Abstract
A critical step toward understanding autism spectrum disorder (ASD) is to identify both genetic and environmental risk factors. A number of rare copy number variants (CNVs) have emerged as robust genetic risk factors for ASD, but not all CNV carriers exhibit ASD and the severity of ASD symptoms varies among CNV carriers. Although evidence exists that various environmental factors modulate symptomatic severity, the precise mechanisms by which these factors determine the ultimate severity of ASD are still poorly understood. Here, using a mouse heterozygous for Tbx1 (a gene encoded in 22q11.2 CNV), we demonstrate that a genetically-triggered neonatal phenotype in vocalization generates a negative environmental loop in pup-mother social communication. Wild-type pups used individually diverse sequences of simple and complicated call types, but heterozygous pups used individually invariable call sequences with less complicated call types. When played back, representative wild-type call sequences elicited maternal approach, but heterozygous call sequences were ineffective. When the representative wild-type call sequences were randomized, they were ineffective in eliciting vigorous maternal approach behavior. These data demonstrate that an ASD risk gene alters the neonatal call sequence of its carriers and this pup phenotype in turn diminishes maternal care through atypical social communication. Thus, an ASD risk gene induces, through atypical neonatal call sequences, less than optimal maternal care as a negative neonatal environmental factor.
Introduction
Autism spectrum disorder (ASD) is characterized by concurrent deficits in reciprocal social communication and interaction, as well as deficits in cognitive and behavioral flexibility. Clinical diagnosis of ASD can be made in children by two years of age. Identification of even earlier signs of ASD is critical as shown by the proven effectiveness of early intervention1–3. Infant behaviors such as decreased eye contact, atypical preverbal vocalizations and atypical development of other behaviors are prognostic of ASD even before formal ASD diagnosis4–6.
Vocalization is a very early, primary means of social communication in that its expression in newborns signals the need for care7,8. Early neonatal vocalization is thought to have an innate component9–11, as vocalization emitted by human infants and rodent pups occurs without auditory feedback12–15. Compared to infants with intellectual disability or typically developing infants, cries in infants with incipient ASD are characterized by high-pitch, lower waveform modulation and rhythm, and more dysphonation; in turn, atypical cries of incipient ASD infants are more negatively perceived by mothers16,17. It is, however, difficult to establish the causative role of atypical vocalizations as a genuinely functional component of ASD in humans, as they are embedded in many atypical features in the cognitive, motor, and social domains18. When separated from dams, mouse pups also emit ultrasonic vocal calls, which elicit maternal approach19. Thus, genetic mouse models of ASD represent an alternative approach for elucidation of a causative role of early atypicalities in ASD.
Hemizygous deletion at human 22q11.2 is one of rare copy number variants that are robustly associated with ASD20. Up to 27% of hemizygous deletion carriers of chromosome 22q11.2 are diagnosed with ASD20,21. TBX1 is a contributory gene among approximately 30 protein-coding genes in a commonly deleted 22q11.2 hemizygous region20. Several private mutations of TBX1 are associated with ASD22–24. In mice, Tbx1 heterozygosity causes all symptomatic elements of ASD, including reduced levels of reciprocal social interaction, pup vocalizations and working memory capacity and heightened repetitive and anxiety-related behavioral traits25. However, while atypical pup calls have been described in this and many other genetic mouse models of ASD26, precise structural components critical for functional impact on maternal behavior have not been determined to date. We report here that normal pup vocalization has a distinct sequence structure and its atypicality in this genetic mouse model of ASD causes decreased maternal responses. Our data suggest that atypical pup vocal sequences induced by a genetic ASD risk factor negatively alter maternal care, which in turn acts as a negative environmental factor in social communication.
Materials and Methods
We used vocal call data from a Tbx1 mouse model of ASD25 to test the hypothesis that call type sequences have functional impacts on maternal approach. The sample size was determined by our previous demonstration to detect statistically significant differences19,25. Pups that emitted no call during the test periods were excluded from analysis. After determining the call and sequence structures of the two genotypes using Partial Least Square Discriminant Analysis, Shannon entropy analysis, Markov model and Sparse Partial Least Squares Discriminant Analysis, we evaluated their functional impact on maternal approach behaviors using our standard experimental paradigm and an emitter composed of a surface-heating thin film electrode, a nanocrystalline silicon (ns-Si) layer, and a single-crystalline silicon wafer19. We measured the fidelity of sound reproduction from our sound emitter and calibrated sound before initiating experiments. The emitter reproduces pup calls with remarkably similarity in terms of amplitude, pitch and duration with a correlation coefficient of 0.9627. The genotypes of pups were blinded until structural and functional analyses were completed. All experimental procedures are detailed in Supplementary Information.
Results
Elements of pup vocalizations
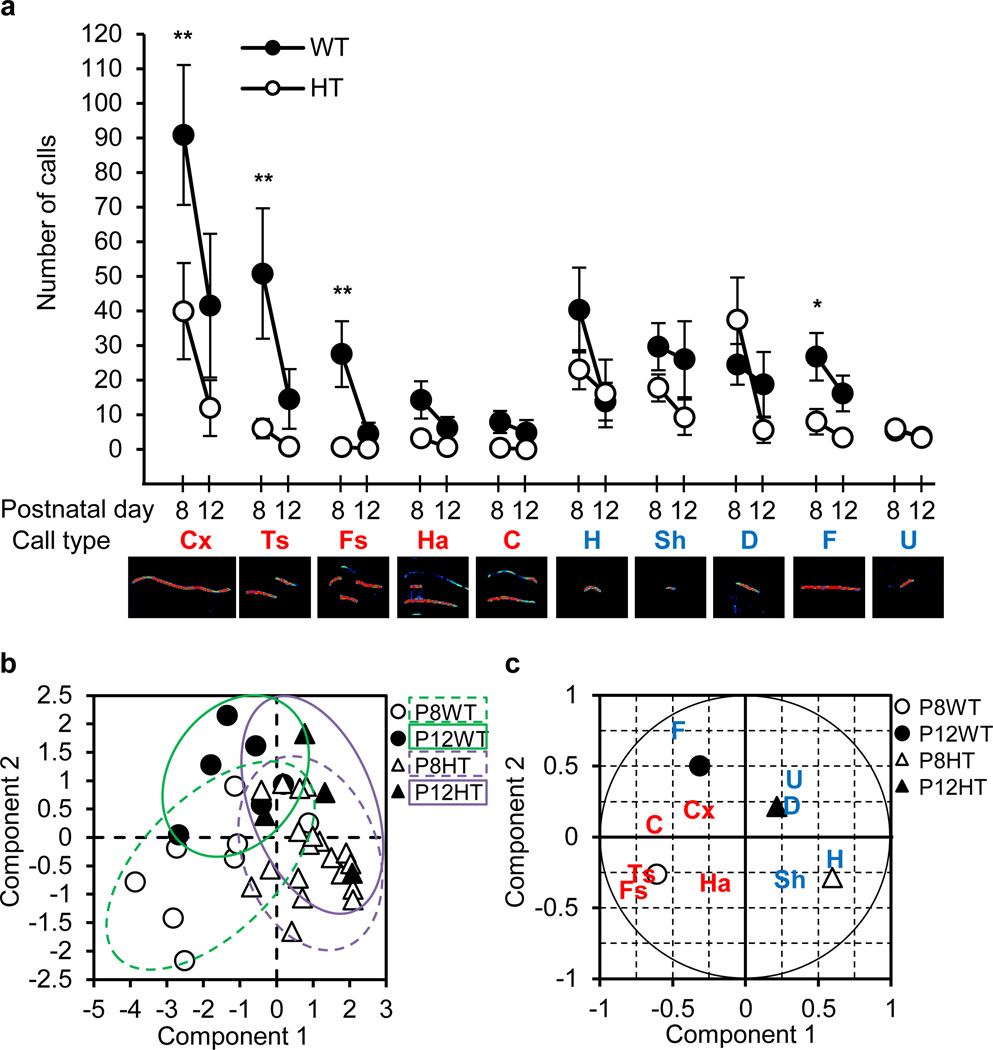
Postnatal days up to around day 3 and days 7–10 in mice correspond to preterm and term human infants, respectively28. Because we wish to model vocalization after birth, we chose postnatal days 8 and 12 for recording and analysis. We classified neonatal call types according to a system used by Scattoni and colleagues29. Normal pup vocal calls during maternal separation include “complicated call types” that are made up of several sounds at different frequencies (two-syllable (Ts), frequency steps (Fs), harmonics (Ha), and composite (C)) or contain more than one frequency change in a sound (complex (Cx)) and “simple call types” that are composed of single waves (hump (H), short (Sh), downward (D), flat (F) and upward (U))29. Tbx1 heterozygous pups emitted significantly fewer Cx, Ts, Fs and F, compared to wild-type pups at P8; vocal calls considerably declined thereafter for wild-type pups so that the two groups were indistinguishable for any call type by P12 (Figure 1a). Wild-type pups emitted longer Ts, Fs, Ha, and C than heterozygous pups at P8 (Supplementary Figure S1a). Wild-type pups exhibited decreased lengths of these calls by P12 so that the two genotypes no longer differed at that time. Wild-type and heterozygous pups did not differ in the pitch (Supplementary Figure S1b) or peak amplitude (Supplementary Figure S1c) of vocal calls.
Figure 1. Neonatal ultrasonic vocalization.
a) The mean (±SEM) number of 10 distinct call types. Five complicated call types (red) and five simple call types (blue) are indicated as: Cx, complex; Ts, two-syllable; Fs, frequency steps; Ha, harmonics; C, composite; H, hump (a.k.a., chevron); Sh, short; D, downward; F, flat; and U, upward. Typical spectrograms are shown below each label. As homogeneity of variance was violated (Cochran’s C=0.15, p<0.01), statistical analyses were applied to square-root transformed data. For clarity, the averages of raw data are shown. Genotype was used as an independent factor and age and call types were used as repeated factors in a two-way, one repeated measure design ANOVA. Interaction was significant among genotype, age and call types (F(9,369)=2.74, P = 0.0041). * and ** indicate statistically significant differences between wild-type (WT) and heterozygous (HT) pups at 5% and 1%, respectively, as determined by Newman-Keuls post-hoc comparisons. Postnatal day (P)8, WT pups, n = 8; HT pups, n = 20. P12, WT pups; n = 8, HT pups, n = 9. Partial Least Square Discriminant Analysis (PLS-DA) scores plot (b) and correlation plot (c) of P8 and P12 vocalization data. Correlation of ten call types and four genotype/age groups with the two components is indicated within the correlation circle (c).
Partial least square discriminant analysis (PLS-DA) of the number of calls revealed two components that separated pups in terms of genotype and age (Figure 1b). The primary component (i.e., Component 1) separated Cx, Ts, Fs, Ha, C and F from H, Sh, D and U (Figure 1c), largely reflecting call types for which wild-type and heterozygous pups did and did not differ, respectively. The second component separated Cx, C, D, F and U from Ts, Fs, Ha, H and Sh, that is all but D of the former call types declined in parallel from P8 to P12 between wild-type and heterozygous pups, but all the latter call types showed non-parallel declines (see Figure 1a). Thus, the primary effect of this ASD risk factor is to preferentially reduce the number and duration of complicated call types, with simultaneously increased relative representation of simple call types.
Sequence structure of pup vocalization
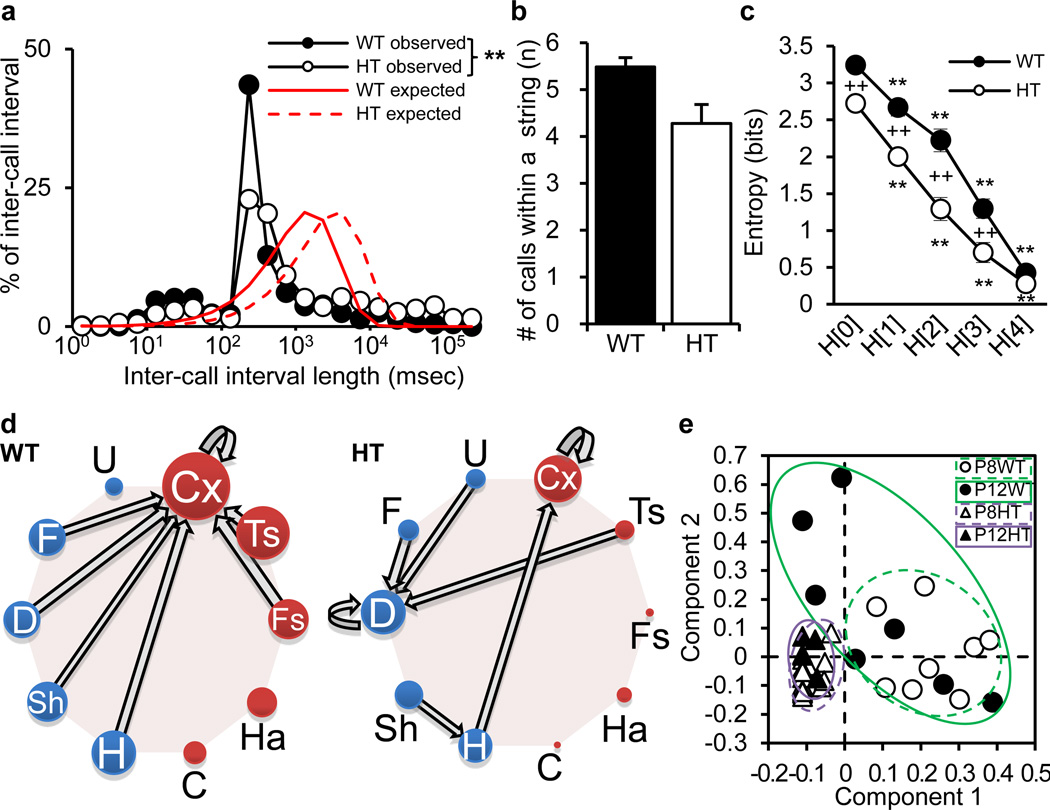
We next analyzed the sequence structure of ultrasonic calls at P8. We noticed that calls were not evenly distributed; instead, calls were clustered, creating periods of silence (Supplementary Figure S2). If two calls separated by a long pause were included in a sequence and counted for analysis, the actual sequence structure would be distorted; thus, we reduced inclusion of two widely separated calls in a sequence as follows. We first determined the theoretically expected distribution of inter-call intervals with a given number of calls for the 5-min test (Figure 2a). As expected from the smaller number of calls emitted by heterozygous pups compared to wild-type pups, the expected distribution of the heterozygous group shifted to the right relative to that of wild-type pups. We then compared these theoretical curves to the distributions of observed inter-call intervals, and quantitatively defined a call cluster, termed a “string”, as a series of calls with inter-call intervals below the intersection between the theoretical and observed distribution curves (Supplementary Table S1; Figure 2a). Wild-type pups and heterozygous pups emitted a statistically indistinguishable numbers of calls per string (Figure 2b).
Figure 2. Sequence structure of vocal calls at P8.
a) Proportions of expected and observed inter-call intervals. The distributions of observed inter-call intervals differed between wild-type (WT) and heterozygous (HT) calls, as determined by Kolmogorov-Smirnov test (KS-Z=6.96, P < 0.0001). b) The numbers of calls per string did not differ between the two genotype groups (t(26)=1.82, P = 0.080), as determined by two-sided student’s t-test (Figure 2B). c) Entropy scores of vocal calls within strings. Entropy values were analyzed by a two-way, one repeated measure ANOVA with genotype as an independent factor and H levels as a repeated measure. The interaction between genotype and model order was significant (F(4,104)=5.28, P = 0.0007). ++ indicates a statistically significant difference between wild-type and heterozygous pups and ** indicates a statistically significant difference at an H[x] level from an H[x−1] level at 1%, as determined by Newman-Keuls post-hoc comparisons. (d) Transitions and their directions between two calls as identified by the Markov model. Thickness of arrows and size of call circles represent the relative proportion of a transition and call numbers, respectively. The diagram depicts connections of the 7 most frequently uttered call types of each genotype and transitions of the highest degree for each call type (see Supplementary Figure S7ab). e) sPLS-DA analysis of string data, based on cross validation of models (see Supplementary Figure S4) and loading plots (see Supplementary Figure S8). Abbreviations: Cx, complex; Ts, two-syllable; Fs, frequency steps; Ha, harmonics; C, composite; H, hump (a.k.a., chevron); Sh, short; D, downward; F, flat; and U, upward
Using Shannon entropy analysis, we next determined whether any sequence structure existed in call strings and, if so, at what sequence level. In the zero-order model (H[0]), we calculated entropy based on the number of call types used, and computed the average for each genotype. Tbx1 heterozygous pups had lower entropy scores than wild-type pups, reflecting a narrower call type repertoire (Figure 2c), consistent with the finding of fewer call types used by heterozygous pups than by wild-type pups (see Figure 1a). In the first-order model, H[1], entropy scores declined from H[0] at a similar rate in wild-type and heterozygous pups, indicating that both wild-type and heterozygous pups emitted some call types more frequently than others within their call repertoires. In the second-, third- and fourth-order models (H[2] to H[4]), entropy scores further declined in both groups, indicating that pups non-randomly chose call types to emit two, three and four successive calls, respectively, within strings. Strings had lower entropy values than raw data (F(1,26)=187.07, P < 0.0001 between Supplementary Figure S3 and Figure 2c), validating that the string significantly reduced inclusion of non-structural elements of inter-call intervals. A sequence structure of calls exists in normal mouse pups and Tbx1 heterozygous pups have a higher degree of non-random sequence.
To determine the predominant sequences of calls of wild-type and heterozygous pups, we applied Markov modeling to the string data. We chose the two-call sequences (i.e. H[2]) within strings, because the two genotypes differed most widely at this level among the multiple-call strings (see Figure 2c). Wild-type pups more frequently connected complicated call types (Cx, Ts and Fs) than heterozygous pups (Figure 2d). In contrast, heterozygous pups more frequently formed connections among simple call types (i.e., U, F, D, Sh and H) than wild-type pups. Although Cx among complicated calls served as a hub for connections with simple call types (i.e., F, D, Sh and H) in wild-type pups, D was a hub in heterozygous calls. Moreover, heterozygous pups repeated D calls, but wild-type pups did not. Thus the predominant sequences of call types are altered in this mouse model of ASD.
To further explore the structure of call sequences, we built a classifier model to determine the most important predictors among two-call combinations within strings. Using a sparse version of PLS-DA (sPLS-DA) to select and identify the most robust predictors in constructing direction vectors, we identified a seven component model with five two-call combinations, which showed the smallest predictive error rate based on leave-one out cross validation (Supplementary Figure S4). The first two components accounted for most of the variance. Wild-type pup call sequences were more individually variable along the two identified components, compared to call sequences of heterozygous pups, despite the fact that all wild-type pups were littermates of heterozygous pups (Figure 2e). Mouse pups do not develop hearing capacity until P1112, and deaf pups normally develop ultrasonic vocalizations13. Although the difference between wild-type and heterozygous pups at P12 is likely to be influenced by hearing impairments in Tbx1 heterozygous mice30, a lack of individually variable vocal call sequencing at P8 is a very early genetically-determined sign of behavioral inflexibility in this mouse model of ASD.
Functional effect of atypical pup vocalizations on maternal approach
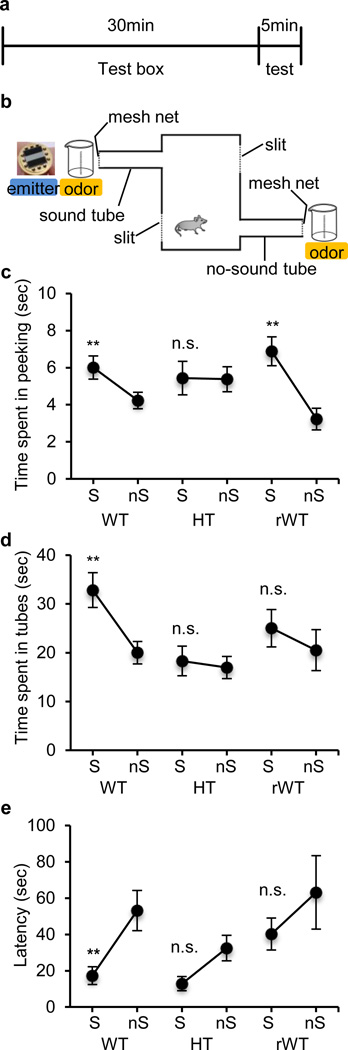
To date, the functional impact of typical and atypical call structures on maternal behavior has not been demonstrated experimentally in mouse models of ASD. Here, we used lactating C57BL/6J mothers 5–7 days postpartum to assess their response to the representative call sequences of Tbx1 wild-type and heterozygous pups (see Supplementary Figure S5) on two consecutive days for 5-minute testing periods (Figure 3ab)19,31. C57BL/6J mothers spent more time peeking into the tube in which wild-calls were played back (i.e., sound tube) rather than in the tube in which no sound was emitted (no sound tube) (Figure 3c, WT); the mothers also spent more time staying at the end of the sound tube than at the end of the no sound tube (Figure 3d, WT) and approached the sound tube more quickly than the no-sound tube (Figure 3e, WT). Heterozygous calls did not induce such a preference for the sound tube compared to the no-sound tube in terms of any of these parameters (Figure 3c,d,e, HT).
Figure 3. Maternal approach.
a) On two consecutive days, following a 30-min habituation period, we administered a 5-min test to each mother. b) In the experimental apparatus, slits were placed on the facing walls of the tubes to prevent reverberation of sound in the open area. The bedding odor of mothers’ own pups was placed at the end of both tubes. Time spent peeking at the entrance of (c), time spent at the end of (d), and latency to enter (e) the sound tube (S) and no-sound tube (nS). WT, wild-type calls; HT, heterozygous calls; rWT, randomized wild-type calls. Both wild-type call groups used with heterozygous calls and with randomized wild-type calls were combined for analysis, as they did not differ in peeking time (P = 0.315), time spent at the end of the tubes (P = 0.125) and latency to the first entry (P = 0.930). The time spent in the sound tube and no-sound tube was significantly different at 5% (*) and 1 % (**) levels, as determined by Wilcoxon Signed Rank test. N = 11 C57BL/6J mothers for a pair of wild-type calls (WT) and heterozygous calls (HT) and N = 13 for a pair of the original wild-type calls and randomized wild-type calls. One HT call data point and one random WT call data point were not recorded due to malfunction of the speaker.
To evaluate the significance of call sequences rather than mere presence of various call types of the wild-type pup, we randomized the original sequences of the representative wild-type pup used above in 100 different ways and chose a series of sequences that least resembled the original call sequences (Supplementary Figure S6). When the randomized wild-type sequences were presented, mothers spent more time in peeking at the sound tune than the no-sound tune (Figure 3c, rWT). However, mothers did not show a preference for the sound tube compared to the no-sound tube in terms of time spent exploring at the end of the tubes (Figure 3d, rWT) or latency to enter the sound tube (Figure 3e, rWT). Given that the original wild-type calls and randomized wild-type calls were identical in terms of the number of the 10 call types, inter-call intervals, and their amplitude, but differ only in sequence of the various call types, our data indicate that call sequence is a more critical determinant for how much time -and how quickly-- mothers approached the call source than the call wave types present. Moreover, our observation that heterozygous calls did not induce an orienting response or preference, indicate that this ASD risk gene renders the call sequence less effective in eliciting maternal approach.
Discussion
Using a genetic mouse model of ASD, we identified early atypicality and inflexibility in call sequences and a negative functional effect of such a pup phenotype on maternal approach. A genetic ASD risk factor influences, via its carrier’s atypical pup call sequences, the level of maternal care. The developmental trajectory of ASD is likely to be influenced by such a self-generated environmental factor in social communication, as well as accidental environmental factors.
The various call types have been analyzed in some mouse models of ASD-associated genetic variants, including Tsc132, Tbx125, Shank233, Fmr126, 16p11.2 CNV34 and Cntnap235. A novel observation of our sPLS-DA analysis is that call sequences are remarkably less varied among individual pups of a Tbx1 mutant model of ASD compared to individual control pups (see Figure 2e). This inflexibility can be considered very early atypicality in this mouse model of ASD. Inflexibility is considered a cardinal sign of ASD; for instance, preclinical studies have modeled this dimensional feature in memory in spontaneous alternation25 and reversal of various learned behaviors36. Our analysis revealed that inflexibility appears in early neonatal call sequences, as well.
Previous studies demonstrated that sequence structures exist among various call types in other mouse models of ASD33,35, and that when played back, mouse calls elicit maternal approach in inbred and other mouse strains19,31,37. The most salient aspects of our observation are that the sequence of call types is a functional determinant for maternal approach and the sequence structure is functionally disrupted in one genetic mouse model of ASD. We implemented two experimental procedures to control for factors other than the sequence structure of pup calls. First, we used C57BL/6J mothers, and these were not the dams of Tbx1 wild-type and mutant pups whose calls were analyzed and used. Thus, mothers’ approach was not determined by familiarity of the pup calls, as the mother had never been exposed to wild-type and heterozygous pup calls. Second, we used a randomized wild-type call sequence to determine the functional importance of the sequence structure; this ensured that we were not simply analyzing the effect of the number, duration and amplitude of various call types and inter-call intervals of wild-type calls, as these parameters were not changed in the randomized wild-type sequence. Given that the randomized wild-type sequence was ineffective in eliciting maternal approach, this control experiment rules out the possibility that mothers approached wild-type pup calls but not heterozygous pup calls simply because the former call types were similar to her own C57BL/6J pups’ calls; even when all familiar calls were used, if the sequence structure was randomized and broken, mothers didn’t approach such calls.
Our observation also suggested the precise aspects of maternal approach that are affected by call contents and call sequences. While mothers peeked at the sound tube more frequently than the no-sound tube when wild-type calls were presented, they also did so in response to randomized wild-type calls, but not to heterozygous calls; in contrast, wild-type calls were more efficient than heterozygous or randomized wild-type calls in terms of the time mothers spent at the end of the tube in the closest vicinity to the emitter and the latency to enter the sound tube. This dissociation suggests that the wild-type call sequence was critical for the motivational aspect of maternal approach, but not for the orienting peeking response to the sound and that the call type content, rather than their sequence, might be a trigger for an initial orienting response. The idea that call type contents are a determinant for an initial orienting response is consistent with previous studies that showed that mothers initiate approach (similar to our peeking measure) toward some artificially-generated ultrasonic sounds that lack natural sequence38–40. However, our study cannot be compared to those previous studies due to several procedural differences. Those studies used a test apparatus in which the mother stayed with own pups in a nest, and the response was measured as departure away from her pups in a nest to explore a distant sound source; it remains unclear if such a response reflects --or includes-- an alert and defensive behaviors (e.g., risk assessment and defensive threat), rather than maternal approach. More work is needed to evaluate the possibility that the call sequence and contents are determinants for motivational and orienting aspects of maternal care in other mouse models of ASD.
One methodological, and consequently interpretative, limitation of this observation is that we used a single representative mouse for each genotype to test impact on maternal approach. We objectively defined the representative call sequences as those that most closely resemble the group average as determined by the proportion of the ten call types and by call-to-call connections. This methodological choice was needed for two reasons. First, our pilot study indicated that calls from many pups cannot be used for each mother, as mothers show a rapid habituation to calls presented (see also41). Second, our pilot study showed that 10 or more mothers were needed to achieve statistically reliable data, because there is a certain degree of inherent variability in approach responses among individual mothers. As our method is the only practical strategy, the same experiment should be conducted in many other mouse models of ASD to critically evaluate the validity and generality of our observation.
Pup calls are induced by maternal separation, through many inseparable intermediate factors such as a drop in body temperature and lack of contact with and smell of mother42–44. ASD risk genes could affect normal vocal call production by altering perception of environmental stimuli (e.g., ambient temperature and mother’s presence or absence), neuronal systems for arousal, motivation and emotion, and motor production of vocalization9,41; phenotypic differences in body weight and neonatal vocal calls are not consistently correlated with each other, however34,45–47. The precise underlying mechanisms for atypical call sequences in Tbx1 heterozygous pups remain unknown. It is, however, unlikely that Tbx1 heterozygous pups emit atypical call sequences entirely due to anatomical abnormalities. First, Tbx1 heterozygous pups, unlike homozygous pups, do not have cleft palate30 or abnormality in the nucleus ambiguous (the origin of the vagus nerve), which controls the larynx48. Second, Tbx1 heterozygous pups are capable of emitting all call types with a normal pitch and amplitude, but simply emit fewer, shorter calls (see Figure 1a and Supplementary Figure S1). A future challenge is to identify the precise mechanisms, among numerous possible factors, through which an ASD risk gene alters vocal sequences in this and other mouse models of ASD.
Postnatal days 8 and 12 correspond to the new born human baby (i.e., term infant) in terms of many developmental milestones in the brain; while pups do emit vocal calls when separated from mother at earlier postnatal days, those murine postnatal days correspond to the pre-term human infant28. Interestingly, Tbx1 wild-type and heterozygous pups differ in their calls at P8, but not at P12. Given that the atypical call sequences found at P8 alter maternal approach, this could represent a critical period during which early atypicality in vocalizations affects the later developmental course of ASD through social communication between pups and mothers.
The precise sequence structure of various cry sounds is not well understood in humans, but human infant crying contains a graded, quantitative variation (i.e., melody)7,49 and acoustic characteristics are atypical in many pathological conditions7. In both humans and mice, crying appears without any auditory feedback12–15. Across cultures, infant crying increases and peaks at about age 6 weeks followed by a gradual decline until 3–4 months7. Similarly, mouse pups increase ultrasonic vocal calls during the first week and decreases such calls thereafter. Maternal responses are also similarly affected by atypical cries in humans. Mothers negatively perceive atypical cries of incipient ASD infants and respond verbally rather than with tactile or vestibular stimulation16,17. However, the precise causal role of atypical infant cries in maternal responses has been difficult to isolate in humans, as they are only one aspect of many signs babies exhibit7. Our data showed that functional consequences of atypical crying in human infants can be experimentally isolated and modeled in mouse pups to identify the causative functional role of atypical vocalizations in social communication in ASD and many other pathological conditions. A future challenge is to understand how different vocal sequences are processed in the brains of mothers.
A corollary of our finding is that caregivers’ improved understanding of atypical vocalizations in babies with incipient ASD might be an entry point for effective therapeutic intervention. This interpretation is consistent with a clinical observation that individualized parent coaching is highly effective in improving social communication, adaptive behaviors and developmental level in children with ASD3. Moreover, as mothers’ responses to atypical vocalizations of incipient ASD babies are likely to individually vary7, such variation might be one of the reasons why symptomatic severity worsens or normalizes among babies with ASD risk50–52.
Supplementary Material
Acknowledgements
We thank Dr. Bernice Morrow for providing us with the original line of Tbx1 heterozygous breeders. This work was supported by the NIH (HD053114 and MH099660), a NARSAD Independent Investigator Award to N.H. and a Maltz Foundation award to N.H., a Grant-in-Aid for Scientific Research on Innovative Areas (No. 4501) from the Japan Society for the Promotion of Science, in Japan to T.K.; M.B. was supported by the NIH (DC007690) award to J.L.P.
Footnotes
Author Contributions:
TT, AN, SA and NH contributed to the overall design and execution of experiments and analyses. TT, SO, PÓ, AN, KY, MVB, JLP, AG, TK and NH wrote the manuscript. GK and AN recorded pup vocalization and annotated call types. TT, TI and AN constructed all data files that were used for analyses. KY applied PLS-DA analysis to the proportion of vocal call types. PÓ and AG determined the sequence structure of vocal calls using sPLS-DA and entropy analyses. MVB and JLP analyzed call sequences using Markov chains. SO, AM and TK conducted the maternal approach experiment.
Conflict of Interest.
We declare that there is no competing financial interests regarding this paper by myself or my coauthors.
Supplementary information is available at Molecular Psychiatry’s website
References
- 1.Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, et al. Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial. Lancet Psychiatry. 2015;2:133–140. doi: 10.1016/S2215-0366(14)00091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord. 2014;44:2981–2995. doi: 10.1007/s10803-014-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wetherby AM, Guthrie W, Woods J, Schatschneider C, Holland RD, Morgan L, et al. Parent-implemented social intervention for toddlers with autism: an RCT. Pediatrics. 2014;134:1084–1093. doi: 10.1542/peds.2014-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- 6.Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. J Autism Dev Disord. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- 7.Soltis J. The signal functions of early infant crying. Behav Brain Sci. 2004;27:443–458. [PubMed] [Google Scholar]
- 8.Zeifman DM. An ethological analysis of human infant crying: answering Tinbergen's four questions. Dev Psychobiol. 2001;39:265–285. doi: 10.1002/dev.1005. [DOI] [PubMed] [Google Scholar]
- 9.Arriaga G, Jarvis ED. Mouse vocal communication system: are ultrasounds learned or innate? Brain Lang. 2013;124:96–116. doi: 10.1016/j.bandl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav. 2011;10:17–27. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portfors CV, Perkel DJ. The role of ultrasonic vocalizations in mouse communication. Curr Opin Neurobiol. 2014;28:115–120. doi: 10.1016/j.conb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brors D, Hansen S, Mlynski R, Volkenstein S, Aletsee C, Sendtner M, et al. Spiral ganglion outgrowth and hearing development in p75-deficient mice. Audiol Neurootol. 2008;13:388–395. doi: 10.1159/000148202. [DOI] [PubMed] [Google Scholar]
- 13.Hammerschmidt K, Reisinger E, Westekemper K, Ehrenreich L, Strenzke N, Fischer J. Mice do not require auditory input for the normal development of their ultrasonic vocalizations. BMC Neurosci. 2012;13:40-. doi: 10.1186/1471-2202-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheiner E, Fischer J. Emotion Expression: The Evolutionary Heritage in the Human Voice. 2011:105–129. [Google Scholar]
- 15.Volkenstein S, Brors D, Hansen S, Berend A, Mlynski R, Aletsee C, et al. Auditory development in progressive motor neuronopathy mouse mutants. Neurosci Lett. 2009;465:45–49. doi: 10.1016/j.neulet.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Esposito G, Venuti P. How is crying perceived in children with Autistic Spectrum Disorder. Research in Autism Spectrum Disorders. 2008;2:371–384. [Google Scholar]
- 17.Esposito G, Venuti P. Comparative analysis of crying in children with autism, developmental delays, and typical development. Focus on Autism and Other Developmental Disabilities. 2009;24:240–247. [Google Scholar]
- 18.Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, et al. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okabe S, Nagasawa M, Kihara T, Kato M, Harada T, Koshida N, et al. Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behav Neurosci. 2013;127:432–438. doi: 10.1037/a0032395. [DOI] [PubMed] [Google Scholar]
- 20.Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy Number Variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry. 2013;18:1153–1165. doi: 10.1038/mp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree MB, et al. Psychiatric Disorders From Childhood to Adulthood in 22q11.2 Deletion Syndrome: Results From the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171:627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong W, Gottlieb S, Collins J, Blescia A, Dietz H, Goldmuntz E, et al. Mutation analysis of TBX1 in non-deleted patients with features of DGS/VCFS or isolated cardiovascular defects. J Med Genet. 2001;38:E45-. doi: 10.1136/jmg.38.12.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata T, Niihori T, Tanaka N, Kawai M, Nagashima T, Funayama R, et al. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One. 2014;9:e91598-. doi: 10.1371/journal.pone.0091598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci U S A. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiramoto T, Kang G, Suzuki G, Satoh Y, Kucherlapati R, Watanabe Y, et al. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum Mol Genet. 2011;20:4775–4785. doi: 10.1093/hmg/ddr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai JK, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA. Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res. 2014;259:119–130. doi: 10.1016/j.bbr.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 27.Kihara T, Harada T, Kato M, Nakano K, Murakami O, Kikusui T, et al. Reproduction of mouse-pup ultrasonic vocalzations by nanocrystalline silicon thermoacoustic emitter. Applied Physics Letters. 2006;88:1–3. [Google Scholar]
- 28.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067-. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, et al. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- 31.Uematsu A, Kikusui T, Kihara T, Harada T, Kato M, Nakano K, et al. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res. 2007;1163:91–99. doi: 10.1016/j.brainres.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 32.Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci U S A. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ey E, Torquet N, Le Sourd AM, Leblond CS, Boeckers TM, Faure P, et al. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res. 2013;256:677–689. doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Yang M, Mahrt EJ, Lewis F, Foley G, Portmann T, Dolmetsch RE, et al. 16p11.2 Deletion Syndrome Mice Display Sensory and Ultrasonic Vocalization Deficits During Social Interactions. Autism Res. 2015 doi: 10.1002/aur.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burkett ZD, Day NF, Penagarikano O, Geschwind DH, White SA. VoICE: A semi-automated pipeline for standardizing vocal analysis across models. Sci Rep. 2015;5:10237-. doi: 10.1038/srep10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brigman JL, Graybeal C, Holmes A. Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front Neurosci. 2010;4 doi: 10.3389/neuro.01.013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410-. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- 38.Ehret G, Haack B. Categorical perception of mouse pup ultrasound by lactating females. Naturwissenschaften. 1981;68:208–209. doi: 10.1007/BF01047208. [DOI] [PubMed] [Google Scholar]
- 39.Ehret G. Categorical perception of mouse-pup ultrasounds in the temporal domain. Anim Behav. 1992;43:409–416. [Google Scholar]
- 40.Ehret G, Haack B. Ultrasonic recognition in house mice: key-stimulus configuration and recognition mechanism. J Comp Physiol. 1982;148:245–251. [Google Scholar]
- 41.Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic 'songs' with approach behaviour. Biol Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehret G. Infant rodent ultrasounds -- a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- 43.Blumberg MS, Sokoloff G. Do infant rats cry? Psychol Rev. 2001;108:83–95. doi: 10.1037/0033-295x.108.1.83. [DOI] [PubMed] [Google Scholar]
- 44.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 45.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 46.Roy S, Watkins N, Heck D. Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One. 2012;7:e44816-. doi: 10.1371/journal.pone.0044816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spruijt NE, Rana MS, Christoffels VM, Mink van der Molen AB. Exploring a neurogenic basis of velopharyngeal dysfunction in Tbx1 mutant mice: No difference in volumes of the nucleus ambiguus. Int J Pediatr Otorhinolaryngol. 2013;77:1002–1007. doi: 10.1016/j.ijporl.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Wermke K, Mende W, Manfredi C, Bruscaglioni P. Developmental aspects of infant's cry melody and formants. Med Eng Phys. 2002;24:501–514. doi: 10.1016/s1350-4533(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 50.Fountain C, Winter AS, Bearman PS. Six developmental trajectories characterize children with autism. Pediatrics. 2012;129:e1112–e1120. doi: 10.1542/peds.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gotham K, Pickles A, Lord C. Trajectories of autism severity in children using standardized ADOS scores. Pediatrics. 2012;130:e1278–e1284. doi: 10.1542/peds.2011-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szatmari P, Georgiades S, Duku E, Bennett TA, Bryson S, Fombonne E, et al. Developmental Trajectories of Symptom Severity and Adaptive Functioning in an Inception Cohort of Preschool Children With Autism Spectrum Disorder. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2463. Online January 28, 2015: Text Si- [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.