redox state in human brain at 4 T. This technical advancement opens new opportunities for non-invasively investigating the critical roles of NAD in human brain functions and diseases at lower field strengths, for instance, using a clinical platform at 3 T.
Acknowledgments and Grant Sponsors
National Institute of Health (NIH) grants: NS057560, NS070839, R24 MH106049, R24 MH106049-02S1, P41 EB015894, P30 NS076408, S10 RR023730 and S10 RR027290; WM Keck Foundation; and Academic Health Center (AHC) Faculty Research Development (FRD) Grant at the University of Minnesota.
Abbreviations
- ATP
Adenosine triphosphate
- GPC
Glycerophosphocholine
- GPE
Glycerophosphoethanolamine
- NAD
Nicotinamide adenine dinucleotide
- NAD+
Oxidized NAD form
- NADH
Reduced NAD form
- PC
Phosphocholine
- PCr
Phosphocreatine
- PE
Phosphoethanolamine
- Pi
Inorganic phosphate
- RX
Redox state ratio
- HLW
Half linewidth
- SNR
Signal to noise ratio
- SAR
Specific absorption rate
- TR
Repetition time
References
- 1.Berger F, Ramirez-Hernandez MH, Ziegler M. The new life of a centenarian: signalling functions of NAD(P) Trends Biochem Sci. 2004;29:111–118. doi: 10.1016/j.tibs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Ying W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]
- 3.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides--small molecules with a multitude of functions. Biochem J. 2007;402:205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 6.Lee HC. Multiplicity of Ca2+ messengers and Ca2+ stores: a perspective from cyclic ADP ribose and NAADP. Curr Mol Med. 2004;4:227–237. doi: 10.2174/1566524043360753. [DOI] [PubMed] [Google Scholar]
- 7.Ying W. NAD+ and NADH in brain functions, brain diseases and brain aging. Front Biosci. 2007;12:1863–1888. doi: 10.2741/2194. [DOI] [PubMed] [Google Scholar]
- 8.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DC. Colloquium paper: bioenergetics, the origins of complexity, and the ascent of man. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(Suppl 2):8947–8953. doi: 10.1073/pnas.0914635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng C, Han J, Xia W, Shi S, Liu J, Ying W. NAD+ administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett. 2012;512:67–71. doi: 10.1016/j.neulet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Wang SY, Nottke AC, Rocheleau JV, Piston DW, Goodman RH. Redox sensor CtBP mediates hypoxia-induced tumor cell migration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9029–9033. doi: 10.1073/pnas.0603269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Chen H, He X, Nie H, Hong Y, Sheng C, Wang Q, Xia W, Ying W. NAD+ metabolism and NAD+-dependent enzymes: promising therapeutic targets for neurological diseases. Curr Drug Targets. 2012;13:222–229. doi: 10.2174/138945012799201711. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie W, Xu A, Yeung ES. Determination of NAD+ and NADH in a single cell under hydrogen peroxide stress by capillary electrophoresis. Anal Chem. 2009;81:1280–1284. doi: 10.1021/ac802249m. [DOI] [PubMed] [Google Scholar]
- 17.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 18.Skala MC, Riching KM, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, White JG, Ramanujam N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19494–19499. doi: 10.1073/pnas.0708425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu M, Zhu XH, Zhang Y, Chen W. Intracellular redox state revealed by in vivo 31P MRS measurement of NAD+ and NADH contents in brains. Magn Reson Med. 2014;71:1959–1972. doi: 10.1002/mrm.24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XH, Lu M, Zhang Y, Chen W. In vivo 31P MRS imaging of intracellular NAD contents and NAD+/NADH redox states in normal and ischemic brains. Proc Int Soc Magn Reson Med. 2013:861. [Google Scholar]
- 21.Lu M, Zhu XH, Zhang Y, Chen W. In vivo 31P MRS study of altered intracellular NAD content and NAD+/NADH redox state in hypoxic brain. Proc Int Soc Magn Reson Med. 2014:3778. doi: 10.1002/mrm.24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NMR of intracellular NAD contents and redox state in healthy human brain. Proc Int Soc Magn Reson Med. 2013:859. [Google Scholar]
- 23.Zhu XH, Lee BY, Rolandelli S, Lu M, Tuite P, Chen W. Preliminary study of cerebral NAD metabolism and redox state in Parkinson’s patients. Proc Int Soc Magn Reson Med. 2014:480. [Google Scholar]
- 24.Lu M, Chen W, Zhu XH. Field dependence study of in vivo brain 31P MRS up to 16.4 T. NMR Biomed. 2014;27:1135–1141. doi: 10.1002/nbm.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luyten PR, Bruntink G, Sloff FM, Vermeulen JW, van der Heijden JI, den Hollander JA, Heerschap A. Broadband proton decoupling in human 31P NMR spectroscopy. NMR Biomed. 1989;1:177–183. doi: 10.1002/nbm.1940010405. [DOI] [PubMed] [Google Scholar]
- 26.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiao H, Zhang X, Zhu XH, Du F, Chen W. In vivo 31P MRS of human brain at high/ultrahigh fields: a quantitative comparison of NMR detection sensitivity and spectral resolution between 4 T and 7 T. Magn Reson Imaging. 2006;24:1281–1286. doi: 10.1016/j.mri.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med. 2003;49:199–205. doi: 10.1002/mrm.10379. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf RA, Behar KL. Detection of cerebral NAD+ by in vivo 1H NMR spectroscopy. NMR Biomed. 2014;27:802–809. doi: 10.1002/nbm.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]