Abstract
Hemorrhagic transformation (HT) is a devastating complication for patients with acute ischemic stroke who are treated with tissue plasminogen activator (tPA). It is associated with high morbidity and mortality, but no effective treatments are currently available to reduce HT risk. Therefore, methods to prevent HT are urgently needed. In this study, we used TWS119, an inhibitor of glycogen synthase kinase 3β (GSK-3β), to evaluate the role of the Wnt/β-catenin signaling pathway in recombinant tPA (rtPA)-induced HT. Sprague–Dawley rats were subjected to a middle cerebral artery occlusion (MCAO) model of ischemic stroke and then were administered rtPA, rtPA combined with TWS119, or vehicle at 4 h. The animals were sacrificed 24 h after infarct induction. Rats treated with rtPA showed evident HT, had more severe neurologic deficit, brain edema, and blood–brain barrier breakdown, and had larger infarction volume than did the vehicle group. Rats treated with TWS119 had significantly improved outcomes compared with those of rats treated with rtPA alone. In addition, Western blot analysis showed that TWS119 increased the protein expression of β-catenin, claudin-3, and ZO-1 while suppressing the expression of GSK-3β. These results suggest that TWS119 reduces rtPA-induced HT and attenuates blood–brain barrier disruption, possibly through activation of the Wnt/β-catenin signaling pathway. This study provides a potential therapeutic strategy to prevent tPA-induced HT after acute ischemic stroke.
Keywords: TWS119, rtPA, Hemorrhagic transformation, Wnt/β-catenin signaling pathway, Ischemic stroke
Introduction
Hemorrhagic transformation (HT) is a major complication of acute ischemic stroke that occurs in 10 to 40 % of patients [1]. The risk of HT is even higher in patients treated with tissue plasminogen activator (tPA), the only therapy for ischemic stroke approved by the United States Food and Drug Administration [2]. Although many preclinical studies have attempted to find ways of reducing HT risk, no effective treatment has yet been developed. Therefore, research into the mechanisms of HT and into potential therapies that could reduce HT risk and improve prognosis of patients with acute ischemic stroke is greatly needed.
Previous studies have focused primarily on breakdown of the blood–brain barrier (BBB) after acute ischemic stroke [3–5]. Evidence has shown that tPA aggravates disruption of the BBB and increases the risk of HT [6–8]. Therefore, protection of the BBB is crucial to reducing the risk of tPA-induced HT.
It has been shown that a dysfunctional Wnt/β-catenin signaling pathway contributes to breakdown of the BBB and that activation of signaling limits the effects of BBB breakdown in degenerative and inflammatory diseases of the brain [9–11]. However, the role of Wnt/β-catenin signaling in the BBB breakdown during post-stroke HT is unknown. Glycogen syn-thase kinase 3β (GSK-3β), a component of the adenomatous polyposis coli/axin/GSK-3β complex, is involved in the ubiquitination and proteasomal degradation of β-catenin, which is the key molecule of the Wnt/β-catenin pathway [12–14]. In this study, to investigate the role of the Wnt/β-catenin pathway in tPA-induced HT, we used 4,6-disubstituted pyrrolo-pyrimidine (TWS119), a specific inhibitor of GSK-3β, to increase the protein level of β-catenin and activate the Wnt signaling pathway. We used a rat middle cerebral artery occlusion (MCAO) model to mimic the clinical scenario of acute ischemic stroke and administered recombinant tPA (rtPA) or rtPA plus TWS119. Our results showed that activation of the Wnt/β-catenin signaling pathway can improve early neurologic function, relieve cerebral edema, decrease permeability of the BBB, and reduce the risk of HT at 24 h after MCAO. We propose that the administration of TWS119 may become a potential clinical treatment to reduce the risk of HT and improve the prognosis of patients with acute ischemic stroke.
Materials and Methods
Animals
All protocols used in this study were approved by the Institutional Animal Care and Use Committee at Wuhan University. Adult male Sprague–Dawley rats weighing 250–280 g were purchased from the Wuhan University Center for Animal Experiments and housed under standard conditions with a 12:12 h light/dark cycle. Food and water were provided to all animals ad libitum. The operators were blinded to the treatment status of the animals in all experiments.
Cerebral Ischemia
Permanent focal cerebral ischemia was produced by endovascular occlusion of the left middle cerebral artery (MCAO) as described previously [15]. Briefly, animals were anesthetized by intraperitoneal injection of pentobarbital sodium (Dainippon Sumitomo Pharma, Osaka, Japan). Body temperature was maintained at 36.5–37.5 °C throughout surgery. After a midline neck incision, the left common carotid artery was isolated under a microscope and ligated with a 4–0 silk suture (Ethicon, Issy-Les-Moulineaux, France). The external and internal carotid arteries were temporarily ligated with a 4–0 silk suture. An arteriotomy was performed proximal to the bifurcation of the common carotid artery. A silicone-coated nylon monofilament (40 mm long, 0.26 mm diameter, Beijing Sunbio Biotech, China) was introduced through the arteriotomy and advanced into the internal carotid artery up to a distance of 18–20 mm to occlude the origin of the middle cerebral artery. Four hours after this procedure, the rats were reanesthetized and the middle cerebral artery blood flow was restored by withdrawing the nylon monofilament. After surgery, the rats were returned to their home cages and maintained at 30 °C with free access to food and water.
Experimental Groups and Drugs
All rats (136) were randomly divided into four groups as follows: Sham group—rats underwent the same surgical procedure, but the filament was not inserted and they received 1 mL of dimethyl sulfoxide (1 % DMSO in saline); Vehicle group—rats underwent MCAO and received 1 mL of DMSO; rtPA group—rats underwent MCAO and received rtPA (10 mg/kg, Actilyse®, Boehringer-Ingelheim, Biberach an der Reiss, Germany) at 4 h after MCAO; and rtPA+TWS119 group—rats underwent MCAO and received intraperitoneal TWS119 (30 mg/kg, dissolved in 1 mL 1 % DMSO, Selleck, Houston, TX, USA) [16, 17] immediately after rtPA injection at 4 h after MCAO.
Neurologic Deficit Score
An investigator blinded to experimental group examined the animals for neurologic function at 24 h after MCAO using a modified scoring system that was developed by Longa [18]. Scoring was as follows: 0, no deficits; 1, difficulty in fully extending the contralateral forelimb; 2, unable to extend the contralateral forelimb; 3, mild circling to the contralateral side; 4, severe circling; and 5, falling to the contralateral side. The higher the neurologic deficit score, the more severe impairment of motor function.
Brain Water Content
Brain water content was measured with the standard wet–dry method [19, 20]. Rats were anesthetized and sacrificed by decapitation at 24 h after MCAO. The brains were quickly removed and placed on a dry surface. After a 4-mm-thick section of frontal pole was dissected, the brain was cut with a brain slicer (Beijing Sunny Instrument Co., Ltd., Beijing, China) and divided into the ipsilateral and contralateral hemispheres. The two hemisphere slices were wrapped in preweighed aluminum foil and immediately weighed on an electronic balance to obtain the wet weight. After they were dried for 24 h in an oven at 100 °C, they were reweighed to obtain the dry weight. Brain water content was calculated as a percentage using the following formula: (wet weight-dry weight)/wet weight×100 % [20, 21].
Brain Infarct Volume
Infarct volume after MCAO was determined by 2,3,5-triphe-nyltetrazolium chloride (TTC, Sigma-Aldrich, St. Louis, MO) staining at 24 h after MCAO. Animals were euthanized and sacrificed, and the brains were quickly removed. Brain tissue was sliced into seven coronal sections (2 mm thick), stained with a 2 % solution of TTC at 37 °C for 20 min, and then fixed in 4 % paraformaldehyde. Normal tissue was stained red, whereas the infarct area remained a pale gray color. A different solution of TTC (1 %) was used to reveal the hemorrhage/bleeding in the infarct area on the frozen brain sections [22, 23]. TTC-stained sections were photographed, and Image J image-processing software (NIH, Bethesda, MD) was used to calculate the infarct volume. The ratio of infarct volume= infarct area of the ipsilateral hemisphere/total area of the ipsilateral hemisphere×100 % [24, 25].
Evaluation of BBB Permeability
Evans blue (EB) dye (2 %, 3 mL/kg, Sigma-Aldrich) was administered intravenously 3 h before the brain was harvested. After the brain was perfused with saline, each hemisphere was weighed and homogenized in 4 mL of 50 % trichloroacetic acid solution. Then the homogenate was centrifuged at 10, 000γ for 30 min, and the supernatant was collected and diluted with ethanol (1:3). EB content was determined on a spectrophotometer (Epoch™ &Take3™, Biotek, USA) at 620 nm and calculated from a standard curve of EB. EB extravasation was expressed as EB extravasation index (EBI): the ratio of absorbance intensity in the ischemic hemisphere to that in the nonischemic hemisphere [26].
Spectrophotometric Assay of Hemoglobin Content
Rats were administered an overdose of pentobarbital sodium and transcardially perfused with cold saline at 20 h after re-perfusion. Brains were immediately removed and cut into the ipsilateral and contralateral hemispheres. After 10 mL/g of saline was added to individual samples, they were homogenized and centrifuged (13,000 rpm for 30 min). A 200 μL volume of reagent (QuantiChrom Hemoglobin Assay Kit; BioAssay Systems, CA, USA) was then added to 50 μL of supernatant. After 15 min, optical density was measured at 400 nm with a spectrophotometer (Skan It RE for Varioskan 133 Flash 2.4; Thermo Fisher Scientific, Waltham, MA, USA). Hemoglobin content index was calculated as ipsilateral hemoglobin content divided by contralateral hemoglobin content [26].
Western Blot Analysis
Equal amounts of protein (100 μg) were separated by Tris-glycine SDS-PAGE and transferred to polyvinylidene fluoride membranes. Based on our established protocols [27, 28], membranes were blocked with 5 % skim milk and then incubated with the following primary antibodies overnight at 4 °C: (1) rabbit polyclonal anti-GAPDH antibody (1:1000, Cell Signaling Technology, Danvers, MA, USA); (2) rabbit poly-clonal anti-claudin-3 antibody (1:100, Abcam, Cambridge, MA, USA); (3) rabbit polyclonal anti-β-catenin antibody (1:5000, Abcam); (4) mouse polyclonal anti-GSK-3β antibody (1:1000, Abcam); and (5) rabbit polyclonal anti-ZO-1 antibody (1:100, Santa Cruz Biotechnology, Dallas, TX, USA). After incubation with the secondary antibody (1:1000, Cell Signaling Technology) for 2 h, immunoblots were probed with ECL kit reagents (Millipore, Billerica, MA, USA). Membranes were then washed three times with phosphate buffered saline (10 min×3), and the relative density of bands was analyzed on an Odyssey infrared scanner (LICOR Bioscience, Lincoln, NE, USA).
Statistical Analysis
All data were expressed as mean±SD. SPSS for Windows 16.0 software package was used to analyze the data. Differences among groups were determined by one-way ANOVA followed by Tukey post-hoc tests. Student’s t test was used to compare the difference between two groups. Differences were considered significant at p values less than 0.05.
Results
Mortality Rates
Mortality was 0 (0/34) in the sham group, 11.7 % (4/34) in the vehicle group, 17.6 % (6/34) in the rtPA group, and 11.7 % (4/34) in the rtPA+TWS119 group. Mortality rate was not significantly different among the four groups (p>0.05).
TWS119 Decreased Neurologic Deficit Score in rtPA-Treated Rats
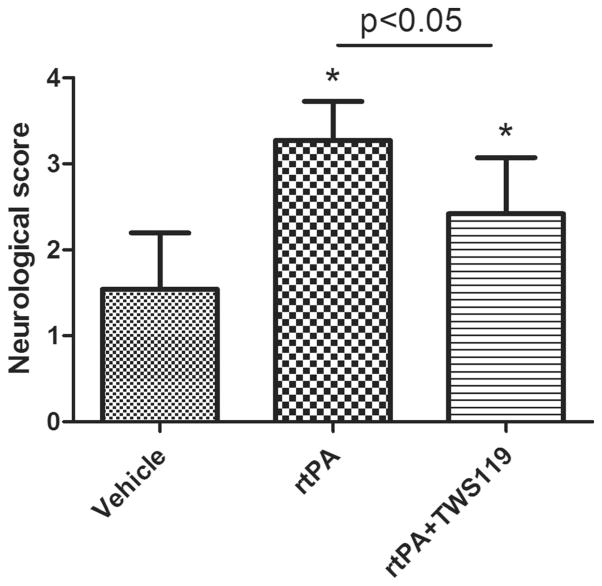
At 24 h after MCAO, neurologic deficit score of the vehicle group was lower than that of the rtPA group (n=34 rats/group, p<0.05, Fig. 1). However, the rtPA+TWS119 group (n=34 rats) had significantly lower neurologic deficit scores than did the rtPA group (p<0.05, Fig. 1). Thus, TWS119 improved the neurologic function in rtPA-treated MCAO rats. No neurologic deficit was present in the sham group (data not shown).
Fig. 1.
Neurologic deficit score of rats at 24 h after MCAO (n=26), MCAO with rtPA treatment (n=25), and MCAO with rtPA and TWS119 treatment (n=25). Modified Longa score was used. Absence of neurologic deficit is scored as 0; maximal score is 5. The results are expressed as means±SD. *p<0.05 vs. vehicle group
TWS119 Effectively Relieved Cerebral Edema in Rats Treated With rtPA
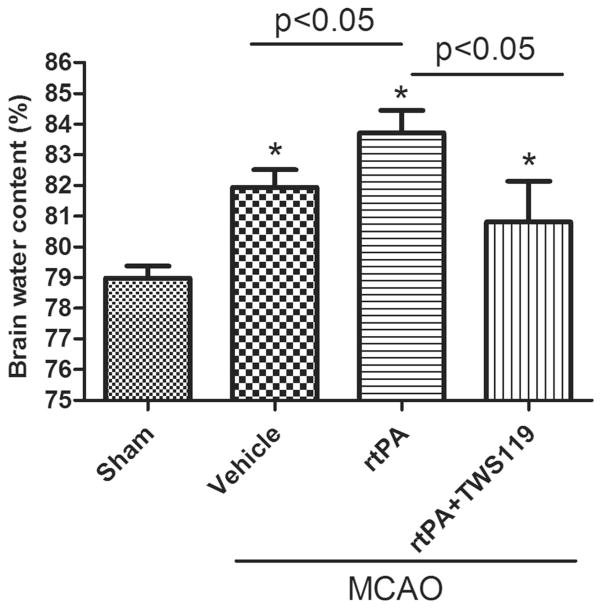
At 24 h after MCAO, the brain water content in rats that received rtPA was significantly higher than that of vehicle-treated rats (n=6 rats/group, p<0.05, Fig. 2). Administration of TWS119 immediately after rtPA significantly decreased the brain water content compared to that in the group that received only rtPA (n=6 rats/group, p<0.05, Fig. 2). Brains from the sham group exhibited no cerebral edema.
Fig. 2.
TWS119 decreases brain water content. Brain water content was measured at 24 h after MCAO or sham surgery with the standard wet-dry method and was calculated as a percentage as follows: (wet weight-dry weight)/wet weight×100 %. The results are expressed as means±SD. *p<0.05 vs. sham group; n=6/group
TWS119 Reduced Cerebral Infarction in rtPA-Treated Rats
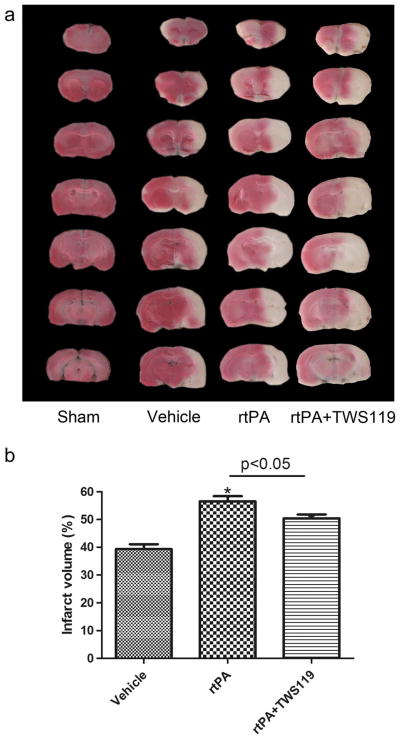
TTC staining was used to reveal the infarct area in the brain sections. Infarct volume in the rtPA group was significantly greater than that of the vehicle-treated MCAO group (n=8 rats/group, p<0.05, Fig. 3). Administration of TWS119 reduced the infarct volume compared with that in the rtPA group (n=8 rats/group, p<0.05, Fig. 3). No infarct area was detected in the sham group (n = 8 rats/group, Fig. 3a).
Fig. 3.
TWS119 decreases infarct volume. a TTC-stained, 2-mm coronal brain sections. b Quantification of the infarct volume in groups that underwent MCAO and treatment with vehicle (MCAO), rtPA, and rtPA plus TWS119. The ratio of infarct volume was calculated as a percentage as follows: infarct area of the ipsilateral hemisphere/total area of the ipsilateral hemisphere×100 %. No infarction was detected in the sham group. The results are expressed as means±SD. *p<0.05 vs. vehicle group; n=8/group
TWS119 Effectively Decreased Blood–Brain Barrier Permeability in rtPA-Treated MCAO Rats
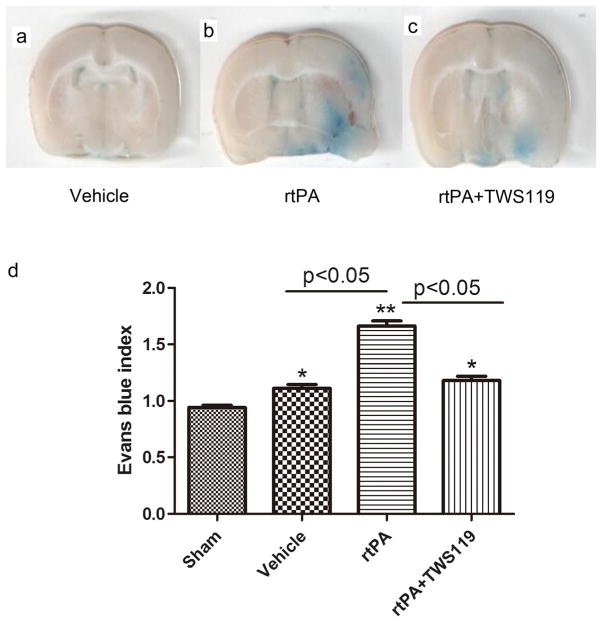
EB dye leakage, used as an indicator of BBB permeability, was mainly concentrated in the regions of the ischemic hemisphere. We found that EBI in the rtPA group was significantly greater than that in the vehicle group (n=6 rats/group, p<0.05, Fig. 4) but was reduced by TWS119 treatment (n=6 rats/group, p<0.05, Fig. 4).
Fig. 4.
TWS119 protects against BBB breakdown. Representative images of brain sections showing extravasation of Evans blue dye: a vehicle group; b rtPA group; c rtPA+TWS119 group. The Evans blue index (the ratio of absorbance intensity in the ischemic hemisphere to that in the nonischemic hemisphere) was used to evaluate blood–brain barrier permeability. The results are expressed as means±SD. *p<0.05, **p<0.01 vs. sham group; n=6/group
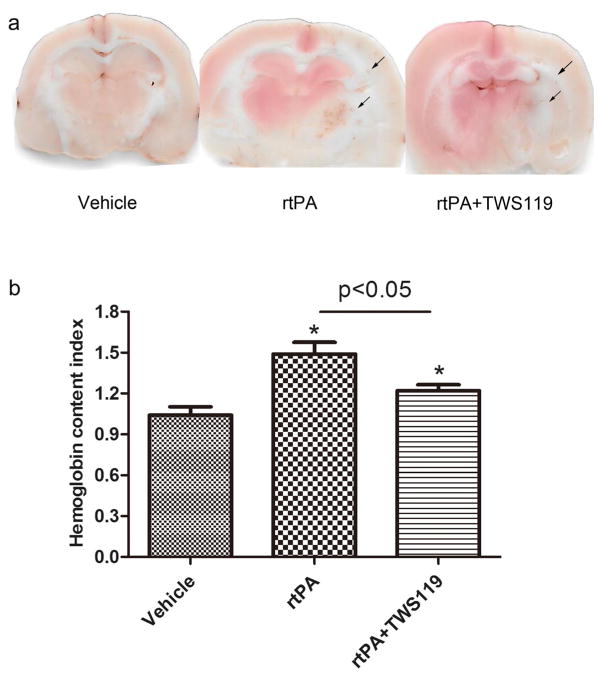
TWS119 Attenuated rtPA-Induced Hemorrhage in Ischemic Brain Tissue
Hemoglobin content index, which reflects the hemorrhage in ischemic brain tissues, was significantly higher in the rtPA group than in the vehicle group (n =6 rats/group, p<0.05, Fig. 5) but was significantly reduced by TWS119 treatment (n=6 rats/group, p<0.05, Fig. 5).
Fig. 5.
TWS119 protects against hemorrhagic transformation. a representative images of brain sections showing hemorrhage in the ischemic brain tissue of rats that underwent MCAO and were treated with vehicle (MCAO), rtPA, or rtPA+TWS119. b The hemoglobin content index was calculated as hemoglobin content in the ipsilateral hemisphere divided by that in the contralateral hemisphere. The results are expressed as means±SD. *p<0.05 vs. vehicle group; n=6/group
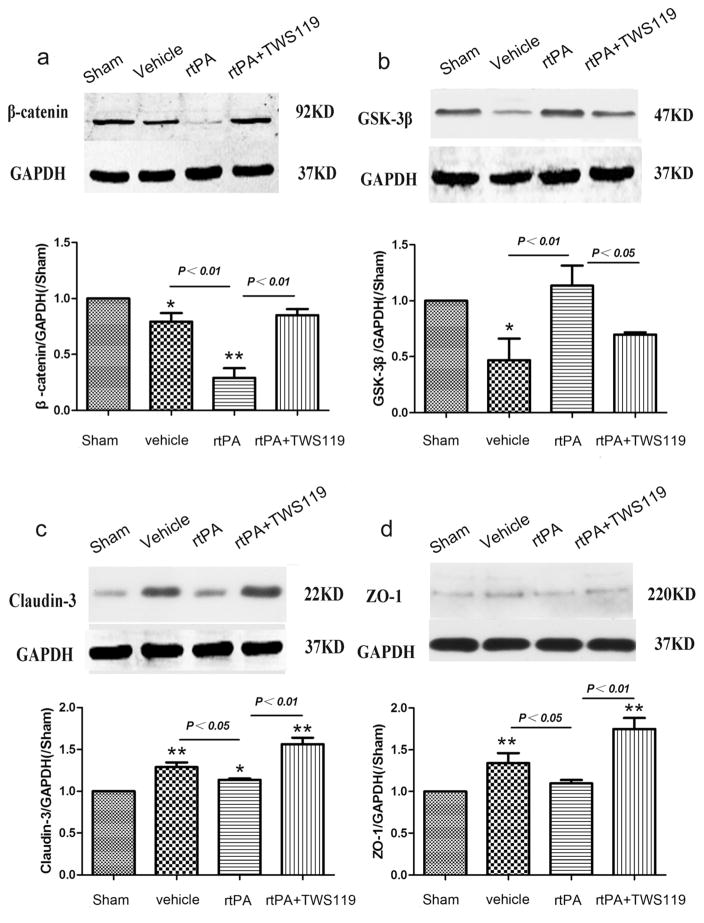
TWS119 Activated the Wnt/β-Catenin Signaling Pathway and Increased the Expression of Claudin-3 and ZO-1
Western blot analysis showed that the expression of tight-junction proteins claudin-3 and ZO-1 was significantly higher in the rtPA+TWS119 group than in the rtPA group (n=6 rats/group, p<0.01, Fig. 6). Additionally, β-catenin was up-regulated and GSK-3β was downregulated in the rtPA+ TWS119 group compared to levels in the rtPA group (n=6 rats/group, p<0.05, Fig. 6). These results suggest that TWS119 activates the Wnt/β-catenin signaling pathway and protects the BBB via regulation of claudin-3 and ZO-1.
Fig. 6.
Western blot analysis of proteins in the Wnt/β-catenin signaling pathway and tight-junction proteins of the blood–brain barrier 24 h after MCAO. Representative Western blots and densitometric analysis of (a) β-catenin, (b) GSK-3β, (c) claudin-3, and (d) ZO-1. Data are representative of at least three independent experiments. *p<0.05, **p<0.01 vs. sham group; n=6/group
Discussion
Reducing the risk of HT in patients treated with rtPA for is-chemic stroke is a worldwide health challenge [3, 29]. In pre-clinical studies, a number of methods have decreased tPA-induced HT, including granulocyte colony-stimulating factor [2], an optimized human apyrase [4], and angiopoietin-1 [30]. However, no therapy has reduced the risk of HT clinically. In this study, we found that TWS119 activated the Wnt/β-catenin signaling pathway and protected the BBB from rtPA-induced HT after acute ischemic stroke in rats. According to our results, TWS119 significantly reduced neurologic deficit, infarction volume, and brain water content; decreased EB extravasation; and attenuated the rtPA-induced hemorrhage 24 h after MCAO. To our knowledge, this study is the first to report that TWS119 can provide protection after ischemic stroke with rtPA treatment.
Previous studies have shown that BBB breakdown is the major cause of tPA-induced HT after acute ischemic stroke [31–33]. Damage to the composition or tight-junction proteins of the BBB usually leads to structural and functional disorders [34]. Therefore, focusing on protection of the BBB is quite important to reducing the risk of HT after tPA.
The conserved Wnt/β-catenin pathway regulates stem cell pluripotency and cell fate decisions during development [23, 35]. It is also involved in the brain’s vascular development and BBB formation [9, 36]. Under pathologic conditions, a dysfunctional Wnt/β-catenin signaling pathway has been reported to contribute to BBB breakdown [9, 32]. However, no study has reported on the relationship between Wnt/β-catenin signaling pathway activation and tPA-induced HT in acute ischemic stroke. As such, we designed our experiments to close this gap. GSK-3β is involved in the degradation of β-catenin, which is a co-transcriptional factor and the key factor of the Wnt/β-catenin pathway [13, 14]. TWS119 is a specific inhibitor of GSK-3β. Therefore, by inhibiting GSK-3β, TWS119 will also inhibit the degradation of β-catenin and theoretically activate the Wnt/β-catenin signaling pathway. Indeed, we found that TWS119 decreased GSK-3β expression at 24 h after MCAO while increasing the amount of β-catenin protein [37].
Because β-catenin is a key component of the Wnt/β-catenin pathway, enhancing its expression activates the downstream signaling pathway [38]. In our study, we found that TWS119 increased the expression of claudin-3 compared with that in the rtPA group. Claudin-3 is a gene downstream of the Wnt/β-catenin pathway. Its protein decreases paracellular permeability and increases transcellular electrical resistance, showing that it changes the tight-junction network and seals the paracellular pathway against the passage of small ions [39]. However, the expression of claudin-3 in the whole brain is rather low [40], and its function in tPA-induced HT is still unresolved [41, 42]. ZO-1 is another important protein that controls endothelial adherens junctions, cell–cell tension, an-giogenesis, and brain barrier formation [43, 44]. Both claudin-3 and ZO-1 are involved in induction and maintenance of the BBB and are the major transmembrane proteins that form the tight junctions of the BBB [45]. However, the changes in the expression of claudin-3 and ZO-1 during rtPA-induced HT and their relationship with Wnt/β-catenin signaling are currently unknown. Our results showed that expression levels of both claudin-3 and ZO-1 were increased in the TWS119 group compared with those in the rtPA group, suggesting that TWS119 protects against BBB disruption. Additional investigation is needed into the respective roles of these proteins and the mechanisms by which their expression increases. However, no standard in vitro or ex vivo model of HT is yet available with which to study deeper cellular and molecular mechanisms [46].
In addition to protecting the integrity of the BBB, our results revealed that TWS119 treatment reduced infarct volume, brain edema and tPA-induced HTafter MCAO. A recent study showed that the Wnt/β-catenin signaling pathway is associated with angiogenesis by regulating the expression of angio-genic factors such as vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP)-9 [47]. Another study reported that the Wnt/β-catenin signaling pathway is involved in the regulation of neuroinflammation [48]. Further research is needed to determine whether the Wnt/β-catenin signaling regulates neuroinflammation and MMP-9 activity, thereby underpinning TWS119-mediated protection after ischemic stroke.
Conclusions
Our study showed that TWS119 activates the Wnt/β-catenin signaling pathway by inhibiting the expression of GSK-3β, thereby reducing rtPA-induced BBB disruption and subsequent HT after acute ischemic stroke. One possible mechanism by which this pathway might reduce HT is through increased expression of claudin-3 and ZO-1. Thus, the activation of Wnt/β-catenin signaling has the potential to protect the BBB and reduce HT in ischemic stroke after clinical rtPA administration. However, the specific mechanism needs additional study.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81171112, 81371272 to M.C.L., 81372683 to Q.X.C.) and grants from the National Institutes of Health (R01NS078026, R01AT007317 to J. W.). We thank Jiarui Wang and Claire Levine, MS, ELS, for assistance with the manuscript preparation.
Footnotes
Compliance with Ethical Standards All protocols used in this study were approved by the Institutional Animal Care and Use Committee at Wuhan University.
Conflict of Interest The authors declare that they have no competing interests.
Contributor Information
Qianxue Chen, Email: chenqx666@sohu.com.
Jian Wang, Email: jwang79@jhmi.edu.
References
- 1.Beslow LA, Smith SE, Vossough A, Licht DJ, Kasner SE, Favilla CG, Halperin AR, Gordon DM, et al. Hemorrhagic transformation of childhood arterial ischemic stroke. Stroke. 2011;42(4):941–946. doi: 10.1161/STROKEAHA.110.604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.dela Pena IC, Yoo A, Tajiri N, Acosta SA, Ji X, Kaneko Y, Borlongan CV. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J Cereb Blood Flow Metab. 2015;35(2):338–346. doi: 10.1038/jcbfm.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozkul-Wermester O, Guegan-Massardier E, Triquenot A, Borden A, Perot G, Gerardin E. Increased blood–brain barrier permeability on perfusion computed tomography predicts hemorrhagic transformation in acute ischemic stroke. Eur Neurol. 2014;72(1–2):45–53. doi: 10.1159/000358297. [DOI] [PubMed] [Google Scholar]
- 4.Tan Z, Li X, Turner RC, Logsdon AF, Lucke-Wold B, DiPasquale K, Jeong SS, Chen R, et al. Combination treatment of r-tPA and an optimized human apyrase reduces mortality rate and hemorrhagic transformation 6h after ischemic stroke in aged female rats. Eur J Pharmacol. 2014;738:368–373. doi: 10.1016/j.ejphar.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang Y, Zuo Z, Wang Z, Roy J, Hou Q, Tong E, Hoffmann A, et al. Effects of tissue plasminogen activator timing on blood–brain barrier permeability and hemorrhagic transformation in rats with transient ischemic stroke. J Neurol Sci. 2014;347(1–2):148–154. doi: 10.1016/j.jns.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Rosell A, Foerch C, Murata Y, Lo EH. Mechanisms and markers for hemorrhagic transformation after stroke. Acta Neurochir Suppl. 2008;105:173–178. doi: 10.1007/978-3-211-09469-3_34. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, Lo EH. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35(11 Suppl 1):2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for is-chemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. 2015;52(3):1572–1579. doi: 10.1007/s12035-014-8952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Wan W, Xia S, Kalionis B, Li Y. Dysfunctional Wnt/β-catenin signaling contributes to blood–brain barrier breakdown in Alzheimer’s disease. Neurochem Int. 2014;75:19–25. doi: 10.1016/j.neuint.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Polakis P. Formation of the blood–brain barrier: Wnt signaling seals the deal. J Cell Biol. 2008;183(3):371–373. doi: 10.1083/jcb.200810040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood–brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31(2):248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caspi M, Perry G, Skalka N, Meisel S, Firsow A, Amit M, Rosin-Arbesfeld R. Aldolase positively regulates of the canonical Wnt signaling pathway. Mol Cancer. 2014;13:164. doi: 10.1186/1476-4598-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi A. Canonical Wnt signaling pathway and cellular responses. Clin Calcium. 2013;23(6):799–807. CliCa1306799807. [PubMed] [Google Scholar]
- 14.Han P, Ivanovski S, Crawford R, Xiao Y. Activation of the canonical Wnt signaling pathway induces cementum regeneration. J Bone Miner Res. 2015;30(7):1160–1174. doi: 10.1002/jbmr.2445. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Yu L, Jiang C, Chen M, Ou C, Wang J. Bone marrow mononuclear cells exert long-term neuroprotection in a rat model of ischemic stroke by promoting arteriogenesis and an-giogenesis. Brain Behav Immun. 2013;34:56–66. doi: 10.1016/j.bbi.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22(7):833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 17.Grassilli E, Ianzano L, Bonomo S, Missaglia C, Cerrito MG, Giovannoni R, Masiero L, Lavitrano M. GSK3A is redundant with GSK3B in modulating drug resistance and chemotherapy-induced necroptosis. PLoS One. 2014;9(7):e100947. doi: 10.1371/journal.pone.0100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T, Sun S, Wang T, Tong X, Bi J, Wang Y, Sun Z. Piperlonguminine is neuroprotective in experimental rat stroke. Int Immunopharmacol. 2014;23(2):447–451. doi: 10.1016/j.intimp.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Zan L, Wu H, Jiang J, Zhao S, Song Y, Teng G, Li H, Jia Y, et al. Temporal profile of Src, SSeCKS, and angiogenic factors after focal cerebral ischemia: correlations with angiogenesis and cerebral edema. Neurochem Int. 2011;58(8):872–879. doi: 10.1016/j.neuint.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thal SC, Luh C, Schaible EV, Timaru-Kast R, Hedrich J, Luhmann HJ, Engelhard K, Zehendner CM. Volatile anesthetics influence blood–brain barrier integrity by modulation of tight junction protein expression in traumatic brain injury. PLoS One. 2012;7(12):e50752. doi: 10.1371/journal.pone.0050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Wu T, Li M, Wang J. Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiol Dis. 2012;45(1):388–394. doi: 10.1016/j.nbd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu A, Suofu Y, Guan F, Broderick JP, Wagner KR, Clark JF. Matrix metalloproteinase-2 deletions protect against hemorrhagic transformation after 1h of cerebral ischemia and 23h of reperfusion. Neuroscience. 2013;253:361–367. doi: 10.1016/j.neuroscience.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, et al. CXCR4+ CD45- BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice. Brain Behav Immun. 2015;45:98–108. doi: 10.1016/j.bbi.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Davis CM, Edin ML, Lee CR, Zeldin DC, Alkayed NJ. Role of endothelial soluble epoxide hydrolase in cerebro-vascular function and ischemic injury. PLoS One. 2013;8(4):e61244. doi: 10.1371/journal.pone.0061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Zhang Z, Sun W, Koehler RC, Huang J. 17β-estradiol attenuates breakdown of blood–brain barrier and hemorrhagic transformation induced by tissue plasminogen activator in cerebral ischemia. Neurobiol Dis. 2011;44(3):277–283. doi: 10.1016/j.nbd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Li Q, et al. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun. 2015;46:293–310. doi: 10.1016/j.bbi.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W, et al. PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiol Aging. 2015;36(3):1439–1450. doi: 10.1016/j.neurobiolaging.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran-Dinh A, Levoye A, Lambert G, Louedec L, Journe C, Meilhac O, Amarenco P. Low levels of low-density lipoprotein-C associated with proprotein convertase subtilisin kexin 9 inhibition do not increase the risk of hemorrhagic transformation. Stroke. 2014;45(10):3086–3088. doi: 10.1161/STROKEAHA.114.005958. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura K, Takahashi T, Kanazawa M, Igarashi H, Nakada T, Nishizawa M, Shimohata T. Effects of angiopoietin-1 on hemorrhagic transformation and cerebral edema after tissue plas-minogen activator treatment for ischemic stroke in rats. PLoS One. 2014;9(6):e98639. doi: 10.1371/journal.pone.0098639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artus C, Glacial F, Ganeshamoorthy K, Ziegler N, Godet M, Guilbert T, Liebner S, Couraud PO. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab. 2014;34(3):433–440. doi: 10.1038/jcbfm.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paolinelli R, Corada M, Ferrarini L, Devraj K, Artus C, Czupalla CJ, Rudini N, Maddaluno L, et al. Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PLoS One. 2013;8(8):e70233. doi: 10.1371/journal.pone.0070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegler JE, Alvi M, Boehme AK, Lyerly MJ, Albright KC, Shahripour RB, Rawal PV, Kapoor N, et al. Hemorrhagic transformation (HT) and symptomatic intracerebral hemorrhage (sICH) risk prediction models for postthrombolytic hemorrhage in the stroke belt. ISRN Stroke. 2013;2013:681673. doi: 10.1155/2013/681673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin X, Liu J, Liu W. Early ischemic blood brain barrier damage: a potential indicator for hemorrhagic transformation following tissue plasminogen activator (tPA) thrombolysis? Curr Neurovasc Res. 2014;11(3):254–262. doi: 10.2174/1567202611666140530145643. [DOI] [PubMed] [Google Scholar]
- 35.Song M, Lim J, Yu HY, Park J, Chun JY, Jeong J, Heo J, Kang H, et al. Mesenchymal stem cell therapy alleviates interstitial cystitis by activating Wnt signaling pathway. Stem Cells Dev. 2015;24(14):1648–1657. doi: 10.1089/scd.2014.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319(9):1317–1323. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Chen Y, Cao C, Chen X, Gao W, Zhang L. Inactivation of glycogen synthase kinase-3β up-regulates β-catenin and promotes chondrogenesis. Cell Tissue Bank. 2015;16(1):135–142. doi: 10.1007/s10561-014-9449-6. [DOI] [PubMed] [Google Scholar]
- 38.Ji XK, Xie YK, Zhong JQ, Xu QG, Zeng QQ, Wang Y, Zhang QY, Shan YF. GSK-3β suppresses the proliferation of rat hepatic oval cells through modulating Wnt/β-catenin signaling pathway. Acta Pharmacol Sin. 2015;36(3):334–342. doi: 10.1038/aps.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, Amasheh S, Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798(11):2048–2057. doi: 10.1016/j.bbamem.2010.07.014. doi:10.j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Adal A, Qi M, Toh J, et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008;1203:133–148. doi: 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazmierski R, Michalak S, Wencel-Warot A, Nowinski WL. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology. 2012;79(16):1677–1685. doi: 10.1212/WNL.0b013e31826e9a83. [DOI] [PubMed] [Google Scholar]
- 42.Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood–brain barrier: structural and functional aspects. Semin Cell Dev Biol. 2014;38:16–25. doi: 10.1016/j.semcdb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Zan L, Zhang X, Xi Y, Wu H, Song Y, Teng G, Li H, Qi J, et al. Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion. Neuroscience. 2014;262:118–128. doi: 10.1016/j.neuroscience.2013.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, et al. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208(6):821–838. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuhaus W, Wirth M, Plattner VE, Germann B, Gabor F, Noe CR. Expression of Claudin-1, Claudin-3 and Claudin-5 in human blood–brain barrier mimicking cell line ECV304 is inducible by glioma-conditioned media. Neurosci Lett. 2008;446(2–3):59–64. doi: 10.1016/j.neulet.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Won S, Lee JH, Wali B, Stein DG, Sayeed I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway. J Cereb Blood Flow Metab. 2014;34(1):72–80. doi: 10.1038/jcbfm.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu B, Liu BR, Du YJ, Chen J, Cheng YQ, Xu W, Wang XH. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol Lett. 2014;7(4):1175–1178. doi: 10.3892/ol.2014.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ajmone-Cat MA, D’Urso MC, di Blasio G, Brignone MS, De Simone R, Minghetti L. Glycogen synthase kinase 3 is part of the molecular machinery regulating the adaptive response to LPS stimulation in microglial cells. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.11.012. [DOI] [PubMed] [Google Scholar]