Abstract
Aim
12/15-lipoxygenase (12/15-LO) metabolizes arachidonic acid (AA) into several vasoactive eicosanoids. In mouse arteries, we previously characterized the enzyme’s 15-LO metabolites 12(S)-hydroxyeicosatetraenoic acid (HETE), 15-HETE, hydroxyepoxyeicosatrienoic acids (HEETAs) and 11,12,15-trihydroxyeicosatrienoic acids (11,12,15-THETAs) as endothelium-derived relaxing factors. However, the observed 12-LO metabolites remained uncharacterized. The purpose of this study was to determine the structure and biological functions of eicosanoids generated by the enzyme’s 12-LO activity.
Methods
Metabolites extracted from aortas of C57BL/6 male mice were separated using a series of reverse and normal phase chromatographic steps and identified as hepoxilin A3, trioxilin A3 and trioxilin C3 by mass-spectrometry. Activities of these natural compounds were tested on isometric tension and intracellular calcium release. The role of thromboxane (TP) receptor was determined in HEK293 cells overexpressing TPα receptor (TPα-HEK).
Results
All identified vascular 12-LO metabolites were biologically active. In mouse mesenteric arteries, trioxilin A3, C3 and hepoxilin A3 (3μM) relaxed arteries constricted with the thromboxane mimetic, U46619 (maximum relaxations of 78.9±3.2, 29.7±4.6, 82.2±5.0 and 88.0±2.4% respectively), but not phenylephrine-constricted arteries. In TPα-HEK cells, trioxilin A3, C3 and hepoxilin A3 (10μM) inhibited U46619 (10nM)-induced increases in intracellular calcium by 53.0±7.2%, 32.8±5.0% and 37.9±13.5%, respectively. In contrast, trioxilin B3 and hepoxilin B3 were not synthesized in arteries and exhibited little biological activity.
Conclusion
Trioxilin A3 and C3 and hepoxilin A3 are endogenous vascular relaxing factors. They are not endothelium-derived hyperpolarizing factors but mediate vascular relaxation by inhibiting TP agonist induced increases in intracellular calcium. Thus, they regulate vascular homeostasis by acting as endogenous TP antagonists.
Keywords: arachidonic acid, endothelium, lipoxygenase, smooth muscle, thromboxane A2 (TP) receptor, trioxilins
Introduction
Vascular relaxation is important to oppose vasoconstriction for homeostatic balance and to reduce the impact of cardiovascular diseases such as coronary artery disease and hypertension (Feletou and Vanhoutte, 1999, McGuire et al., 2001, Campbell and Falck, 2007). Several arachidonic (AA) metabolites cause vascular relaxation and have been targeted for therapeutic development. Endothelial cell cytochrome P450 (CYP450) epoxygenase and cyclooxygenase (COX) metabolize AA to the vasoactive epoxyeicosatrienoic acids and prostacyclin (PGI2), respectively (Campbell and Falck, 2007). A third enzyme, lipoxygenase (LO), is well known for generating leukotrienes, major mediators of inflammation and bronchoconstriction; however, less is known about LO metabolites and their roles in regulating vascular tone (Brash, 1999, Chawengsub et al., 2009a).
Lipoxygenases catalyze dioxygenation of AA into several eicosanoids (Brash, 1999). Leukocyte-type 12/15-LO, platelet-type 12-LO and 15-LO are major LOs found in the vasculature (Gauthier et al., 2011, Kim et al., 1995, Chawengsub et al., 2009a). The product of 12/15-LO metabolism of AA, 12(S)-hydroperoxyeicosatetraenoic acid (12-HPETE) is reduced to 12(S)-hydroxyeicosatetraenoic acid (12-HETE) or converted to hydroxyl-epoxide containing eicosanoids called hepoxilins (Pace-asciak and Martin, 1984, Pace-asciak et al., 1983). The epoxy group undergoes hydrolysis resulting in trihydroxy eicosanoids called trioxilins (Pace-asciak, 1986). Several 15-LO products of AA were identified from the endothelium of rabbit arteries including 15(S)-hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA), 11,12,15-trihydroxyeicosatrienoic acid (11,12,15-THETA), 12-HETE and 15-HETE, the major metabolite (Chawengsub et al., 2008, Campbell et al., 2003, Chawengsub et al., 2009b, Chawengsub et al., 2009a). 15-H-11,12-EETA and 11,12,15-THETA relaxed rabbit arteries through activation of potassium channels and thus function as endothelium-derived hyperpolarizing factors (EDHF) (Chawengsub et al., 2008, Gauthier et al., 2008, Campbell et al., 2003, Chawengsub et al., 2009a). In the mouse, 12(S)-HETE, 15-HETE, HEETAs and 11,12,15-THETA were isolated from AA metabolism by abdominal aorta, carotid, femoral and mesenteric arteries (Gauthier et al., 2011). While 15-LO-derived products of AA are more predominant in rabbits, the 12-LO pathway is predominant in mice. We previously showed that 12(S)-HETE, the major LO metabolite in mouse arteries, relaxed mouse mesenteric arteries by antagonizing thromboxane A2 (TP) receptors (Siangjong et al., 2013).
Hepoxilins were first identified in rat lung by Pace-Asciak et al (Pace-asciak et al., 1983). Hepoxilin A3 was found in rat pancreatic islets, lung, brain, aorta, human platelets and neutrophils (Pace-asciak and Martin, 1984, Pace-asciak, 1988, Bryant and Bailey, 1979, Laneuville et al., 1991, Dho et al., 1990). Biological functions of hepoxilin A3 include stimulation of insulin secretion (Pace-asciak and Martin, 1984), modulation of intracellular calcium (Dho et al., 1990), membrane calcium transport across (Reynaud et al., 1999), and neutrophil migration (Reynaud et al., 1999). The vascular activities of hepoxilin A3 have not been characterized, particularly in mice. The allylic epoxide group of hepoxilins is reactive and is hydrolyzed to stable trioxilins. Trioxilin C3 caused concentration-dependent relaxation of phenylephrine-contracted rabbit aorta (Pfister, 2003). In this present study, we identified and characterized 12/15(S)-LO metabolites of AA in mouse arteries.
Methods
Animals
Eight to fourteen-week-old male C57BL6 mice (24–28 g) were obtained from Jackson Laboratory (Bar Harbor, Maine). Animal protocols were approved by the Animal Care Committee of the Medical College of Wisconsin and procedures were carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (2011).
[14C]-Arachidonic acid metabolism
Mouse aortas were cleaned of connective tissue and placed in cold HEPES buffer (in mM: 10 HEPES, 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 6 glucose, pH 7.4) (Gauthier et al., 2011). The aortas were then incubated with indomethacin (10 μM) for 10 min at 37°C. Subsequently, [14C]-arachidonic acid (AA, 0.05 μCi) and unlabeled AA (0.1 μM) were added and further incubated for 5 min. Calcium ionophore A23187 (20 μM) was then added. The reaction was continued at 37°C for another 15 min. There after, ice-cold ethanol was added to a final concentration of 15% to stop the reaction. The incubation buffer was transferred to a glass tube and acidified (pH < 3.5) with glacial acetic acid. The buffer containing [14C]-AA was extracted on Bond Elut C18 extraction columns as previously described (Chawengsub et al., 2008, Gauthier et al., 2008). The extracts were dried under a stream of nitrogen gas and stored at −30°C until analysis by HPLC.
Reverse phase (RP)-HPLC separation of AA metabolites
The C18 column extracts containing AA metabolites were reconstituted and separated on a Nucleosil-C18 column (5 μm, 4.6 × 250 mm) using solvent system I (Gauthier et al., 2008, Gauthier et al., 2011). Solvent A was deionized water and solvent B was acetonitrile containing 0.1% glacial acetic acid. The program was a 40-min linear gradient from 50% solvent B in solvent A to 100% solvent B. The flow rate was 1 ml/min. Column elutes of 0.2 ml/min were collected. Aliquot radioactivity was measured by a liquid scintillation counter. UV absorbance was monitored at 205 nm. Fractions corresponding to THETA (trioxilins) and HEETAs (hepoxilins) were collected and further extracted with 50:50 cyclohexane and ethyl acetate. The extracts were dried under a stream of nitrogen gas and stored at −30°C until further analysis.
HPLC Determination of THETAs and trioxilins
Column elutes corresponding to THETAs and trioxilins from RP-HPLC using solvent system I were pooled and further resolved on the Nucleosil-C18 column using solvent system II as previously described (Gauthier et al., 2008). Solvent A was deionized water containing 0.1% glacial acetic acid and solvent B was acetonitrile. The program was a 5-min isocratic phase with 35% solvent B in solvent A followed by a 35-min gradient to 85% solvent B. The flow rate was 1 ml/min. The absorbance was detected at 205 nm. Fractions of 0.2 ml/min were collected and aliquots was measured for radioactivity by a liquid scintillation counter.
The purified THETA peak (15.8–18.0 min) from solvent system II was subsequently re-chromatographed on normal phase (NP)-HPLC (a Nucleosil silica column, 5 μm, 4.6 × 250 mm) using solvent system III (Gauthier et al., 2008). The solvent was 95.9: 4: 0.1 mixture of hexane: isopropanol: glacial acetic acid. The system was an 80 min isocratic elution with a flow rate of 1 ml/min. The absorbance was monitored at 205 nm. Elute fractions of 0.5 ml/min were collected and radioactivity measured by a liquid scintillation counter.
Mass spectrometric analysis
The incubation and extraction of mouse aorta were repeated with 0.1 mM AA, in a similar manner as described above, in the absence of [14C]-AA (Gauthier et al., 2011, Chawengsub et al., 2008). The extracts were resolved on RP-HPLC as described above. Fractions corresponding to HEETA/hepoxilin and THETA/trioxilin standards were collected and extracted with 50:50 cyclohexane and ethyl acetate. The organic phase containing HEETAs/hepoxilins or THETAs/trioxilins was collected and evaporated under a stream of nitrogen gas.
The unknown metabolites corresponding to THETAs/trioxilins from HPLC were identified by liquid chromatography-electrospray-Fourier transform ion cyclotron resonance mass spectrometry (LC-ESI-FTICR) (7.0 Tesla FTICR, IonSpec) as previously described (Cui et al., 2008, Chawengsub et al., 2009b). Detection was made in the negative ion mode and the ion guides were optimized for m/z 300. The m/z 353 ions were isolated in the ICR cell and fragmented by sustained off-resonance irradiation collision-induced dissociation (SORI-CID) using a nitrogen gas pulse of 20 ms. The FTICR provides a high mass accuracy of fragments for identification of THETAs/trioxilins.
The HEETAs/hepoxilins were analyzed by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-MS/MS, Waters Quattro mass spectrometer with a 2695 liquid chromatograph) (Chawengsub et al., 2008, Chawengsub et al., 2009b). The eicosanoids were separated on a Kromasil C18 column, 250 × 2.0 mm 5μm (Phenomenex, Torrance, CA) using deionized water (A) and acetonitrile (B) containing 0.005% acetic acid as a mobile phase with a flow rate of 0.2 ml/min. The mobile phase gradient started at 50% B, increased to 70% B over 40 minutes, and to 100% B over 5 minutes. Detection was made in the negative ion mode. The cone voltage and capillary voltage were set at 19V and 2.4kV, respectively. MS/MS ionization was obtained by collision-induced dissociation of the precursor ion m/z 335 using argon as the collision gas. Product ions were detected for the m/z of 50 to 400. Sample MS/MS spectra and retention times were compared with known standards.
Isometric tension recording
Mouse mesenteric arteries (150 to 300 μm in diameter) were dissected and cleaned of connective tissues. The arteries were cut into 1.5 to 1.8 mm rings and mounted in a four-chamber wire myograph (Danish MyoTechnology A/S) (Gauthier et al., 2011, Siangjong et al., 2013). The arteries were maintained in physiological saline solution (PSS, in mM: 119 NaCl, 4.7 KCl, 2.5 CaCl2, 1.17 MgSO4, 24 NaHCO3, 1.18 KH2PO4, 0.026 EDTA and 5.5 glucose), at 37°C, supplied with 95% O2/5% CO2. After 30 min, tension recording was performed as previously described (Gauthier et al., 2011, Siangjong et al., 2013). Briefly, the arteries were stretched to a tension of 0.80 mN, where optimum isometric length-tension was achieved (Gauthier et al., 2011). The arteries were challenged with KCl (60 mM) and the thromboxane mimetic, U46619 (100 nM) 3–4 times until the maximum active tension was established. Thereafter, U46619 or phenylephrine (in the presence of the COX inhibitor indomethacin (10 μM) and the nitric oxide synthase inhibitor, nitro-L-arginine (30 μM)) was added to partially constrict the arteries to approximately 50–70% of maximum active tension. Upon a stable constriction, increasing concentrations of test compounds were added and tension was recorded. In some experiments, the arteries were pretreated with AUDA (1 μM), a soluble epoxide hydrolase (sEH) inhibitor, for 30 min prior to the constriction (Chawengsub et al., 2009b, Chawengsub et al., 2008). Results are expressed as percent relaxation with basal tension representing 100% relaxation. In other experiments, arteries were treated with vehicle or trioxilin C3 (10 μM) for 30 min. Then, constrictor responses to U46619 (10−8-10−6M) were determined. U46619 constriction responses were expressed as % constriction with maximum active tension being 100%.
Cell culture
HEK293 cells overexpressing the human TPα receptor (TPα-HEK) were produced as previously described (Wilson et al., 2004). The cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 25 mM HEPES, 4.5 g/L D-glucose, 4 mM L-glutamine and 0.5 mg/ml G418. The cells were kept at 37°C in a humidified chamber with 5% CO2 and 95% O2.
Fluorometric imaging plate reader (FLIPR) measurement of intracellular calcium
The calcium assay was performed on a fluorometric imaging plate reader (FLIPR) (Molecular Devices) as previously described (Siangjong et al., 2013). Briefly, TPα-HEK cells were seeded (5×104 cells per well) in phenol red-free growth media in a black CellBIND surface 96-well plate with clear bottoms (Corning). A monolayer of cells at 80–90% confluency was loaded with Fluo-4 calcium dye according the manufacture’s protocol (Fluo-4 NW calcium assay kit, Molecular Probes). Cells were incubated with the dye for 15 min at 37°C followed by an additional 10 min at room temperature. Test compounds, i.e., vehicle control, hepoxilins and trioxilins were added to the cells and incubated for 4 min. Subsequently, U46619 was added and the fluorescence was measured. After the assay, cells were lysed with NaOH (2 N) for 2 h at 60°C, followed by HCl (2 N). Samples were then collected and used for protein concentration measurement using the BCA assay (Pierce BCA protein assay kit, Thermo scientific). Fluorescence was normalized to total cellular protein. Results are presented as % maximum fluorescence (per μg protein) evoked by U46619 in the presence of a test compound compared to a vehicle control (100%).
Drugs and chemicals
Synthetic trioxilin and hepoxilin analogs were produced in the laboratory of Dr. Falck. [14C]-AA (810 mCi/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). U46619 and 12-[[(tricycle [3.3.1.13,7]dec-1-ylamino)carbonyl]amino]-dodecanoic acid (AUDA) were purchased from Cayman Chemical Company (Ann Arbor, MI). DMEM, penicillin, streptomycin, HEPES, L-glutamine and G418 (Geneticin) were purchased from Invitrogen (New York). Hyclone fetal bovine serum was purchased from Thermo Scientific. Indomethacin, nitro-L-arginine (L-NA), phenylephrine, and other chemicals were purchased from Sigma.
Data analysis
Data are presented as mean ± SEM. Significant differences between mean values were evaluated by Student t test or ANOVA followed by multiple comparison test. P value <0.05 was considered statistically significant.
Results
Incubation of mouse aorta with [14C]-AA in the presence of 10 μM indomethacin resulted in the production of several LO metabolites, which were resolved on RP-HPLC (solvent system I). The products co-migrated with standards for THETAs, HEETAs, 15-HETE and 12-HETE (Fig. 1A). To further purify THETAs, fractions from 5.0–7.4 min were pooled and resolved on RP-HPLC using solvent system II (Fig. 1B). The major peak that eluted at 15.8–18 min was collected and re-chromatographed on NP-HPLC using solvent system III (Fig. 1C). The radiochromatogram revealed two products eluting at 51.8 min (peak 1) and 68.0 min (peak 2). These peaks did not co-migrate with THETA standards (11,12,15-THETA, 13,14,15-THETA) that correspond to 15-LO-derived metabolites (Gauthier et al., 2008).
Figure 1.
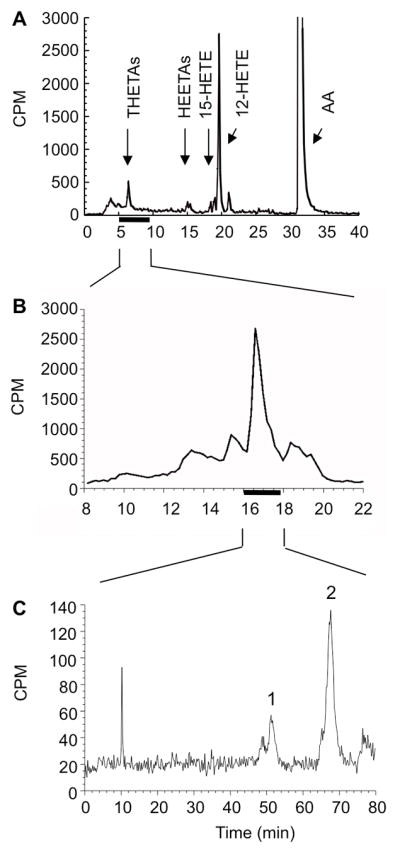
HPLC analysis of [14C]-arachidonic acid (AA) metabolism in mouse aorta. Extracts containing [14C]-AA metabolites were resolved on RP-HPLC using solvent system I (A). Fractions co-migrating with THETA standards (5.0 – 7.4 min) were collected and further separated on RP-HPLC using solvent system II (B). The purified THETA peak (15.8 – 18.0 min) was re-chromatographed on NP-HPLC using solvent system III (C). The radioactive chromatograph shows two unknown products, peak 1 and 2.
To identify the unknown THETAs, the incubation of mouse aorta was repeated with 10−4 M unlabeled AA, and the metabolites were extracted and purified on RP-HPLC using solvent system I and II, and NP-HPLC using solvent system III in the same manner as the radioactive-labeled products (above). NP-HPLC fractions corresponding to peaks 1 and 2 were analyzed by LC-ESI-FTICR. The molecular mass [M-H] of both peaks was 353 m/z and corresponds to the molecular weight of trioxilins (Fig. 2). Fragment ions of 335 m/z [M-H-H2O], 317 m/z [M-H-2H2O], 299 m/z [M-H-3H2O], 291 m/z [M-H-CO2-H2O], 273 m/z [M-H-CO2-2H2O] and 255 m/z [M-H-CO2-3H2O] indicate that the structures of the two metabolites contain three hydroxyls and a carboxyl group. Although the overall major fragment ion spectra of both peaks were similar, the relative ion intensities were clearly different. The most abundant ions of peak 1 and 2 were 155 m/z (Fig. 2A) and 195 m/z (Fig. 2B), respectively. This fragmentation profile is useful for predicting their chemical structure. Trioxilin A3 and C3 contain hydroxyl (-OH) groups at C8 and C12, but the location of third –OH group differs. While this –OH group is located at C9 in trioxilin C3, it is positioned at C11 for trioxilin A3. The bond between C8 and C9 of trioxilin C3 is reactive due to the flanking OH groups. Breakage of this bond gives rise to the fragment ion 155 m/z (Fig. 2C), the most abundant fragment in peak 1 (Fig. 2A). Likewise, the C11 – C12 breakage in trioxilin A3 results in the ion 195 m/z as the most abundant fragment ion (Fig. 2D); the same ion profile was observed with the metabolite in peak 2 (Fig. 2B). On this basis, peak 1 and 2 were identified as trioxilin C3 and trioxilin A3, respectively.
Figure 2.
LC-ESI-FTICR mass spectrometry analysis of THETAs. Mass spectrum of peak 1 (A), peak 2 (B), the trioxilin C3 standard (C), and the trioxilin A3 standard (D). Detection was made in the negative mode.
The discovery of trioxilin C3 and A3 suggests the synthesis of their precursor, the HEETA-like compound, hepoxilin A3. To identify the structure of the unknown HEETA-like products, incubation of mouse aorta was repeated with 10−4 M of AA, and the metabolites were extracted and resolved on RP-HPLC using solvent system I. we collected the fractions corresponding to HEETAs from RP-HPLC solvent system I (12.8 – 15.8 min) (Fig. 3A). The HEETA fractions were pooled and subjected to LC-MS/MS using the negative ion mode. The mass spectrum of the HEETA-like compound is shown in (Fig. 3B). The structure prediction of the unknown metabolite was achieved in the same manner as trioxilins (above). The [M-H] ion 335 m/z represents the molecular weight of HEETA/hepoxilin. Ion fragments of 317 m/z [M-H-H2O], 299 m/z [M-H-2H2O], 273 m/z [M-H-H2O-CO2] and 255 m/z [M-H-2H2O-CO2] suggest two hydroxyl groups and a carboxyl group. The most abundant fragment ion of 195 m/z that corresponds to fragmentation at the C11-C12 bond, the location of the reactive epoxy group. Other major fragmentation ions include 155, 163 and 179 m/z. Major ions of the unknown eicosanoid were consistent with hepoxilin A3 [8-hydroxy-11(S),12(S)-epoxy-5(Z),14(Z),9(E)-eicosatrienoic acid] as described in the Lipidmaps database (The LIPID MAPS Lipidomics Gateway, http://www.lipidmaps.org/). We did not detect any additional structure that correspond with hepoxilin B3 or other HEETAs. The major fragment ions of the 12/15-LO metabolites and standards are shown in (Table 1).
Figure 3.
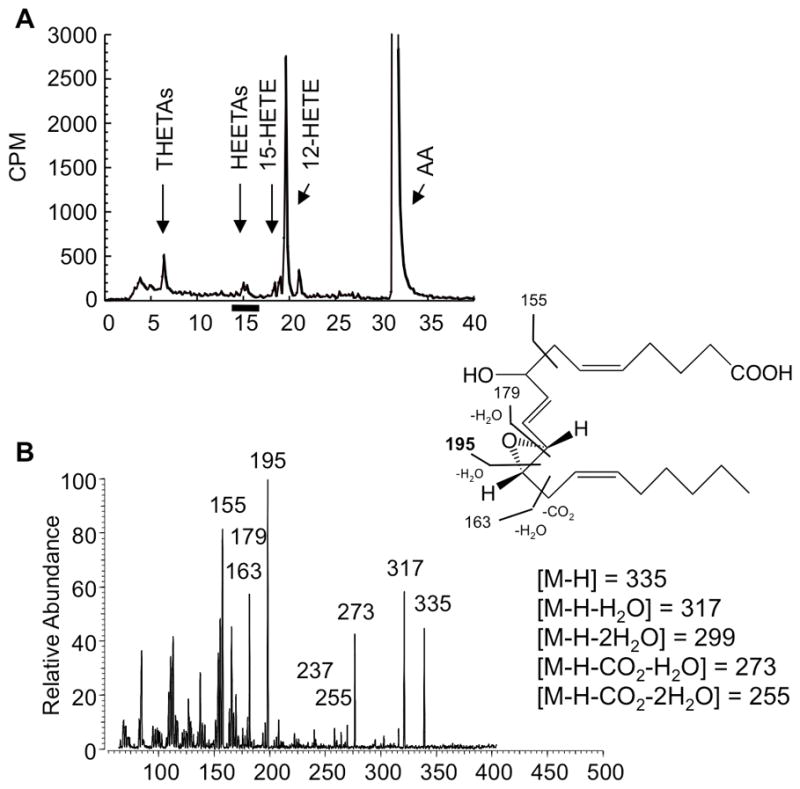
RP-HPLC analysis and mass spectrum of the HEETA fraction. (A) Fractions co-migrating with the HEETA standard were collected from RP-HPLC solvent system I. (B) LC-MS/MS analysis of the HEETA fraction using the negative ion mode.
Table 1.
Major ion fragmentation of trioxilin A3, trioxilin C3 and hepoxilin A3 from mouse aorta (m/z, mass/charge). The fragment ions of the hepoxilin A3 standard were adapted from Lipidmaps database.
| Eicosanoids | Aorta | Standard | ||
|---|---|---|---|---|
| [M-H]− | Major fragment ions [m/z] | [M-H]− | Major fragment ions [m/z] | |
| Trioxilin A3 | 353 | 111(22), 127(34), 155(79), 177(14), 195(100), 255(6), 273(58), 299(10), 317(31), 335(27), 353(22) | 353 | 111(28), 127(37), 155(27), 177(12), 195(100), 255(8), 273(33), 291(4), 299(7), 317(22), 353(22), 353(3) |
| Trioxilin C3 | 353 | 111(18), 127(10), 155(100), 177(12), 195(13), 255(6), 273(38), 299(7), 317(16), 335(1), 353(10) | 353 | 111(24), 127(14), 155(100), 177(6), 195(13), 255(6), 273(31), 291(2), 299(3), 317(8), 335(2), 353(1) |
| Hepoxilin A3 | 353 | 155(81), 163(45), 179(57), 195(100), 255(7), 273(42), 299(4), 317(57), 335(45) | 353 | 151(10), 163(35), 179(22), 195(100), 273(24), 299(8), 217(49), 335(1) |
Having identified the 12/15-LO metabolites of AA in mouse arteries, we next examined the vascular activity of trioxilins and hepoxilins in mouse mesenteric arteries. Synthetic trioxilins were tested for vasorelaxation in arteries constricted with either U46619, a thromboxane A2 mimetic or phenylephrine, an α1-adrenergic receptor agonist. Trioxilin A3, B3 and C3 caused concentration-related relaxations of U46619, but not phenylephrine, constricted arteries (Fig. 4A, 4B and 4C). Maximum relaxations were 78.9 ± 3.2%, 29.7 ± 4.6% and 82.2 ± 5.0% for 3 μM trioxilin A3, B3 and C3, respectively. The EC50 values, a concentration that causes 50% maximum effect, were 1.26, 7.15 and 1.00 μM for trioxilin A3, B3 and C3, respectively. Interestingly, trioxilin B3, which was not found in the AA metabolites from mouse aorta, was less potent than trioxilin A3 and C3 in relaxing the U46619-constricted arteries. These finding that trioxilins relax mouse mesenteric arteries constricted with a TP agonist, but not an α1-adrenergic agonist, suggests that these AA metabolites may competitively displace the thromboxane mimetic U46619 from the TP receptors, thus antagonizing the contraction induced by U46619. This assumption was tested on mesenteric arteries pre-incubated with 10 μM trioxilin C3 or vehicle. The presence of trioxilin C3 shifted a constriction curve to increasing concentration of U46619 to the right as compared to vehicle-treated vessels (Fig 4D).
Figure 4.
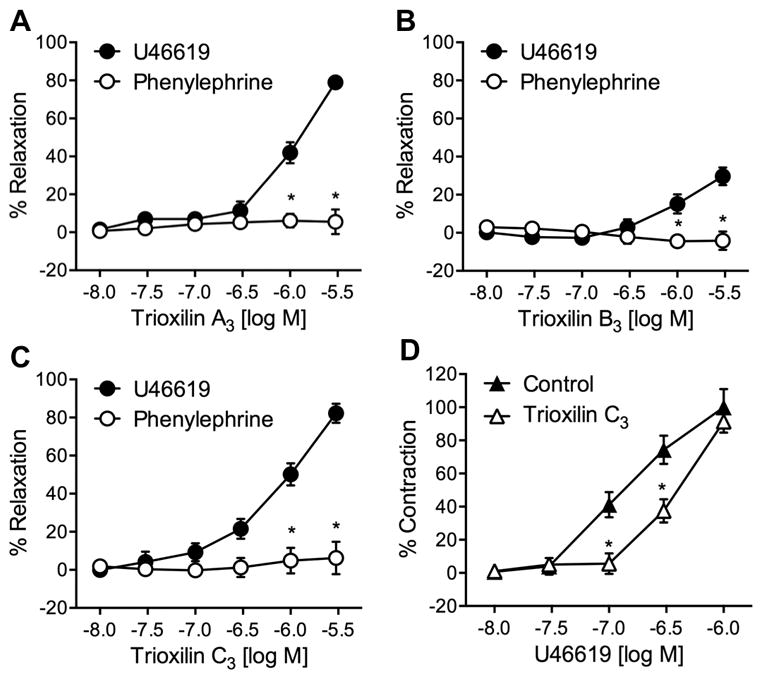
Relaxation responses of trioxilin A3 (A), trioxilin B3 (B), and trioxilin C3 (C) in mouse mesenteric arteries constricted with either U46619 (●) or phenylephrine (○). (D) Constriction of mouse mesenteric arteries by U46619 with (△) or without (▲) pre-incubation with 10 μM trioxilin C3. Each value represents the mean ± SEM. n = 8–12. * p < 0.05 compared to U46619 constricted arteries.
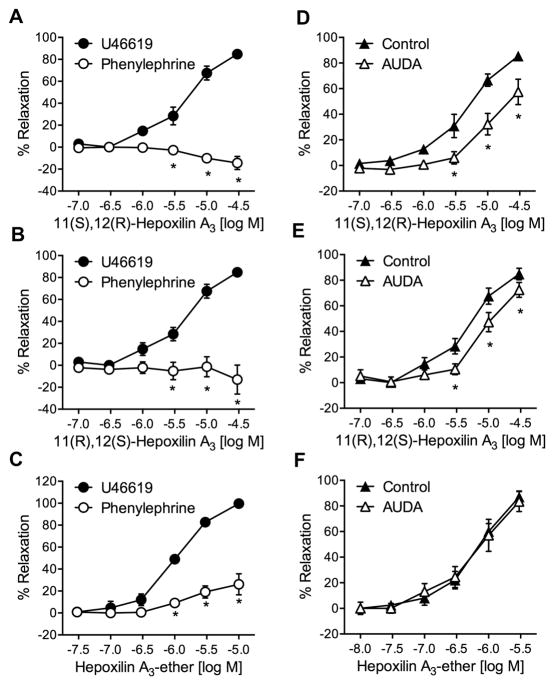
The cis-epoxy isomers of hepoxilin A3, 11(R),12(S)- and 11(S),12(R)-hepoxilin A3 also relaxed U46619, but not phenylephrine, constricted arteries (Fig. 5A and 5B). Thus, the stereochemical configuration of the cis-epoxide does not alter relaxation. Their relaxation responses at 3 μM were 20.0 ± 5.1% and 27.8 ± 6.3% for 11(R),12(S)- and 11(S),12(R)-hepoxilin A3, respectively. Hepoxilin A3–ether, a stable hepoxilin analog with an ether group between C12 and C13, also caused relaxation of U46619-constricted arteries (Fig. 5C), with maximal relaxation of 88.0 ± 2.4% at 3 μM. Hepoxilin A3-ether also relaxed phenylephrine-constricted arteries by approximately 20%; however, this effect was not statistically significant. Additionally, the relaxations to the hepoxilin A3 isomers were attenuated by the sEH inhibitor, AUDA (1 μM) (maximal relaxations = 10.4 ± 4.3% and 7.1 ± 3.3%, respectively) (Fig. 5D and 5E). However, AUDA did not alter relaxations of hepoxilin A3-ether (Fig. 5F). This response correlates with the hepoxilin A3-ether structure since replacement of the epoxy group with an ether group renders it resistant to sEH metabolism. These data indicate that a portion of the relaxations to hepoxilin A3 isomers was mediated by the sEH hydrolysis products. A summary of the trioxilin and hepoxilin relaxation responses is shown in (Table 2).
Figure 5.
Relaxation responses of 11(S),12(R)-hepoxilin A3 (A), 11(R),12(S)-hepoxilin A3 (B), and hepoxilin A3-ether (C) in mouse mesenteric arteries constricted with either U46619 (●) or phenylephrine (○). Relaxation responses of 11(S),12(R)-hepoxilin A3 (D), 11(R),12(S)-hepoxilin A3 (E), and hepoxilin A3-ether (F) in mouse mesenteric arteries constricted with U46619 with (△) or without (▲) the sEH inhibitor, AUDA (1 μM). Each value represents the mean ± SEM. n = 7–15, * p < 0.05 compared to control.
Table 2.
Chemical structures of trioxilin and hepoxilin analogs and their effect on relaxation at a concentration of 3 μM on mesenteric arteries constricted with U46619.
| Eicosanoid | Chemical Structure | Log EC50 (M) | Relaxation at 3 μM (%) |
|---|---|---|---|
| Trioxilin A3 |

|
−5.90 | 78.9±3.2 |
| Trioxilin B3 |

|
−5.15 | 29.7±4.6 |
| Trioxilin C3 |

|
−6.00 | 82.2±5.0 |
| Hepoxilin A3-ether |

|
−5.99 | 88.0±2.4 |
| 11(R),12(S)-Hepoxilin A3 |

|
−5.14 (−4.93) | 20.0±5.1 (10.4±4.3) |
| 11(S),12(R)-Hepoxilin A3 |

|
−5.20 (−4.66) | 27.8±6.3 (7.1±3.3) |
Values represent Mean ± SEM (n=8). Values in parenthesis were determined in the presence of 1μM AUDA. Log EC50 represents concentration that causes 50% of the maximum response.
To prove further that trioxilins and hepoxilins act as TP receptor antagonists, we examined intracellular calcium concentration responses in HEK293 cells overexpressing the TPα receptor. U46619 (10 nM) increased the calcium-associated fluorescence signal in HEK293 cells overexpressing the TP receptor (Fig. 6A) but was without effect in HEK293 cells without the TP receptor (Fig. 6B). When the cells were pre-treated with trioxilin A3 and trioxilin C3 (10 nM – 10 μM), calcium increases induced by U46619 were significantly inhibited in a concentration-dependent manner (Fig. 6C and 6E). The calcium-associated fluorescence signals were 53.0 ± 7.2 and 32.8 ± 5.0% for 10 μM trioxilin A3, and C3, respectively, when compared to 100% of vehicle control. In contrast, trioxilin B3 slightly reduced the calcium increase induced by U46619 at the highest concentration tested (% maximum = 62.7 ± 5.3, Fig. 6D); however, this decrease was not statistically significant. These data are consistent with the vascular studies, in which the relaxation response of trioxilin A3 and C3 were more potent than trioxilin B3. Hepoxilin A3-ether at a concentration of 10 μM significantly reduced the U46619-evoked calcium increase (% maximum = 37.9 ± 13.5, Fig. 6F). The LO metabolites did not alter fluorescence when U46619 was not present (data not shown). The calcium assays indicate that 12/15-LO metabolites inhibit intracellular calcium increases induced by the TP receptor agonist.
Figure 6.
Effect of trioxilins and hepoxilin on fluorometric measurement of intracellular calcium in HEK293 cells overexpressing the human TPα receptor. Representative fluorescence signals induced by 10 nM U46619 in HEK293 cells in the presence (A) or absence (B) of the human TPα receptor. Effect of trioxilin A3 (C), trioxilin B3 (D), trioxilin C3 (E) and hepoxilin A3-ether (F) on intracellular calcium increases induced by U46619 (10 nM). Data are presented as percent of the maximum fluorescence signal (per μg protein) evoked by U46619 in the presence of test compounds compared to vehicle control. Each value represents the mean ± SEM. n = 6–12. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Discussion
Previous studies indicate that vascular LO metabolites are formed in endothelium and their production is greatly diminished if endothelium is removed (Gauthier et al., 2011). These endothelial LO metabolites of AA regulate vascular tone in arteries from a number of different vasculatures and from various species (Chawengsub et al., 2009a, Chawengsub et al., 2008, Miller et al., 2003, Gauthier et al., 2011). Recent studies indicate the importance of LO metabolites in the regulation of vascular tone of mouse arteries (Gauthier et al., 2011, Kriska et al., 2012, Siangjong et al., 2013, Stapleton et al., 2007). 12(S)-HETE is the major LO metabolite of AA in mouse arteries (Siangjong et al., 2013, Gauthier et al., 2011) but numerous other LO metabolites are also present. Therefore, we undertook the identification and characterization of the other 12-LO metabolites.
Trioxilin A3, trioxilin C3 and hepoxilin A3 were identified using a series of HPLC and mass spectrometric analyses. The two trihydroxy eicosanoids were purified using reverse and normal phase HPLC. The unknown products did not co-migrate with a standard of the previously identified mouse THETA, 11,12,15-THETA (Gauthier et al., 2011, Gauthier et al., 2008). Subsequently, the structures of trioxilins were identified by mass spectrometry. The relative intensity and major ion fragments of trioxilins from mouse aorta samples were identical to those of the trioxilin A3 and C3 standards. The aortic synthesis of trioxilins suggested the existence of their unstable precursor, hepoxilin A3. Therefore, the aortic HEETA-like metabolites were purified from HPLC and analyzed on LC-MS/MS. Hepoxilin A3 was identified. The identification of hepoxilin and trioxilins along with 12(S)-HETE supports the dominant role of 12-LO in mouse arteries. This contrasts with the rabbit vasculature in which 15-LO metabolites are predominant (Campbell et al., 2003, Chawengsub et al., 2008, Chawengsub et al., 2009b).
In rabbit arteries, we previously identified several 15-LO derived metabolites of AA that are relaxing factors (Campbell et al., 2003, Chawengsub et al., 2008, Chawengsub et al., 2009b, Chawengsub et al., 2009a). 15-H-11,12-EETA and 13-H-14,15-EETA were isolated and identified as endothelial 15-LO metabolites of AA (Campbell et al., 2003, Chawengsub et al., 2008, Chawengsub et al., 2009b). 15-H-11,12-EETA mediated AA- and acetylcholine-induced relaxations in phenylephrine-constricted rabbit aorta and promoted membrane hyperpolarization of rabbit smooth muscle, suggesting that it is an EDHF (Chawengsub et al., 2008, Chawengsub et al., 2009a). It is hydrolyzed by sEH to 11,12,15-THETA, which also hyperpolarizes and relaxes rabbit arteries. 11,12,15-THETA relaxation was inhibited by the K channel blocker apamin (Campbell et al., 2003, Gauthier et al., 2008). Therefore, 11,12,15-THETA also represents an EDHF in rabbit arteries (Campbell et al., 2003, Chawengsub et al., 2009a). Endothelial 15-LO is induced by hypoxia, cholesterol, interleukin, estrogen and other factors resulting in enhanced hyperpolarization and relaxation of smooth muscle (Campbell and Gauthier, 2013). Thus, the 15-LO pathway functions as an inducible EDHF (Campbell and Gauthier, 2013). In comparison, the 15-LO/HEETA/THETA enzymatic pathway in the rabbit vasculature is similar to the 12/15-LO/hepoxilin/trioxilin pathway in mouse arteries.
We investigated the vascular activity of the hepoxilins and trioxilins in mouse mesenteric arteries. In arteries constricted with the thromboxane mimetic, U46619, the hepoxilins and trioxilins caused concentration-related relaxations; however, their potencies varied. Trioxilin A3 and C3, identified as mouse aortic AA metabolites, were more active than trioxilin B3. Their potencies were comparable to the stable hepoxilin A3-ether. The two epoxy-containing hepoxilin A3 isomers were less active than the stable hepoxilin-ether analog and trioxilin A3 and C3. Their reduced activity may be due to their chemical instability in buffer or metabolism by sEH. Instead of enhancing hepoxilin-induced relaxation, sEH inhibition reduced the relaxation responses of the hepoxilin isomers. This indicates that chemical instability, rather than sEH metabolism, accounts for the reduced vasorelaxant activity. Additionally, these data suggest that hepoxilin relaxations in the absence of sEH inhibition are partially mediated by trioxilins. In our previous study of 12(S)-HETE relaxation in mouse mesenteric arteries, the comparison of relaxation responses in either phenylephrine or U46619-constricted arteries provided a useful tool to investigate the role of TP receptors in vasorelaxation (Siangjong et al., 2013). While 12(S)-HETE had no effect on arteries constricted with the α1-adrenergic receptor agonist phenylephrine, it competed with TP receptor agonists to cause relaxation in a concentration-dependent manner (Siangjong et al., 2013). Similar to 12(S)-HETE, trioxilin A3, trioxilin B3, trioxilin C3 and hepoxilin A3 isomers and ether analog relaxed the mouse mesenteric arteries constricted with U46619, but not phenylephrine. This suggests a TP receptor-dependent mechanism of relaxation. Contrary to the mouse, trioxilin C3 caused concentration-dependent relaxation in phenylephrine-contracted rabbit aorta whereas trioxilin A3 was inactive (Pfister, 2003). This highlights differences in vascular activities of LO metabolites in rabbits and mice. In agreement with our findings in mouse arteries, Qiao et al showed that the hepoxilin analog, PBT-3 [10(S)-hydroxy-11,12-cyclopropyleicosa-5(Z),8(Z),14(Z)-trienoic acid methyl ester], inhibited binding of the TP antagonist [3H]-SQ29548 to TPα, but not TPβ, receptors in transiently transfected COS-7 cells (Qiao et al., 2003). These data and ours indicate that the structure around carbon 11 and 12 may vary greatly and still inhibit TP receptors. The presence of a 12-hydroxy (12-HETE and trioxilin C3), 11,12-dihydroxy (trioxilin A3), 11(R),12(S) or 11(S),12(R) –epoxy (hepoxilin A3), an ether between carbons 11 and 12 (hepoxilin A3-ether) or 11,12 cyclopropyl (PBT-3) all inhibit TP receptors. In contrast, the 10,11,12-trihydroxy group of hepoxilin B3 is not active.
Activation of TP receptors leads to signaling cascades that involve intracellular calcium and Rho kinase (Klages et al., 1999, Dorn and Becker, 1993, Nakahata, 2008). Since calcium is a key second messenger in regulating vascular tone, we used a calcium-sensitive fluorescence dye assay to analyze changes in intracellular calcium in HEK293 cells stably expressing the human TPα receptor (Wilson et al., 2004). We previously showed that the addition of U46619 to TPα-HEK cells caused concentration-dependent increases of calcium-associated fluorescence, which were inhibited by the TP receptor antagonist SQ29548 (Siangjong et al., 2013). In the current study, trioxilin C3 was the most potent 12/15-LO metabolite at inhibiting U46619-induced intracellular calcium increases, which was consistent with the relaxation studies. Trioxilin C3 was also used to demonstrate the competitive nature of antagonism of U46619-mediated constriction. Hepoxilin A3-ether also significantly reduced the calcium increases to U46619. An advantage of using hepoxilin A3-ether is that it is more stable than natural hepoxilins that undergo hydrolysis to trioxilins. Similar to our results, Qiao et al showed that the hepoxilin analog PBT-3 inhibited intracellular calcium release induced by collagen and I-BOP, a TP receptor agonist (Qiao et al., 2003). Trioxilin B3, which is not synthesized from AA in mouse arteries, was a weak relaxing factor and had no effect on intracellular calcium release evoked by U46619.
Finally, EDHF activity has been defined as endothelium-dependent relaxation that occur in the presence of NO synthase and cyclooxygenase inhibition and that are absent in arteries constricted by high extracellular potassium (Feletou and Vanhoutte, 1999, Hecker, 2000, McGuire et al., 2001, Campbell and Falck, 2007). The current studies indicate that this definition is inadequate since it cannot distinguish EDHF from an endogenous antagonist of a constrictor receptor such as a TP receptor antagonist. Measurements of smooth muscle cell membrane potential may also not be useful in defining EDHF since a TP antagonist would repolarize smooth muscle depolarized by U46619. By definition vasodilators such as EDHF act by a mechanism distal to constrictor receptors and inhibit vasoconstriction by all constrictors. Of course, EDHF will not inhibit vasoconstriction by potassium by virtue of its mechanism of action to activate potassium channels. Thus, in defining EDHF, multiple constrictors, other than potassium, should be tested. EDHF causes endothelium-dependent hyperpolarization and relaxation that occurs in the presence of several vasoconstrictors and that are not inhibited by NO synthase or cyclooxygenase inhibitors.
In summary, we isolated and identified three 12-LO metabolites of AA in mouse arteries; trioxilin A3, trioxilin C3 and hepoxilin A3. They reduced intracellular calcium increases induced by a TP receptor agonist and promoted vascular relaxation in mouse mesenteric arteries through TP receptor antagonism. Thus, these 12-LO metabolites have characteristics of endogenous TP receptor antagonists.
Conclusions
In mouse arteries and platelets, LOs metabolize AA to produce 12(S)-HETE, hepoxilin A3, trioxilin A3 and trioxilin C3, which act as TP receptor antagonists. On the other hand, under pathological conditions such as stroke, COX is activated in platelets and metabolizes AA to TXA2, an endogenous agonist of the TP receptor (Chamorro, 2009). During oxidative stress, such as hypertension, hypercholesterolemia, or ischemia-reperfusion, AA is oxidized by reactive oxygen species to isoprostanes, which also function as TP receptor agonists (Oguogho et al., 1999, Yamada et al., 1999, Dobrian et al., 2001, Xiao et al., 2001). While TXA2 and isoprostanes promote vasoconstriction, the 12-LO-derived metabolites act as TP receptor antagonists counteracting the effect of these constrictors. The contrasting effects of these AA metabolites on the same receptor suggest that 12-LO metabolites may represent biologically important endogenous TP antagonists. Vascular homeostasis is tightly regulated by AA metabolites as well as other mechanisms. When TP receptor agonist and antagonist concentrations are balanced, vascular tone and blood pressure are normal. If TXA2 production is increased or if the 12/15-LO pathway is disrupted, the balance is disturbed and may lead to increased vascular constriction and hypertension.
Acknowledgments
The authors thank Ms. Gretchen Barg for her secretarial assistance and Mr. Cody J. Cepura and Ms. Jessica L. Kelliher for technical assistance. We also thank Mrs. Marilyn Isbell and Dr. Kasem Nithipatikom for mass spectrometry analysis. We thank the Ministry of Science and Technology of Thailand for supporting a scholarship for the graduate studies for Ms. L. Siangjong. These studies were supported by grants from the National Heart Lung and Blood Institute (HL-103673, HL-37981 and HL-66233), Institute of General Medicine (GM-31278), and the Robert A. Welch Foundation (GL625910).
Footnotes
Conflict of Interest
The authors declared no conflicts of interest, financial or otherwise.
References
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. Journal of Biological Chemistry. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- Bryant RW, Bailey M. Isolation of a new lipoxygenase metabolite of arachidonic acid, 8,11,12-trihydroxy-5,9,14-eicosatrienoic acid from human platelets. Prostaglandins. 1979;17:9–18. doi: 10.1016/0090-6980(79)90071-6. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gauthier KM. Inducible endothelium-derived hyperpolarizing factor: Role of the 15-lipoxygenase-EDHF pathway. J Cardiovasc Pharmacol. 2013;61:176–187. doi: 10.1097/FJC.0b013e31828165db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. 11,12,15-Trihydroxyeicosatrienoic acid mediates ACh-induced relaxations in rabbit aorta. American Journal of Physiology: Heart and Circulatory Physiology. 2003;285:H2648–H2656. doi: 10.1152/ajpheart.00412.2003. [DOI] [PubMed] [Google Scholar]
- Chamorro A. TP Receptor Antagonism: A New Concept in Atherothrombosis and Stroke Prevention. Cerebrovasc Dis. 2009;27:20–27. doi: 10.1159/000209262. [DOI] [PubMed] [Google Scholar]
- Chawengsub Y, Aggarwal NT, Nithipatikom K, Gauthier KM, Anjaiah S, Hammock BD, Falck JR, Campbell WB. Identification of 15-hydroxy-11,12-epoxyeicosatrienoic acid as a vasoactive 15-lipoxygenase metabolite in rabbit aorta. American Journal of Physiology: Heart and Circulatory Physiology. 2008;294:H1348–H1356. doi: 10.1152/ajpheart.01326.2007. [DOI] [PubMed] [Google Scholar]
- Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. American Journal of Physiology: Heart and Circulatory Physiology. 2009a;297:H495–H507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawengsub Y, Gauthier KM, Nithipatikom K, Hammock BD, Falck JR, Narsimhaswamy D, Campbell WB. Identification of 13-hydroxy-14,15-epoxyeicosatrienoic acid as an acid-stable endothelium-derived hyperpolarizing factor in rabbit arteries. Journal of Biological Chemistry. 2009b;284:31280–31290. doi: 10.1074/jbc.M109.025627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Isbell Ma, Chawengsub Y, Falck JR, Campbell WB, Nithipatikom K. Structural characterization of monohydroxyeicosatetraenoic acids and dihydroxy- and trihydroxyeicosatrienoic acids by ESI-FTICR. Journal of the American Society for Mass Spectrometry. 2008;19:569–585. doi: 10.1016/j.jasms.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dho S, Grinstein S, Corey EJ, Su WG, Pace-asciak CR. Hepoxilin A3 induces changes in cytosolic calcium, intracelllar pH and membrane potential in human neutrophils. Biochemical Journal. 1990;266:63–68. doi: 10.1042/bj2660063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension. 2001;37:554–60. doi: 10.1161/01.hyp.37.2.554. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Becker MW. Thromboxane A2 stimulated signal transduction in vascular smooth muscle. Journal of Pharmacology and Experimental Therapeutics. 1993;265:447–56. [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. The alternative: EDHF. J Mol Cell Cardiol. 1999;31:15–22. doi: 10.1006/jmcc.1998.0840. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Chawengsub Y, Goldman DH, Conrow RE, Anjaiah S, Falck JR, Campbell WB. 11(R),12(S),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid: an endothelium-derived 15-lipoxygenase metabolite that relaxes rabbit aorta. American journal of physiology: Heart and circulatory physiology. 2008;294:H1467–H1472. doi: 10.1152/ajpheart.01052.2007. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Goldman DH, Aggarwal NT, Chawengsub Y, Falck JR, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in acetylcholine-induced relaxations of mouse arteries. American Journal of Physiology: Heart and Circulatory Physiology. 2011;300:H725–H735. doi: 10.1152/ajpheart.00696.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M. Endothelium-derived hyperpolarizing factor-fact or fiction. News Physiol Sci. 2000;15:1–5. [PubMed] [Google Scholar]
- Kim JA, Gu JL, Natarajan R, Berliner JA, Nadler JL. A Leukocyte type of 12-Lipoxygenase is expressed in human vascular and mononuclear cells: evidence for upregulation by angiotensin II. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15:942–948. doi: 10.1161/01.atv.15.7.942. [DOI] [PubMed] [Google Scholar]
- Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. Journal of Cell Biology. 1999;144:745–54. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriska T, Cepura C, Magier D, Siangjong L, Gauthier KM, Campbell WB. Mice lacking macrophage 12/15-lipoxygenase are resistant to experimental hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H2428–38. doi: 10.1152/ajpheart.01120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneuville O, Corey EJ, Couture R, Pace-Asciak CR. Hepoxilin A3 (HxA3) is formed by the rat aorta and is metabolized into HxA3-C, a glutathione conjugate. Biochimica et Biophysica Acta. 1991;1084:60–68. doi: 10.1016/0005-2760(91)90056-n. [DOI] [PubMed] [Google Scholar]
- McGuire JJ, Ding H, Triggle CR. Endothelium-derived relaxing factors: A focus on endothelium-derived hyperpolarizing factor(s) Canad J Physiol Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- Miller AW, Lee HC, Weintraub NL. Arachidonic acid-induced vasodilation of rat small mesenteric arteries is lipoxygenase-dependent. Journal of Pharmacology and Experimental Therapeutics. 2003;304:139–144. doi: 10.1124/jpet.102.041780. [DOI] [PubMed] [Google Scholar]
- Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacology & therapeutics. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Oguogho A, Mehrabi M, Sinzinger H. Increased plasma, serum and urinary 8-epi-prostaglandin F2 alpha in heterozygous hypercholesterolemia. Wiener Klinische Wochenschrift. 1999;111:113–8. [PubMed] [Google Scholar]
- Pace-asciak CR. Formation of hepoxilin A4, B4 and the corresponding trioxilins from 12(S)-hydroperoxy-5,8,10,14,17-icosapentaenoic acid. Prostaglandins, Leukotrienes and Medicines. 1986;22:1–9. doi: 10.1016/0262-1746(86)90017-x. [DOI] [PubMed] [Google Scholar]
- Pace-asciak CR. Formation and metabolism of hepoxilin A3 by the rat brain. Biochemical and biophysical research communications. 1988;151:493–498. doi: 10.1016/0006-291x(88)90620-1. [DOI] [PubMed] [Google Scholar]
- Pace-asciak CR, Granstrom E, Samuelsson B. Arachidonic acid epoxides. Isolation and structure of two hydroxy epoxide intermediates in the formation of 8,11,12- and 10,11,12-trihydroxyeicosatrienoic acids. Journal of Biogical Chemistry. 1983;258:6835–6840. [PubMed] [Google Scholar]
- Pace-asciak CR, Martin JM. Hepoxilin, a new family of insulin secretagogues formed by intact rat pancreatic islets. Prostaglandins, Leukotrienes and Medicines. 1984;16:173–180. doi: 10.1016/0262-1746(84)90069-6. [DOI] [PubMed] [Google Scholar]
- Pfister S. Metabolism of 12-hydroperoxyeicosatetraenoic acid to vasodilatory trioxilin C3 by rabbit aorta. Biochimica et Biophysica Acta. 2003;1622:6–13. doi: 10.1016/s0304-4165(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Qiao NA, Reynaud D, Demin P, Halushka PV, Pace-asciak CR. The Thromboxane receptor antagonist PBT-3, a hepoxilin stable analog, selectively antagonizes the TPa isoform in transfected COS-7 cells. Journal of pharmacology and experimental therapeutics. 2003;307:1142–1147. doi: 10.1124/jpet.103.056705. [DOI] [PubMed] [Google Scholar]
- Reynaud D, Demin PM, Sutherland M, Nigam S, Pace-Asciak CR. Hepoxilin signaling in intact human neutrophils: biphasic elevation of intracellular calcium by unesterified hepoxilin A3. FEBS letters. 1999;446:236–8. doi: 10.1016/s0014-5793(99)00225-2. [DOI] [PubMed] [Google Scholar]
- Siangjong L, Gauthier KM, Pfister SL, Smyth EM, Campbell WB. Endothelial 12(S)-HETE vasorelaxation is mediated by thromboxane receptor inhibition in mouse mesenteric arteries. Am J Physiol Heart Circ Physiol. 2013;304:H382–92. doi: 10.1152/ajpheart.00690.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PA, Goodwill AG, James ME, Frisbee JC. Altered mechanisms of endothelium-dependent dilation in skeletal muscle arterioles with genetic hypercholesterolemia. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1110–9. doi: 10.1152/ajpregu.00410.2007. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Roche AM, Kostetskaia E, Smyth EM. Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. Journal of biological chemistry. 2004;279:53036–47. doi: 10.1074/jbc.M405002200. [DOI] [PubMed] [Google Scholar]
- Xiao CY, Hara A, Yuhki KI, Fujino T, Ma H, Okada Y, Takahata O, Yamada T, Murata T, Narumiya S, Ushikubi F. Roles of Prostaglandin I2 and Thromboxane A2 in Cardiac Ischemia-Reperfusion Injury. Circulation. 2001;104:2210–2215. doi: 10.1161/hc4301.098058. [DOI] [PubMed] [Google Scholar]
- Yamada M, Omata K, Abe F, Ito S, Abe K. Changes in prostacyclin, thromboxane A2 and F2-isoprostanes, and influence of eicosapentaenoic acid and antiplatelet agents in patients with hypertension and hyperlipidemia. Immunopharmacology. 1999;44:193–8. doi: 10.1016/s0162-3109(99)00137-x. [DOI] [PubMed] [Google Scholar]