Abstract
Poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) micelles and poly(D,L-lactic-co-glycolic acid)-block-polyethylene glycol)-block-poly(D,L-lactic-co-glycolic acid) (PLGA-b-PEG-b-PLGA) sol-gels have been extensively researched for systemic and localized drug delivery applications, respectively, and they have both progressed into humans for paclitaxel, an important yet poorly water-soluble chemotherapeutic agent. In this review article, preclinical and clinical research on PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels that has focused on paclitaxel will be updated, and recent research on other poorly water-soluble anticancer agents and delivery of drug combinations (i.e. multi-drug delivery) that seeks synergistic anticancer efficacy will be summarized. PEG-b-PLA micelles are a first-generation platform for the systemic multi-delivery of poorly water soluble anticancer agents. PLGA-b-PEG-b-PLGA sol-gels are a first-generation platform for the localized multi-drug delivery of water-soluble and/or poorly water-soluble anticancer agents. In summary, PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels may safely enable pre-clinical evaluation and clinical translation of poorly water-soluble anticancer agents, especially for promising, rapidly emerging anticancer combinations.
Keywords: Block copolymer, controlled release, drug combination, drug solubilization, hydrogels, polymeric micelles, prodrugs
Graphical Abstract
1. Introduction
Anticancer drug combination are commonplace in cancer treatment, and the pace of research on novel drug combinations will likely grow moving from chemotherapy combinations towards more rational drug combinations of chemotherapy and so-called targeted agents, targeted agent combinations, and even combinations that involve compelling anticancer immunotherapies [1, 2]. While drug combinations are changing, primary goals remain the same: Synergy, non-overlapping toxicity, and overcoming drug resistance. Drug combinations require formidable pre-clinical testing, validation and prioritization prior to clinical trials [3]. The Food and Drug Administration (FDA) in the USA has recognized the significance of emerging and innovative anticancer combinations, and it has drafted a guidance on the development of two or more novel anticancer agents in a single development program, termed co-development [4].
While target-centric research has received a lot of attention in anticancer drug development, opportunities in the delivery of drug combinations, i.e. multi-drug delivery, have been largely overlooked and not appreciated in the scope of gaining synergy. Are there safe and simple ways to deliver poorly water-soluble anticancer drug combinations? Do drug combinations reach solid tumors at effective levels without widespread non-target distribution? Can we take advantage of the enhanced permeability and retention (EPR) effect for drug combinations, and in this context is tumor drug ratio important? While often not appreciated, novel concepts and drug delivery systems are emerging that satisfy requirements in solubility, safety and scale-up and may permit advances in drug delivery that move beyond the EPR effect, e.g. tumor priming, ratiometric dosing [5–7].
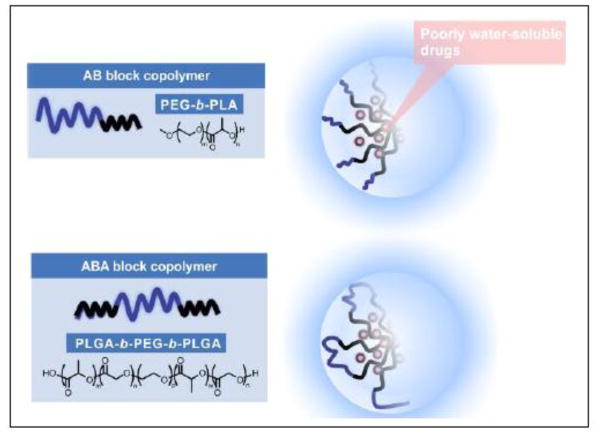
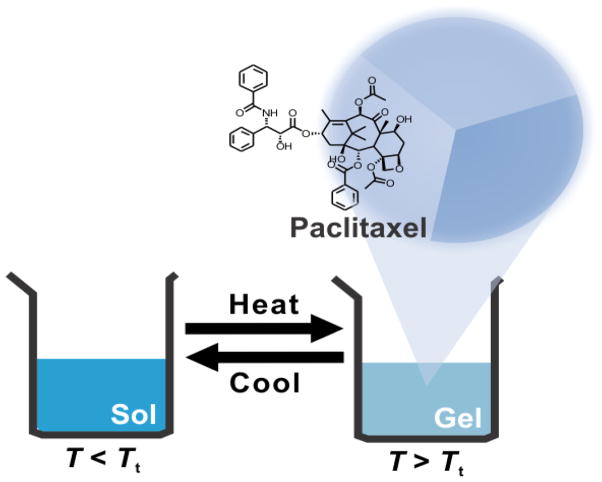
Bock copolymers based on poly(ethylene glycol (PEG) and poly(α-hydroxy acid) such as poly(D,L-lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) are being studied for drug delivery, owing to their biocompatibility, controlled biodegradability of poly(α-hydroxy acid), relative ease of polymer synthesis and preparation of a variety of nano- to macro-scale forms and sizes: Polymeric micelles, nanoparticles, polymersomes, and sol-gels [8–11]. Block copolymers based on PEG and poly(α-hydroxy acid) have been studied for the delivery of hydrophobic and hydrophilic drugs; in the former case, poorly water-soluble paclitaxel, has been widely studied, and there is an approved product based on poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) micelles in Asia (Figure 1). In the latter case, feasibility of controlled release of insulin from a poly(D,L-lactic-co-glycolic acid)-block-polyethylene glycol)-block-poly(D,L-lactic-co-glycolic acid) (PLGA-b-PEG-b-PLGA) sol-gel has been established (Figure 1).
Figure 1.
PEG-b-PLA and PLGA-b-PEG-b-PLGA block copolymers for drug delivery. Both assemble into micelles for drug solubilization. The latter micelles exist with PEG loops in the shell region, and they form a controlled release gel at above the sol-gel transition temperature.
Block copolymers based on PEG and poly(α-hydroxy acid) have been studied for systemic and local drug delivery, and with the advent of the field of nanomedicine, they have played a central role in drug targeting efforts. Local or regional drug delivery is increasingly gained attention, and block copolymers based on PEG and poly(α-hydroxy acid) have potential for localized cancers, such as brain, ovarian and esophageal cancers. Bock copolymers based on PEG and poly(α-hydroxy acid) may be used for the delivery of drug combinations (i.e. multi-drug delivery), and recent efforts in multi-drug delivery have been the subject of compelling review articles that summarize synthetic strategies, physicochemical characterization, controlled release mechanisms and pre-clinical and clinical studies [12–15].
The aim of this review article is to summarize recent progress on PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for systemic and local drug delivery, respectively, and describe efforts in multi-drug delivery. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels have been tested extensively in humans and have proven safety profiles and proven to be amendable to scale-up for human clinical trials. Because PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels have been reviewed nicely elsewhere [8, 11], emphasis will be placed on newly tested anticancer agents and clinical developments, especially for paclitaxel. In addition, recent research has revealed that PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels have a unique capacity for multiple poorly water-soluble anticancer agents, offering a new perspective on delivery of drug combinations. This work will be discussed in the context of drug delivery research that seeks to seamlessly and safely translate novel drug combinations into animal models and ultimately into humans. Lastly, research efforts on PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels that pursue novel drug delivery strategies will be discussed with the goal of showing the feasibility of drug delivery beyond the scope of current paradigms in drug solubilization and local sustained release effects.
2. PEG-b-PLA micelles
Many review articles over the years have described polymeric micelles for drug delivery and have charted progress into clinical trials [8, 14, 15–20]. Most block copolymers have a hydrophilic block that is PEG and hydrophobic blocks are commonly poly(propylene glycol), poly(α-amino acid) or poly(α-hydroxy acid), providing structural variation for chemical (prodrug) and physical drug loading strategies. The first description of PEG-b-PLA dispersions can be found in a European patent application by Churchill and Huchinson in 1985 [21]. Interestingly, it portends ensuing research on PEG-b-PLA micelles for drug delivery of poorly water drugs: In this patent application, they described block copolymers that possess a hydrophobic, biodegradable block and a hydrophilic block that may or may not be biodegradable and that are rapidly self-dispersible in water, which is indicative of polymeric micelles. In example 1, they described an AB block copolymer that has a PEG at 5,900 g/mol and a PLA block at 75% by weight, and it was dissolved in glacial acetic acid and stirred vigorously with excess distilled water to produce an extremely fine dispersion. It could be freeze-dried and subsequently reconstituted with water to form a very fine dispersion. In example 6, it was shown that PEG-b-PLA dispersions (50% PLA by weight) could solubilize a poorly water-soluble antiestrogen, ICU 189150, which is suitable for injection. In claim 9, they described a process for the manufacture of a frozen, stable aqueous dispersion of a copolymer and a water-insoluble drug, characterized by dissolving the drug and the self-dispersible copolymer in a minimum amount of a water-miscible organic solvent, slowly adding an excess of water to the vigorously agitated solution to produce a fine, stable dispersion and then freezing the dispersion. In summary, results in this European patent application were the first to show drug solubilization by PEG-b-PLA dispersions as an alternative strategy for injection, moving beyond the scope of pH adjustment, cosolvents, and surfactants such as Cremophor EL.
2.1. PEG-b-PLA micelles for drug solubilization
Table 1 summarizes anticancer agents that have been incorporated in PEG-b-PLA micelles and fulfill the requirement of drug solubilization in water, along with associated drug targets [22–32]. These anticancer agents hit key targets in cancer: Microtubules, topoisomerase II, mammalian target of rapamycin (mTOR), heat shock protein 90 (Hsp90), androgen receptor (AR), X-chromosome-linked IAP (XIAP), histone deacetylase (HDAC), NAD(P)H dehydrogenase, quinone 1 (NQO1), heat shock protein 70 (Hsp70), and DNA polymerase. Ethanol, chemical modification, Cremophor EL, and DMSO have been used for the solubilization of these anticancer agents for pre-clinical and clinical evaluation as single agents and as drug combinations. For example, a combination of Taxol® and tanespimycin (17-AAG) was evaluated in a phase 1 clinical trial, using Cremophor EL and DMSO/lipid for paclitaxel and 17-AAG, respectively [33]. In contrast, a combination of bicalutamide and embelin using PEG-b-PLA micelles was tested against prostate cancer in vitro and in vivo, as an early example of alternative and safer multi-drug delivery [28].
Table 1.
Anticancer agent delivery by PEG-b-PLA micelles.
| Anticancer agent | Target | In vitro/in vivo | Reference |
|---|---|---|---|
| ICU 189150 | Estrogen receptor | In vitro | 21 |
| Doxorubicin | Topoisomerase II | In vitro | 22 |
| Paclitaxel | Microtubules | In vitro | 23 |
| Docetaxel | Microtubules | In vivo | 24 |
| Etoposide | Topoisomerase II | In vitro | 25 |
| Rapamycin | mTOR | In vitro | 26 |
| 17-AAG | Hsp70 | In vivo | 27 |
| Bicalutamide | AR | In vivo | 28 |
| Embelin | XIAP | In vivo | 28 |
| Suberoylanilide hydroxamic acid | HDAC | In vivo | 29 |
| β-lapachone | NQO1 | In vitro | 30 |
| Pifithrin-μ | Hsp70 | In vitro | Unpublished data |
| Sagopilone | Microtubules | In vitro | 31 |
| Thiocoraline | DNA polymerase | In vivo | 32 |
Drug loading methods for polymeric micelles have been described elsewhere [8]. PEG-b-PLA micelles have been loaded with poorly water-soluble anticancer agents by a solution-casting method akin to a process used for liposomes and by freeze-drying of PEG-b-PLA and anticancer agent in a water/tert-butanol mixture to form a solid that can be reconstituted with water. Because PEG-b-PLA have a hydrophilic fraction, f, > 0.5 [10], they may be loaded with anticancer agents as described and yield polymeric micelles instead of other supramolecular assemblies in water such as nanoparticles that require other methods of drug loading, e.g. emulsion-based method. When looking for compatibility between PLA and a poorly water-soluble anticancer agent, one can consider its size, structural similarity, similarity in polarity, and calculate solubility parameter values for anticancer agents and compare values with PLA (σt = 23.3 Mpa1/2), seeking to minimize the Flory-Huggins interaction parameter, χsc [34, 35]. This latter approach provides guidance, but has limitations built in the calculation of solubility parameters. Nonetheless, when successful, PEG-b-PLA micelles increase the water solubility of anticancer agents by two to three orders of magnitude, enabling > 1.0 mg/mL in water, which is often a requirement for pre-clinical studies on efficacy and toxicity in rodents and eventual testing in human beings.
2.2. Drug release from PEG-b-PLA micelles
In contrast to PEG-b-PLA nanoparticles, PEG-b-PLA micelles have a lower PLA molecular weight, tend to be smaller, less stable and have more rapid release of anticancer agent in blood by disassembly and/or diffusion [36–38]. When paclitaxel in PEG-b-PLA micelles was incubated in human plasma above the critical micelle concentration (CMC) of PEG-b-PLA in vitro, an equal amount of paclitaxel was found in lipoprotein and lipoprotein deficient fractions within 5 minutes, with 70–75% of paclitaxel associated with the high-density lipoprotein fraction [37]. Notably, this rapid distribution among serum proteins was similar to the profile of free paclitaxel, indicating rapid paclitaxel release from PEG-b-PLA micelles. In vivo Förster resonance energy transfer (FRET) imaging of DiIC18 and DiOC18 co-loaded in PEG-b-PLA micelles showed that FRET efficiency is significantly lost within 15 minutes after intravenous injection of mice, indicating rapid release of DiIC18 and DiOC18 from PEG-b-PLA micelles [38].
Owing to rapid drug release, PEG-b-PLA micelles did not significantly modify pharmacokinetic parameters of 17-AAG compared to a conventional formulation of ethanol-Cremophor EL-PEG400 (2:1:1), e.g., no significant difference in mean residence time in rats [27]. However, ethanol-Cremophor EL-PEG400 caused 35% mortality within 24 hours, whereas PEG-b-PLA micelles caused no deaths. Similarly, PEG-b-PLA micelles did not significantly modify pharmacokinetic parameters of docetaxel (Nonoxel-PM™) compared to a Taxotere® in mice, rats and beagle dogs [24]. However, Taxotere® caused hypersensitivity reactions and fluid retention in beagle dogs, whereas Nonoxel-PM™ has no such effects in beagle dogs. Interestingly, PEG-b-PLA micelles did significantly modify the pharmacokinetic parameters of vorinostat, an HDAC inhibitor, compared to a conventional formulation of PEG400 in rats [29]: Mean residence time increased by two orders of magnitude, dramatically increasing exposure of vorinostat. It is noted that the molecular weight of PEG and PLA was relatively high, ca. 5,000 and 2,000 g/mol, respectively, perhaps contributing to stability of PEG-b-PLA micelles in vivo. On the other hand, drug-polymer interaction may be inordinately strong in the case of vorinostat. Thus, while it is reasonable to expect minor changes in pharmacokinetics of anticancer agents due to PEG-b-PLA micelles relative to conventional formulations, pharmacokinetic experiments in rodents are justified and should be done as standard practice in the context of pre-clinical drug development as we gain more knowledge into the properties of PEG-b-PLA micelles in vivo.
While most studies suggest that drug release tends to be rapid for PEG-b-PLA micelles, there is strong evidence for long-circulating PEG-b-PLA micelles, and promising tactics to slow drug release for PEG-b-PLA micelles for drug targeting by the EPR effect have emerged [39–42].
For example, PEG-b-PLA was synthesized by anionic polymerization of ethylene oxide and D,L-lactide using 3,3-diethoxy-1-propanolate as an initiator [39]. PEG-b-PLA had PEG and PLA molecular weight at 5,100 and 5,300 g/mol, respectively, at low polydispersity, ca. 1.10, and it assembled into micelles with a hydrodynamic diameter of 33 nm. Radioiodinated PEG-b-PLA micelles circulated for a prolonged period in mice: 25% injected dose at 24 hours [39]. Integrity of PEG-b-PLA micelles in blood was verified by gel filtration chromatography of plasma samples taken from mice at 1 and 24 hours. PEG-b-PLA micelles with a slight negative surface charge due to a small peptidyl ligand (Tyr-Glu-) had lower uptake at the liver and spleen compared to neutral Tyr-conjugated PEG-b-PLA micelles.
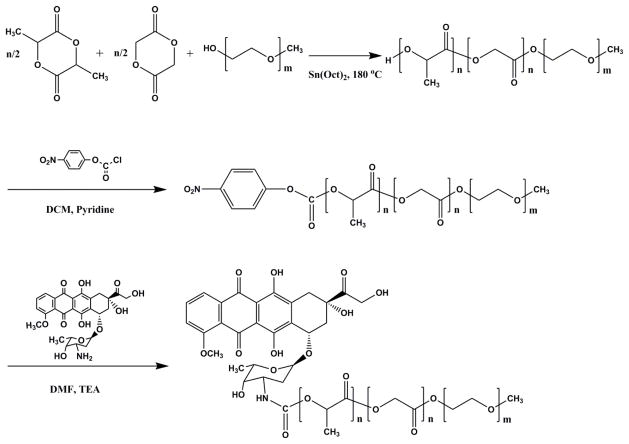
To enhance drug loading and slow drug release, doxorubicin has been coupled onto the terminal hydroxyl group of PEG-b-PLGA by a carbamate linkage (Figure 2), resulting in PEG-b-PLGA micelles that exhibit a sustained release profile of doxorubicin in comparison to PEG-b-PLGA micelles that contain physically loaded doxorubicin [40]. The sustained release profile of doxorubicin for “prodrug” PEG-b-PLGA micelles was attributed to the gradual hydrolysis of PLGA and gradual liberation of doxorubicin-PLGA oligomer conjugates in the incubation medium.
Figure 2.
Synthesis of a PEG-b-PLGA-doxorubicin conjugate (Reproduced with permission from reference 40).
A lipophilic diester prodrug of β-lapachone had higher loading of PEG-b-PLA micelles in comparison to parent β-lapachone, enabling drug loading content at ca. 10%, loading efficiency at >95% and water solubility >7 mg/mL [41]. Loading of β-lapachone was low, ca. 2%. It could be that the prodrug is more hydrophobic, favoring partitioning inside PEG-b-PLA micelles. PEG and PLA had molecular weights of 10,000 and 5,000 g/mol, respectively. Like most prodrugs, esterases convert the diester prodrug of β-lapachone into β-lapachone. In an A549 orthotopic lung cancer model, PEG-b-PLA micelles containing β-lap-dC3 at 50 mg/kg had a tumor area under the curve over the first 2 hours of 7.3±0.61 × 106 ng•min/g of β-lapachone, versus 1.4±0.85 × 106 ng•min/g of β-lapachone for a hydroxypropyl-β-cyclodextrin formulation of β-lapachone at maximum tolerated dose (MTD) of 25 mg/kg. As a result, antitumor efficacy of PEG-b-PLA micelles containing β-lap-dC3 significantly prolonged survival of mice in comparison to a hydroxypropyl-β-cyclodextrin formulation of β-lapachone.
In summary, while PEG-b-PLA micelles typically have minor pharmacokinetic influence on anticancer agents due to rapid drug release, there are exceptions, especially for prodrugs designed for stable integration in PEG-b-PLA micelles. Both options are available for cancer treatment: In the former case, PEG-b-PLA micelles with relatively low molecular weight PEG and PLA blocks might be sufficient for drug solubilization and for a log-kill, MTD strategy [42]. On the other hand, prodrug strategies involving PEG-b-PLA micelles with relatively high molecular weight PEG and PLA blocks and prodrugs may be considered for anticancer agents that may benefit from increasing the time of exposure as a method to maximize tumor cell killing.
2.3. Genexol-PM®
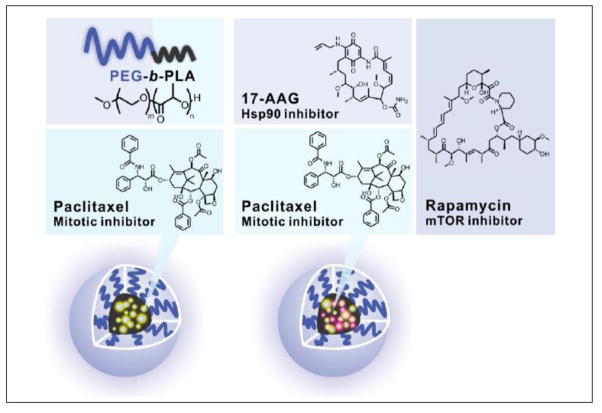
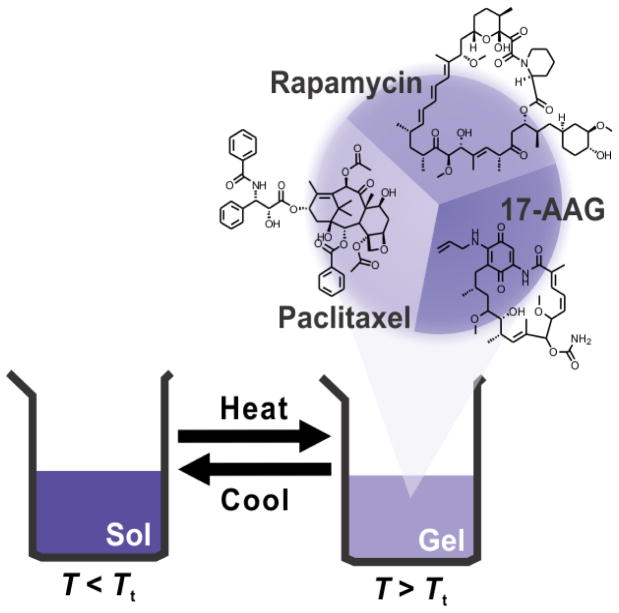
Genexol-PM® is an injectable formulation of paclitaxel based on PEG-b-PLA micelles that has advanced into humans (Figure 3), gaining approval in several Asian countries, and earlier reviews have summarized its properties in vitro and in vivo [14, 43, 44]. In brief, PEG and PLA in Genexol-PM® have molecular weights of 2,000 and 1,750 g/mol, respectively, and Genexol-PM® is a solid state formulation that is reconstituted with sterile water prior to IV infusion. Not surprisingly, Genexol-PM® does not increase the circulation time of paclitaxel, consistent with rapid release of paclitaxel from PEG-b-PLA micelles [45]. However, Genexol-PM® is less toxic than Taxol®, standard injectable formulation of paclitaxel that contains ethanol and Cremophor EL, permitting dose escalation of paclitaxel in pre-clinical studies: MTD in mice of 60 and 20 mg/kg for Genexol-PM® and Taxol®, respectively. As a result, Genexol-PM® was much more effective than Taxol® in ovarian and breast murine tumor models, justifying entry into human clinical trials [45].
Figure 3.
Genexol-PM® and Triolimus.
In clinical studies, MTD of Genexol-PM® was as high as 390 mg/m2, versus 175 mg/m2 for Taxol®, infused every 3-weeks [14, 43, 44]. As a result, Genexol-PM® had high response rates in clinical trials with patients with non-small cell lung, gastric and breast cancers, although antitumor efficacy was not as impressive as results observed in pre-clinical studies [14, 43, 44]. Major toxicities of Genexol-PM® include neutropenia, peripheral neurotoxicity and arthralgia, which are commonplace for Taxol®. These toxicities are known to be caused by paclitaxel. In clinical studies, cases of hypersensitivity reactions have emerged for Genexol-PM®, although seemingly less frequently than for Taxol®. For IV infusion, paclitaxel as Taxol® at a recommended dose of 175 mg/m2 requires ca. 25 mL of Cremophor EL, polyoxyethylated castor oil, resulting in toxic side effects, most notably acute hypersensitivity reactions that necessitate use of steroids and histamine antagonists [46]. Despite premedication, frequency of minor reactions are estimated at ca. 44%, and major reactions that necessitate discontinuation of treatment with Taxol® occur in 1.5 to 3% of patients. In summary, Genexol-PM® has high response rates in clinical trials with patients with non-small cell lung, gastric and breast cancers, and a similar dose-limiting toxicity profile as Taxol® despite a higher dose and reduced acute hypersensitivity reactions.
More recently, Genexol-PM® was licensed by a US company, Sorrento Therapeutics Inc., and they changed the name to Cynviloq™. Cynviloq™ entered a phase 3 bioequivalence study versus nab-paclitaxel (Abraxane®) [47]. Termed TRIBECA™ (TRIal establishing bioequivalence [BE] between Cynviloq™ and Albumin-bound (nab) paclitaxel), this comparative bioequivalence study was designed to be an open-label, randomized, multi-center, single dose, 2-sequence, 2-period, crossover, comparative bioequivalence study of Cynviloq™ and nab-paclitaxel at 260 mg/m2 infused intravenously with an open-label extension of Cynviloq™ in female patients with metastatic or locally recurrent breast cancer. This clinical trial will compare the pharmacokinetics of paclitaxel as Cynviloq™ and nab-paclitaxel, noting that both appear to be unstable in blood and rapidly release paclitaxel into systemic circulation [14, 43, 44, 48, 49]. Upon IV infusion of nab-paclitaxel into pigs at 300 or 900 mg/m2 over 30 minutes, no nanoparticles were detected in plasma by dynamic light scattering measurement at any time point post infusion, indicating rapid dissolution of amorphous paclitaxel in blood [49]. Nab-paclitaxel is approved for breast, non-small cell lung, and pancreatic cancers and it is in clinical trials for melanoma, ovarian and bladder cancers [50]. Thus, bioequivalence between Cynviloq™ and nab-paclitaxel could be a major milestone for paclitaxel-delivery systems and open the door to additional drug development involving PEG-b-PLA micelles as a viable alternative to nab technology for drug delivery.
2.4. PEG-b-PLA micelles for multi-drug delivery
While Cynviloq™ and nab-paclitaxel represent progress in drug delivery, they do not represent major breakthroughs in anticancer treatment, i.e. increased survival time of months to years. However, drug combinations may represent a promising way forward, especially with newer drug combinations that attack aberrant cancer cell signaling [1–4]. In this context, PEG-b-PLA micelles and nab technology may safely deliver drug combinations, considering drug solubility, drug ratio, and drug interaction. Thus, an anticancer agent in Table 1 can be formulated as a PEG-b-PLA micelle (i.e. 1-in-1 micelle) and mixed together with another 1-in-1 micelle, forming 2-in-2 micelle solutions for concurrent drug delivery. Alternatively, a pair of 1-in-1 micelles may be infused as a sequential drug combination in order to maximize synergy [28]. In either case, PEG-b-PLA micelles are an attractive option for drug combinations because of proven safety in humans, multiple examples of drug solubilization (Table 1) and established scale-up, e.g. Genexol-PM®.
PEG-b-PLA micelles also have the capacity for multi-drug solubilization, meaning that they can take up 2 or 3 anticancer agents inside the same micelle, i.e. 2-in-1 or 3-in-1 micelle [25, 26]. In this approach, anticancer drug combinations may be produced in a single formulation process as opposed to a separate formulation process for each individual anticancer agent. This may reduce cost and improve safety by omitting toxic excipients, such as Cremophor EL. Moreover, multi-drug containing PEG-b-PLA micelles as a single IV infusion simplifies drug administration versus sequential infusion of drug combinations that is commonplace in clinical practice. Important considerations for multi-drug containing PEG-b-PLA micelles are maintenance of drug solubility, achievement of synergistic fixed drug ratios and absence of major pharmacokinetic drug interactions. In addition, the goal of non-overlapping drug toxicity becomes a major consideration, given that drug combinations are dosed simultaneously. In summary, PEG-b-PLA micelles represent a drug delivery system for the safe and simplified delivery of drug combinations that merits consideration in drug development, particularly in cancer research where many anticancer agents are poorly water-soluble and where exciting drug combinations are emerging.
The first example of multi-drug containing PEG-b-PLA micelles involved paclitaxel, docetaxel, etoposide and 17-AAG [25]. At PEG and PLA molecular weights of 4,200 and 1,900 g/mol, respectively, PEG-b-PLA micelles individually increased the water solubility of paclitaxel, docetaxel, etoposide and 17-AAG to ca. 4 mg/mL, achieving ca. 10% weight drug/weight polymer. Notably, 2-in-1 PEG-b-PLA micelles involving 17-AAG had drug loading levels in PEG-b-PLA micelles achieved for the individual anticancer agents, e.g. water solubility for both paclitaxel and 17-AAG was ca. 4.0 mg/mL, achieving ca. 25% weight drug/weight polymer while the micelle diameter stayed at 40 nm. Similarly, 3-in-1 PEG-b-PLA micelles containing paclitaxel, etoposide and 17-AAG reached 3.5, 3.2 and 3.6 mg/mL, respectively, achieving ca. 36% weight drug/weight polymer while the micelle diameter stayed at 40 nm. The similarity in drug loading for the individual drugs in multi-drug containing PEG-b-PLA micelles and single-drug loaded PEG-b-PLA micelles is not well understood, but may reflect drug interaction in PEG-b-PLA micelles. Interestingly, 2-in-1 and 3-in-1 PEG-b-PLA micelles were physically stable at room temperature over 24 hours, showing no or little signs of precipitation. In contrast, paclitaxel, docetaxel and etoposide as 1-in-1 PEG-b-PLA micelles precipitated over 24 hours, indicating an absence of thermodynamic stability.
2.5. Triolimus
PEG-b-PLA micelles have enabled a novel 3-drug nanotherapeutic composed of paclitaxel, 17-AAG and rapamycin, termed Triolimus, enabling the investigation of combined anticancer action on microtubules, Hsp90 and mTOR, respectively (Figure 3) [26]. Inhibition of mTOR has been widely studied for cancer treatment, noting that the PI3K/Akt signaling pathway is aberrantly activated in many cancers and contributes to cellular resistance to chemotherapy [51, 52]. A recent phase 3, randomized, double-blind multicenter clinical trial on everolimus (mTOR inhibitor), paclitaxel and trastuzumab as a first-line treatment for patients with HER2-positive breast cancer (Bolero-1) is noteworthy [53]; although progression-free survival was not significantly different between treatment groups, a 7.2 month prolongation of progression-free survival was noted with the addition of everolimus to hormone receptor-negative, HER2-positive breast cancer patients. It is noted that in this clinical trial that Cremophor EL was used to solubilize paclitaxel (Taxol®) for IV infusion.
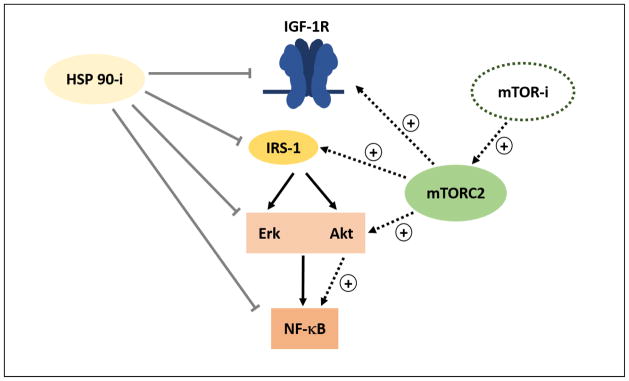
The rationale for combining 17-AAG and rapamycin is shown in Figure 4. Inhibition of mTOR leads to a paradoxical activation of Akt, Ras/MAPK signaling pathway and NFκB and therefore drug resistance [51, 52]. Hsp90 plays a central role in the folding, stability and function of multiple oncogenic proteins, and Hsp90 inhibition essentially interferes with multiple signaling pathways involved in mTOR resistance: IGF-1 receptor, IRS-1, Akt, Raf, NF-κB and HER2. Thus, inhibition of Hsp90 may enhance anti-neoplastic as well as the antiangiogenic activity of mTOR inhibitors. In addition, Hsp90 inhibitors themselves exert potent anti-neoplastic activity; for example, 17-AAG in combination with trastuzumab showed promising anticancer activity in a phase 2 clinical trial for patients with HER2-positive metastatic breast cancer [54]. It is noted that in this clinical trial that Cremophor EL was used to solubilize 17-AAG for IV infusion.
Figure 4.
Combined inhibition of mTOR and Hsp90 by rapamycin and 17-AAG, respectively. Inhibition of Hsp90 blocks oncogenic feedback loops that are associated with mTOR resistance, targeting insulin-like growth factor-2 receptor (IGF-1R), Akt, Erk and NF-κB. Both rapamycin and 17-AAG have potent anticancer and anti-angiogenic activity on their own right (Reproduced with permission from reference 51).
Triolimus consists of a PEG-b-PLA micelle with PEG and PLA molecular weights at 4,200 and 1,900 g/mol, respectively, and paclitaxel, 17-AAG and rapamycin at 15, 16, and 9.0% weight drug/weight polymer, respectively, achieving 3.4, 3.9 and 2.1 mg/mL in water as a 44 nm diameter micelle [26]. Paclitaxel, 17-AAG and rapamycin displayed synergistic cytotoxicity against breast cancer cell lines (MCF-7, 4T1, MDA-MB-231) and an A549 non-small cell lung cancer cell line based on combination index (CI) analysis [26, 55]. For example, IC50 values for paclitaxel, 17-AAG and rapamycin as a 1-in-1 PEG-b-PLA micelle were 11,160±4160, 118±10 and >100,000 nM, respectively, for 4T1 murine breast cancer cells. For 2-in-1 PEG-b-PLA micelles, IC50 values for paclitaxel and 17-AAG (4.7:1 mol ratio), paclitaxel and rapamycin (1:1 mol ratio) and 17-AAG and rapamycin (1:1 mol ratio) were 92±19, 4010±3610 and 147±11 nM, respectively. Triolimus had the lowest IC50 value at 25±1 nM, and a CI value of 0.04±0.001, indicating synergistic anticancer activity.
Triolimus was dosed intravenously at 60, 60 and 30 mg/kg for paclitaxel, 17-AAG and rapamycin, respectively, in mice on days 0, 4 and 8 with <10% weight loss and no death. It is noted that the MTD of Genexol-PM® is 60 mg/kg in mice [45]. Triolimus at 60, 60 and 30 mg/kg strongly inhibited A549 and MDA-MB-231 tumor growth in comparison to paclitaxel-containing PEG-b-PLA micelles at 60 mg/kg, noting tumor cures in the orthotopic MDA-MB-231 tumor model [55]. Triolimus doubled tumor cell apoptosis and halved tumor cell proliferation in vivo in comparison with paclitaxel-containing PEG-b-PLA micelles based on immunohistochemical analysis.
Pharmacokinetic analysis of Triolimus at 60, 60 and 30 mg/kg in mice were consistent with earlier studies on PEG-b-PLA micelles that showed minor changes in pharmacokinetics of anticancer agents due to PEG-b-PLA micelles [56]: Plasma half-lives were 1.31, 0.60 and 7.68 hours for paclitaxel, 17-AAG and rapamycin, respectively based on reverse-phase HPLC analysis. However, there were notable, significant changes: Clearance of paclitaxel as Triolimus was slower than 1-in-1 paclitaxel PEG-b-PLA micelles (60 mg/kg): 0.25±0.02 versus 0.42± 0.007 L/h/kg. Genexol-PM® at 50 mg/kg in mice had a clearance value of 0.72 L/h/kg [45]. In addition, paclitaxel and rapamycin had 1.7- and 1.6-fold higher plasma area under the curves in comparison to 1-in-1 PEG-b-PLA micelles, whereas there was no significant change for 17-AAG [56]. The increased plasma area under the curves for paclitaxel and rapamycin may reflect slowed release of paclitaxel from 3-in-1 PEG-b-PLA micelles versus the 1-in-1 PEG-b-PLA micelle at early time points and preferential metabolism of 17-AAG by cytochrome P450 3A4 (CYP3A4) over rapamycin, respectively [26], noting that the in vitro release of paclitaxel was rapid and lead to precipitation in ca. 1 hour, whereas the t1/2 for paclitaxel release from 3-in-1 PEG-b-PLA micelles was 9.2 hours. Lastly, preferential metabolism of 17-AAG by CYP3A4 over rapamycin produced 17-amino-17-demethoxygeldanamycin, a bioactive metabolite capable of Hsp90 inhibition.
In sequential drug delivery, Genexol-PM® and Triolimus may act as a substitute for Taxol® for “tumor priming,” causing tumor cell apoptosis, reduction in tumor cell density and enhanced intratumoral accumulation of secondarily administered nanoparticles [6, 57]. In a subcutaneous human pharynx FaDu xenograft tumor model, intravenous Taxol® at 40 mg/kg induced ≥10% apoptosis in solid tumors over 24 to 96 hours, with maximal apoptosis over 48 to 72 hours and without significant apoptosis in normal tissues [57]. This is consistent with results in patients undergoing neoadjuvant therapy by Taxol®, where serial fine-needle aspiration showed that apoptosis subsides after 4 days [58]. 100- and 200-nm diameter fluorescent nanoparticles injected 48 hours after Taxol® had significantly increased intratumoral accumulation: 1.73- and 1.74-fold increase in average nanoparticle concentration at tumor, respectively. In contrast, there was no tumor priming for 500 nm diameter fluorescent nanoparticles. Tumor priming by Taxol® at 40 mg/kg increased intratumoral accumulation of liposomal doxorubicin (diameter ca. 85 nm) by 1.4-fold in comparison to liposomal doxorubicin alone, based upon measurement of area under the curve, whereas there were no changes for normal tissues. As a result, tumor priming of liposomal doxorubicin by Taxol® showed the highest therapeutic response based on tumor regression and survival in comparison to liposomal doxorubicin or sequential injection of liposomal doxorubicin followed by Taxol®. While effective, it is noted that tumor priming of liposomal doxorubicin by Taxol® caused serious acute toxicity: >20% weight loss of mice.
Tumor priming by Triolimus has been characterized in a subcutaneous human colon LS180 xenograft tumor model by ex vivo near-infrared optical imaging [59]. A single injection of Triolimus at 60, 60 and 30 mg/kg of paclitaxel, 17-AAG and rapamycin, respectively, caused a 1.6-fold reduction in tumor volume after 6 days, with <10% change in body weight and no death. 1-in-1 paclitaxel containing PEG-b-PLA micelle at 60 mg/kg had about the same tumor volume as the vehicle control, ca. 580 mm3. Tumor priming by Triolimus of 50-nm diameter PEG-b-poly(ε-caprolactone) micelles containing a near-infrared dye, DiR, injected 48 hours afterwards, resulted in a tumor-to-muscle ratio of 149±13, based on ex vivo optical imaging of DiR, whereas tumor priming by paclitaxel at 60 mg/kg resulted in a tumor-to-muscle ratio of 118±10. Vehicle control (targeting by the EPR effect) resulted in a tumor-to-muscle ratio of 68±3. Thus, ex vivo optical imaging of DiR showed a 2.1- and 1.7-fold increase in intratumoral DiR due to tumor priming by Triolimus and 1-in-1 paclitaxel containing PEG-b-PLA micelle, respectively. In summary, Triolimus displays potent antitumor activity in xenograft models and while not long-circulating in itself, it may be used for tumor priming, increasing intratumoral accumulation of secondarily injected nano-particles beyond the EPR effect for drug combination anticancer treatment.
3. PLGA-b-PEG-b-PLGA sol-gels
PLGA-b-PEG-b-PLGA is a thermo-sensitive ABA block copolymer that reversibly transitions into a gel at body temperature for drug delivery [11, 60]. Hydrophilic and hydrophobic drugs can be loaded into PLGA-b-PEG-b-PLGA in a sol state and injected into a diseased site, whereupon it will form a gel that gradually releases drug over a prolonged time period and eventually biodegrades into non-toxic by-products. While PLGA-b-PEG-b-PLGA sol-gels have been studied for systemic drug delivery, e.g. peptides after subcutaneous injection, by far the most significant clinical progress has been made in localized control of cancer as an adjunct to surgery and radiation [61, 62]. Surgical oncology remains the most important treatment option for cancer and the single most important predictor of patient survival, even with major research efforts in anticancer drug development and nanomedicine. Despite the curative potential of surgical resection for localized early stage cancer, undetected micrometastases and ill-defined tumor margins remain major challenges facing surgeons [61]. Thus, localized drug delivery by polymeric delivery systems as an adjunct to surgery, best exemplified by Gliadel® wafer for brain tumors, remains a viable and interesting strategy for cancer treatment. Besides placement in a surgically resected tumor cavity, PLGA-b-PEG-b-PLGA sol-gel could possibly be used in a neoadjuvant setting to reduce tumor burden and enhance tumor resection. Lastly, endoscopic ultrasound-guided injection of PLGA-b-PEG-b-PLGA sol-gel is an interesting drug delivery strategy and may be considered for local control of pancreatic cancer, particularly for patients with unresectable pancreatic cancer [63].
3.1. Regel®
PLGA-b-PEG-b-PLGA termed Regel® has been extensively studies for systemic and local drug delivery and has distinguished itself as a sol-gel by gaining entry into clinical trials, reaching phase 2b clinical trials for the local treatment of esophageal cancer. Pre-clinical and clinical development of Regel® and its paclitaxel product, Oncogel™ have been described elsewhere [60–62].
Briefly, Regel® can be synthesized with poly(ethylene glycol) as a macro-initiator for D,L-lactide and glycolide (3:1 mole ratio, using stannous 2-ethylhexanoate as a catalyst. PLGA-b-PEG-b-PLGA has weight-average molecular weight of 4,200 g/mol and polydispersity index of 1.3 [60]. The transition of PLGA-b-PEG-b-PLGA from a sol into a gel was studied at 15–23% weight polymer/weight water and occurred at ca. 20 °C, decreasing slightly with increased level of PLGA-b-PEG-b-PLGA. All existed as gels at 37 °C. Notably, the sol-gel transition depends on the relative molecular weight of PEG to PLGA of PLGA-b-PEG-b-PLGA and somewhat on the ratio of LA to GA [64]. Hydrophilic and hydrophobic drugs have been incorporated into Regel® [60]. Insulin, porcine growth hormone, granulocyte colony stimulating factor and recombinant hepatitis B surface antigen have been incorporated in Regel® and showed sustained release over 1 to 2 weeks in vitro. On the other hand, Regel® increased the water solubility of paclitaxel and cyclosporine A to ca.10 and 2 mg/mL as a sol at 5 °C, respectively, and in both cases increased chemical stability of the drugs as a gel at 37 °C in comparison to a water-acetonitrile solution.
Regel® (ca. 0.4 mL) at 23% weight polymer/weight water formed a palpable mass and retained its integrity as a gel over a 2-week period following subcutaneous injection in a rat, becoming progressively smaller over time. In the next two weeks, Regel® was a mixture of a gel in a viscous liquid and finally a viscous liquid that was completely resorbed after 4-weeks. Histological evaluation of Regel® after subcutaneous injection over 30 days showed that it causes minimal acute inflammatory reaction. Signs of chronic inflammation, i.e. granulation, foreign body giant cells and fibrous capsule formation were minimal. Histological observations for Regel® were similar to that observed for PLGA microspheres and resorbable suture products [60]. Following extensive pre-clinical safety tests, the FDA approved the entry of Regel® into clinical trials for the delivery of paclitaxel.
3.2. Oncogel™
Oncogel™ is Regel® at 23% weight polymer/weight water containing paclitaxel at 6.0 mg/mL (Figure 5). Oncogel™ releases paclitaxel over 50 days in vitro by diffusion over the first 2-weeks, followed by a combined diffusion and polymer degradation mechanism over the next few weeks. Intratumoral injection of Oncogel™ containing [14C]-paclitaxel showed that the drug clears slowly over a 6-week period with a half-life of 21 days. Minimal recovery of [14C]-paclitaxel was found in major organs, and the major route of elimination of [14C]-paclitaxel or its degradation products was through the feces (68% at day 42). Thus, systemic toxicity due to Oncogel™ was not expected in vivo, and this was confirmed in human clinical trials described below. A single intratumoral injection of Oncogel™ at 10 mg/kg in a MDA-MB-231 breast xenograft model was as effective paclitaxel as Taxol® at 100 mg/kg (20 mg/kg injected intravenously on 5 consecutive days) in terms of survival [60]. Oncogel™-treated mice did not show adverse effects, whereas Taxol®-treated mice showed weight loss and 2 deaths during dosing.
Figure 5.
Oncogel™.
Oncogel™ entered phase 2 clinical trials for the treatment of esophageal cancer based relative ease of access to tumors by endoscopic ultrasound-guided injection and visualization and potential for synergy in combination with radiation treatment [62, 65]. Oncogel™ was provided as ready-to-use single use prefilled glass syringes, stored at −20 °C and thawed at room temperature for 1 hour prior to injection. In a phase 2a multi-center clinical trial involving 11 patients, a dose-escalation study on Oncogel™ was carried out on patients with histologically confirmed adeno- or squamous cell carcinoma who were not candidates for surgery, but were scheduled to receive radiation treatment. Radiation is a common palliative treatment in esophageal cancer, and paclitaxel is a potent radiosensitizer. Oncogel™ at paclitaxel doses at 0.48, 1.0 and 2.0 mg/cm3 was administered by intratumoral injection at one-third of the total tumor volume and in some cases administered into endoscopically-accessible lymph nodes, followed 3 days later by radiation treatment (50.4 Gy as 28 fractions at 1.8 Gy). Tumor volume and toxicity, pharmacokinetics and antitumor efficacy were studied. Encouragingly, low systemic exposure of paclitaxel was found consistent with earlier rodent experiments, and adverse effects of radiation combined with Oncogel™ were not discerningly different than that of radiation treatment alone. Further, Oncogel™ plus radiation treatment seemed to reduce tumor burden, noting improvement in dysphagia (difficulty in swallowing). However, Oncogel™ subsequently failed in a phase 2b study on patients with advanced esophageal cancer, failing to enhance antitumor efficacy of pre-operative chemotherapy and radiation treatment prior to planned surgical resection. It may be that paclitaxel as a single agent is insufficient, and drug combinations may provide a way forward to enhance treatment of esophageal cancer. In summary, while endoscopic ultrasound-guided injection of Oncogel™ combined with radiation was not successful for esophageal cancer in a phase 2b clinical trial, safety and low systemic exposure of paclitaxel was confirmed, showing the feasibility of this strategy for local anticancer delivery of paclitaxel.
The feasibility of endoscopic ultrasound-guided injection of Oncogel™ into porcine pancreas has been demonstrated, showing that Oncogel™ provides clinically significant levels of paclitaxel 30 to 50 mm from the injection site in the tail of the pancreas up to 14 days [62, 63]. Oncogel™ was injected at 1 to 4 mL (paclitaxel at 6.0 mg/mL) through a 22-gauge needle into the tail of the pancreas of 8 Yorkshire breed pigs under EUS-guidance. A linear endoscopic ultrasound device was placed into pigs’ esophagus and advanced into the stomach. After visualization of the pancreatic tail by endoscopic ultrasound, Oncogel™ was injected into the pancreatic tail using a threaded syringe attached to a 22-gauge endoscopic ultrasound needle, slowly turning the lever of the screw syringe in 180° steps every 15 seconds. In this way, 5 of 8 pigs were accurately injected into the pancreatic tail, noting technically difficulty due to the small diameter of the porcine pancreatic tail in comparison to humans. It was noted that endoscopic ultrasound guided injection of Oncogel™ into pancreatic tumor will likely be less challenging due to better differentiation of tumor tissue relative to normal pancreas. Oncogel™ formed a gel with an average diameter of 2.1±0.8 cm based on observation of an intrapancreatic hyperchoic focus by endoscopic ultrasound imaging and a hypodense area visible contrast computed tomography. There were no signs of acute of chronic pancreatitis besides localized tissue reaction next to Oncogel™. Given that the total injected dose of paclitaxel was ca. 0.5 mg/kg, systemic toxicity was not expected. In summary, endoscopic-guided injection of Oncogel™ into porcine pancreas was feasible and safe and produced high local levels of paclitaxel in the tail of pig pancreas, and this local drug delivery strategy is a minimally invasive treatment option for unresectable pancreatic tumors.
Oncogel™ has been evaluated in an intracranial 9L gliosarcoma rat model as an adjunct to radiation [66]. Oncogel™ (6.3 mg/mL) could be safely injected stereotactically into tumors at < 50 μL, but not higher volumes due to death of rats. A intracerebral biodistribution study on Oncogel™ containing [14C]-paclitaxel showed radioactivity in the ipsilaterial hemisphere over 3 weeks and the highest level of radioactivity after 3 hours. Notably, detected levels of [14C]-paclitaxel were orders of magnitude higher than in vitro lethal levels for brain tumor cell lines, whereas plasma levels of [14C]-paclitaxel were low, indicating little systemic exposure. As a monotherapy, Oncogel™ injected on day 0 (same day as 9L cells) and on day 5 had a median survival time of 31 and 17 days, respectively. The control or Regel® had a median survival time of 17 days, and 26 days for radiation treatment given on day 5 (single dose of 20 Gy). Combination of Oncogel™ and radiation treatment (20 Gy) on day 5 had a median survival time of 32 days, which was not significant relative to radiation treatment. The lack of antitumor efficacy of Oncogel™ may be due to limited injection volume of 10 μL in this intracranial 9L gliiosarcoma rat model. In humans with malignant glioblastoma, a higher volume of Oncogel™ may be placed in a resected cavity akin to the use of Gliadel® wafer, and studies on combining surgical resection and Oncogel™ in orthotopic brain tumor models may have great value.
In a recent study, 35 μL of Oncogel™ (6.3 mg/mL) was tested in an intracranial 9L gliosarcoma rat model in combination with oral temozolomide, locally implanted temozolomide incorporated in a biodegradable polyanhydride and radiation treatment [67]. Control rats and rats treated with radiation after 5 days with 20 Gy had a mean survival time of 15 and 19 days, respectively. Oncogel™ injected on day 0 (same day as 9L cells) increased the mean survival time to 33 days. Combination of Oncogel™ and oral temozolomide significantly increased survival times of rats with 57% long term survivors (up to 120 days), whereas oral temozolomide and radiation, a clinical treatment regimen, produced a mean survival time of 36 days. Addition of radiation treatment to Oncogel™ and oral temozolomide produced 100% long-term survivors. Interestingly, combination of Oncogel™ and temozolomide incorporated in a biodegradable polyanhydride placed intratumorally produced 100% long-term survivors, whereas addition of radiation treatment produced 75% long-term survivors. Limitations noted in this study were the use of a single rodent glioma cell line and rodent model, and sequential administration of Oncogel™ and temozolomide (days 0 and 5). In a clinically relevant situation, both anticancer agents would be dosed concurrently, following surgical resection. In summary, combination of Oncogel™ with oral or local temozolomide is safe and highly effective in an intracranial 9L gliosarcoma rat model, and radiation treatment augments antitumor efficacy.
3.3. Triogel
Akin to PEG-b-PLA micelles, PLGA-b-PEG-b-PLGA has a multi-drug capacity as a sol-gel for paclitaxel, 17-AAG and rapamycin (Figure 6) [68]. 150 mg of PLGA-b-PEG-b-PLGA was dissolved in 1.0 mL of distilled water at 4 °C and mixed with paclitaxel, 17-AAG and rapamycin at 6.0, 6.0 and 3.0, respectively, dissolved in 1.0 mL of tert-butanol and freeze-dried. The freeze-dried cake was rehydrated with 1.0 mL of distilled water at 4 °C. In this way, PLGA-b-PEG-b-PLGA sol-gel had a capacity for paclitaxel, 17-AAG and rapamycin at 5.7, 5.7 and 3.0 mg/mL, respectively, based on reverse-phase HPLC analysis. This is the first report showing the multi-drug capacity of a PLGA-b-PEG-b-PLGA sol-gel, enabling concurrent local multi-drug delivery for applications in cancer treatment.
Figure 6.
Triogel.
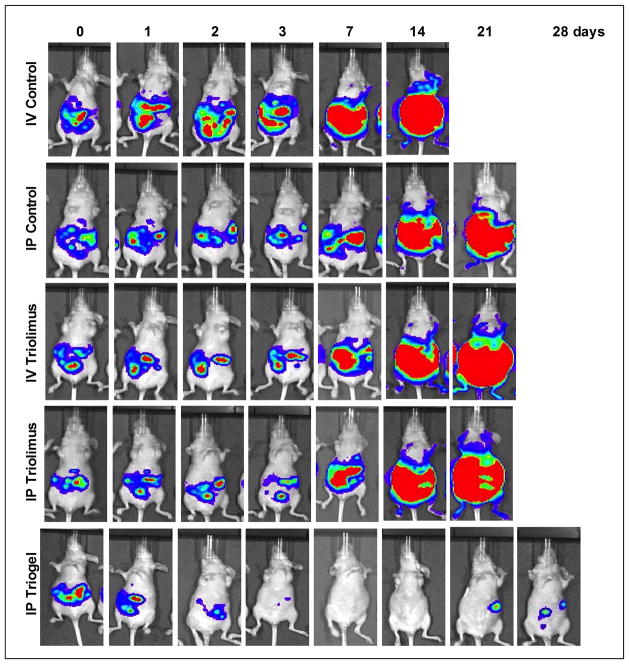
In an intraperitoneal ES-2-luc ovarian tumor model, a single intraperitoneal injection of Triogel with paclitaxel, 17-AAG and rapamycin at 60, 60 and 30 mg/kg, respectively, 4 days after tumor cell inoculation significantly reduced tumor burden based on whole body bioluminescence imaging and doubled survival time of mice in comparison to the control (Figure 7). In contrast, intravenous and intraperitoneal Triolimus were ineffective in this intraperitoneal ES-2-luc ovarian tumor model, suggesting that local sustained release of the 3 anticancer agents is important for antitumor efficacy. Notably, gel remnants, purple in color due to 17-AAG, were visible in the peritoneum after 2 and 8 hours, and while-colored gels depleted of drug were visible after 24 hours. After 8 hours, collected Triogel remnants in the peritoneum of mice had 16, 6 and 8% of paclitaxel, 17-AAG and rapamycin, respectively. In summary, Triogel is the first example of a 3-drug sol-gel for local drug delivery, and it might surpass Oncogel™ for treatment of esophageal, pancreatic and brain cancers.
Figure 7.
Antitumor efficacy of intraperitoneal Triogel based on whole-body bioluminescence imaging in an ES-2-luc ovarian tumor model (Reproduced with permission from reference 68).
4. Conclusions
Genexol-PM®/Cynviloq™ and Oncogel™ represent major milestones in drug delivery, advancing paclitaxel into human clinical trials and approval in the case of Genexol-PM® in Asia. PEG-b-PLA and PLGA-b-PEG-b-PLGA have encouraging safety records in pre-clinical and clinical studies, and both have been scaled-up and manufactured at quantities required for human studies. It is anticipated that the application of PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels will be broadened beyond the scope of paclitaxel. At this point, anticancer agents listed in Table 1 can be considered for clinical development for systemic and local delivery by PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels, respectively. Furthermore, multi-drug delivery, systemic and local, by PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels, respectively, raises significant opportunities for anticancer treatment, enabling potent drug combinations, such as chemotherapy and so-called targeted agents in a uniquely safe manner. However, challenges in chemistry, manufacturing and control will be posed in the pre-clinical development of multi-drug delivery systems and FDA approval. Local intratumoral delivery is primarily limited to resectable solid tumors and does not address metastatic disease. Moving forward, one can consider novel prodrugs for PEG-b-PLA micelles for controlled release and better drug targeting perhaps in the context of tumor priming. Besides a capacity of PLGA-b-PEG-b-PLGA sol-gels for poorly water-soluble anticancer agents, its capacity for hydrophilic anticancer agents should not be overlooked in the context of multi-drug delivery. Lastly, multi-drug delivery by PLGA-b-PEG-b-PLGA sol-gels should be assessed in combination with surgery and radiation, two mainstays in the treatment of cancer.
Acknowledgments
This work was supported in part by NIH (R01AI01157). This work was supported in part by the Global Innovative Research Center program of the National Research Foundation of Korea and by the Intramural Research Program (Global RNAi Carrier Initiative) of KIST. We apologize to authors whose related work was not cited due to omission or oversight in the literature.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nature Biotech. 2012;20:1–13. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 2.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer research. Nature Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decker S, Sausville EA. Preclinical model of combination treatments: fantasy or requirement? Ann NY Acad Sci. 2005;1059:61–69. doi: 10.1196/annals.1339.024. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock J, Griffin JP, Behrman RE. Development of novel combination therapies. N Engl J Med. 2011;364:985–877. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- 5.Sausville EA. Respecting cancer drug transportability: a basis for lead selection. J National Cancer Institute. 2006;98:1098–1099. doi: 10.1093/jnci/djj327. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Lu Z, Gao Y, Wientjes MG, Au JL. Improving delivery and efficacy of nanomedicines in solid tumors: role of tumor priming. Nanomedicine. 2011;6:1605–1620. doi: 10.2217/nnm.11.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 8.Gaucher G, Marchessault RH, Leroux J-C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J Controlled Release. 2010;143:2–12. doi: 10.1016/j.jconrel.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 10.Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng. 2006;8:323–341. doi: 10.1146/annurev.bioeng.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- 11.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Delivery Rev. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 12.Hu CJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Del. 2010;1:323–334. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Wang G, Yang H. Drug delivery systems for differential release in combination therapy. Expert Opin Drug Deliv. 2011;8:171–190. doi: 10.1517/17425247.2011.547470. [DOI] [PubMed] [Google Scholar]
- 14.Kwon GS. Polymeric micelles for multiple-drug delivery. In: Svenson S, Prud’homme RK, editors. Multifunctional Nanoparticles for Drug Delivery Applications. Springer; New York: 2012. pp. 133–152. [Google Scholar]
- 15.Cho H, Lai TC, Tomoda K, Kwon GS. Polymeric micelles for multidrug delivery in cancer. AAPS Pharm Sci Tech. 2015;19:10–20. doi: 10.1208/s12249-014-0251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataoka K, Kwon GS, Yokoyama M, Okano T, Sakurai Y. Block copolymer micelles as vehicles for drug delivery. J Controlled Release. 1993;24:119–132. [Google Scholar]
- 17.Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(l-amino acid) micelles for drug delivery. Adv Drug Del Rev. 2002;54:169–190. doi: 10.1016/s0169-409x(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 18.Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci. 2003;92:1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura Y. The discovery of nanomedicine and its clinical experience. Jpn J Clin Oncol. 2014;44:515–525. doi: 10.1093/jjco/hyu046. [DOI] [PubMed] [Google Scholar]
- 20.Eetezadi S, Ekdawi SN, Allen C. The challenges facing block copolymer micelles for cancer therapy: in vivo barriers and clinical translation. Adv Drug Delivery Reviews. doi: 10.1016/j.addr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Churchill JR, Hutchinson F. Biodegradable amphipathic copolymers. 85304489.9 European patent application.
- 22.Piskin E, Kaitian X, Denkbas EB, Kucukyavuz Z. Novel pdlla/peg copolymer micelles as drug carriers. J Biomater Sci Polymer Ed. 1995;7:359–373. doi: 10.1163/156856295x00373. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Jackson JK, Burt HM. Development of amphiphilic diblock copolymers as micellar carriers of taxol. Int J Pharm. 1996;132:195–206. [Google Scholar]
- 24.Lee SW, Yun MH, Jeong SW, In C-H, Kim JY, Seo M-H, Pai CM, Kim SO. Development of docetaxel-loaded intravenous formulation, nanoxel-pm™ using polymer-based delivery system. J Controlled Release. 2011;155:262–271. doi: 10.1016/j.jconrel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Shin HC, Alani AWG, Rao DA, Rockich NC, Kwon GS. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J Controlled Release. 2009;140:294–300. doi: 10.1016/j.jconrel.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin HC, Alani AWG, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol Pharm. 2011;8:1257–1265. doi: 10.1021/mp2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong MP, Yanez JA, Kwon GS, Davies NM, Forrest ML. A cremophor-free formulation of tanespimycin (17-aag) using peo-b-pdlla micelles: characterization and pharmacokinetics in rats. J Pharm Sci. 2009;98:1577–1586. doi: 10.1002/jps.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danquah M, Li F, Duke CB, III, Miller DD, Mahato RI. Micellar delivery of bicalutamide and embelin for treating prostate cancer. Pharm Res. 2009;26:2081–2092. doi: 10.1007/s11095-009-9903-5. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed EA, Zhao Y, Meshall MM, Remsberg CM, Borg TM, Foda AMM, Takemoto JK, Sayre CL, Martinez SE, Davies NM, Forrest ML. Vorinostat with sustained exposure and high solubility in poly(ethylene glycol)-b-poly(d,l-lactic acid) micelle nanocarriers: characterization and effects on pharmacokinetics in rat serum and urine. J Pharm Sci. 2012;101:3787–3798. doi: 10.1002/jps.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco E, Bey EA, Dong Y, Weinsberg BD, Suttion DM, Boothman DA, Gao J. β-lapachone-containing peg-pla polymer micelles as novel nanotherapeutics against nqo1-overexpressing tumor cells. J Controlled Release. 2007;122:365–374. doi: 10.1016/j.jconrel.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter A, Olbrich C, Krause M, Kissel T. Solubilization of sagopilone, a poorly water-soluble anticancer drug, using polymeric micelles for parenteral delivery. Int J Pharm. 2010;389:244–253. doi: 10.1016/j.ijpharm.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 32.Wyche TP, Dammalapati A, Cho H, Harrison AD, Kwon GS, Shen H, Bugni TS, Jaskula-Sztul R. Thiocoraline activates the notch pathway in carcinoids and reduces tumor progression in vivo. Cancer Gene Therapy. 2014;21:518–525. doi: 10.1038/cgt.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramakingam SS, Egorin MJ, Ramanathan RK, Remick SC, Sikorski RP, Lagattuta TF, Chatta GS, Friedland DM, Stoller RG, Potter DM, Percy Ivy S, Belani CP. A phase I study with 17-allylamino-17-demethoxygeldanamycin combined with paclitaxel in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3456–3461. doi: 10.1158/1078-0432.CCR-07-5088. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Xiao Y, Allen C. Polymer-drug compatibility: a guide to the development of delivery systems for the anticancer agent, ellipticine. J Pharm Sci. 2004;93:132–143. doi: 10.1002/jps.10533. [DOI] [PubMed] [Google Scholar]
- 35.Nagarajan R, Barry M, Ruckenstein E. Unusual selectivity in solubilization by block copolymer micelles. Langmuir. 1986;2:210–215. [Google Scholar]
- 36.Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JL, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a psma-targeted docetaxel nanoparticle with a differential pharmacological profile. Science Transl Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 37.Ramaswamy M, Zhang X, Burt HM, Wasan KM. Human plasma distribution of free paclitaxel and paclitaxel associated with diblock copolymers. J Pharm Sci. 1997;86:460–464. doi: 10.1021/js960333n. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Kim S, He W, Wang H, Low PS, Park K, Chen JX. Fast release of lipophilic agents from circulating peg-pdlla micelles reveale by in vivo förster resonance energy transfer imaging. Langmuir. 2008;24:5213–5217. doi: 10.1021/la703570m. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles with modulated surface charge. J Controlled Release. 2001;77:27–38. doi: 10.1016/s0168-3659(01)00451-5. [DOI] [PubMed] [Google Scholar]
- 40.Yoo HS, Park TG. Biodegradable polymeric micelles composed of doxorubicin conjugated plga-peg block copolymer. J Controlled Release. 2001;70:63–70. doi: 10.1016/s0168-3659(00)00340-0. [DOI] [PubMed] [Google Scholar]
- 41.Ma X, Huang X, Moore Z, Huang G, Kilgore JA, Wang Y, Hammer S, Williams NS, Boothman DA, Gao J. Esterase-activatable β-lapachone prodrug micelles for nqo1-targeted lung cancer therapy. J Controlled Release. 2015;200:201–211. doi: 10.1016/j.jconrel.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blols J, Smith A, Josephson L. The slow death response when screening chemotherapeutic agents. Cancer Chemother Pharmacol. 2011;68:795–803. doi: 10.1007/s00280-010-1549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stirland DL, Nichols JW, Miura S, Bae YH. Mind the gap: a survey of how cancer drug carriers are susceptible to the gap between research and practice. J Controlled Release. 2013;172:1045–1064. doi: 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang D, Yu L, Van S. Clinically relevant anticancer polymer paclitaxel therapeutics. Cancers. 2011;3:17–42. doi: 10.3390/cancers3010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, Seo MH. In vivo evaluation of polymeric micelle paclitaxel formulation: toxicity and efficacy. J Controlled Release. 2001;72:191–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 46.Sparreboom A, Baker SD, Verwell J. Paclitaxel repackaged in an albumin-stabilized nanoparticle: handy or just a dandy? J Clin Oncology. 2005;23:7765–7767. doi: 10.1200/JCO.2005.03.7135. [DOI] [PubMed] [Google Scholar]
- 47.https://clinicaltrials.gov/ct2/show/NCT02064829.
- 48.Sparreboom A, scripture CD, Trieu V, Williams PJ, De T, Young A, Beals B, Figg WD, Hawkins M, Desai N. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (abi-007) and paclitaxel formulated in cremophor (taxol) Clin Cancer Res. 2005;11:4136–4143. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 49.Desai N, De T, Ci S, Louie L, Trieu V. Characterization and in vitro/in vivo dissolution of nab-paclitaxel nanoparticles. Cancer Res. 2008;68:5624. [Google Scholar]
- 50.Kratz F. A clinical update of using albumin as a drug vehicle – a commentary. J Controlled Release. 2014;190:331–336. doi: 10.1016/j.jconrel.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Stoltzing O. Dual-targeting of mtor and hsp90 for cancer therapy. Cell Cycle. 2010;9:2051–2052. doi: 10.4161/cc.9.11.11924. [DOI] [PubMed] [Google Scholar]
- 52.Perego P, Cossa G, Zuco V, Zunino F. Modulation of cell sensitivity to antitumor agents by targeting survival pathways. Biochem Pharmacol. 2010;80:1459–1465. doi: 10.1016/j.bcp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 53.Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, Tseng LM, Zhang Q, Shen K, Liu D, Dreosti LM, Burris HA, Toi M, Buyse ME, Cabaribere D, Lindsay MA, Rao S, Pacaud LB, Taran T, Slamon D. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with her2-positive advanced breast cancer (bolero-1): a phase 3, randomized, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 54.Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D’Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patit S, Norton L, Hannah AL, Hudis C. Hsp90 inhibition is effective in breast cancer: a phase ii trial of tanespimycin (17-aag) plus trastuzumab in patients with her2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 55.Hasenstein JR, Shin HC, Kasmerchak K, Buehler D, Kwon GS, Kozak KR. Antitumor activity of triolimus: a novel multidrug-loaded micelle containing paclitaxel, rapamycin, and 17-aag. Mol Cancer Ther. 2012;11:1–10. doi: 10.1158/1535-7163.MCT-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin HC, Cho H, Lai TC, Kozak KR, Kolesar JM, Kwon GS. Pharmacokinetic study of 3-in-1 poly(ethylene glycol)-block-poly(d,l-lactic acid) micelles carrying paclitaxel, 17-allylamino-17-demethoxygeldanamycin, and rapamycin. J Controlled Release. 2012;163:93–99. doi: 10.1016/j.jconrel.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu D, Wientjes G, Lu Z, Au JLS. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Experimental Ther. 2007;322:80–88. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez-Angulo AM, Hortobagyi GN. Optimal schedule of paclitaxel: weekly is better. J Clin Oncology. 2008;26:1585–1587. doi: 10.1200/JCO.2007.15.7651. [DOI] [PubMed] [Google Scholar]
- 59.Cho H, Kwon GS. Polymeric micelles for neoadjuvant cancer therapy and tumor-primed optical imaging. ACS Nano. 2011;5:8721–8729. doi: 10.1021/nn202676u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, Rhee BG, Mestecky J, Moldoveanu Z, Morgan M, Weitman S. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Controlled Release. 2001;73:203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 61.Wolinsky JB, Colson Y, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Controlled Release. 2012;159:14–26. doi: 10.1016/j.jconrel.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elstad NL, Fowers KD. Oncogel (regel/paclitaxet) – clinical applications for a novel paclitaxel delivery system. Adv Drug Delivery Rev. 2009;61:785–794. doi: 10.1016/j.addr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Matthes K, Mino-Kenudson M, Sahani DV, Holalkere N, Fowers KD, Rathi R, Brugge WR. Eus-guided injection of paclitaxel (oncogel) provides therapeutic drug concentrations in porcine pancreas. Gastrointest Endosc. 2007;65:448–453. doi: 10.1016/j.gie.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 64.Lee DS, Shim MS, Kim SW, Lee H, Park I, Chang T. Novel thermoreversible gelation of biodegradable plga-block-peo-block-plga triblock copolymers in aqueous solution. Macromol Rapid Commun. 2001;22:587–592. [Google Scholar]
- 65.DuVall GA, Tarabar D, Seidel RH, Elstad NL, Fowers KD. Phase 2: a dose-escalation study of Oncogel (regel/paclitaxel), a controlled-release formulation of paclitaxel, as adjunctive local therapy to external-beam radiation in patients with inoperable esophageal cancer. Anti-Cancer Drugs. 2009;20:89–95. doi: 10.1097/CAD.0b013e3283222c12. [DOI] [PubMed] [Google Scholar]
- 66.Tyler B, Fowlers KD, Li KW, Recinos VR, Caplan JM, Hdeib A, Grossman R, Basaldella L, Bekelis K, Pradilla G, Legnani F, Brem H. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. J Neurosurg. 2010;113:210–217. doi: 10.3171/2009.11.JNS08162. [DOI] [PubMed] [Google Scholar]
- 67.Vellimana AK, Recinos VR, Hwang L, Fowers KD, Li KW, Zhang Y, Okonma S, Eberhart CG, Brem H, Tyler BM. Combination of paclitaxel thermal gel depot with temozolomide and radiotherapy significantly prolongs survival in an experimental rodent glioma model. J Neurooncol. 2013;111:229–236. doi: 10.1007/s11060-012-1014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho H, Kwon GS. Thermosensitive poly(d,l-lactide-co-glycolide)-block-poly(ethylene glycol)-block-poly(d,l-lactide-co-glycolide) hydrogels for multi-drug delivery. J Drug Targeting. 2014;22:669–677. doi: 10.3109/1061186X.2014.931406. [DOI] [PMC free article] [PubMed] [Google Scholar]