Abstract
Background
Williams syndrome (WS), a genetic disorder resulting from hemizygous microdeletion of chromosome 7q11.23, has emerged as a model for identifying the genetic architecture of socioemotional behavior. Recently, common polymorphisms in GTF2I, which is found within the WS microdeletion, have been associated with reduced social anxiety in the general population. Identifying neural phenotypes affected by these polymorphisms will help advance our understanding not only of this specific genetic association but also the broader neurogenetic mechanisms of variability in socioemotional behavior.
Methods
Through an ongoing parent protocol, the Duke Neurogenetics Study, we measured threat-related amygdala reactivity to fearful and angry facial expressions using functional MRI (fMRI), assessed trait personality using the Revised NEO Personality Inventory, and imputed GTF2I rs13227433 from saliva-derived DNA using custom Illumina arrays. Participants included 808 non-Hispanic Caucasian, African American, and Asian university students.
Results
The GTF2I rs13227433 AA genotype, previously associated with lower social anxiety, predicted decreased threat-related amygdala reactivity. An indirect effect of GTF2I genotype on the warmth facet of extraversion was mediated by decreased threat-related amygdala reactivity in women but not men.
Conclusions
A common polymorphism in the WS gene GTF2I associated with reduced social anxiety predicts decreased threat-related amygdala reactivity, which mediates an association between genotype and increased warmth in women. These results are consistent with reduced threat-related amygdala reactivity in WS and suggest that common variation in GTF2I contributes to broader variability in socioemotional brain function and behavior, with implications for understanding the neurogenetic bases of WS as well as social anxiety.
Keywords: Amygdala, Williams Syndrome, fMRI, GTF2i, Extraversion, Emotion
Introduction
Hemizygous microdeletion of approximately 25 genes on chromosome 7q11.23 causes Williams syndrome (WS), a developmental disorder characterized by a unique profile of social and cognitive phenotypes, including hyper-sociability and increased approach to strangers (1). Systematic investigation of the genes within the WS microdeletion is underway in effort to better understand the genetic architecture of the disorder specifically and of socioemotional behavior broadly (2, 3). Clinical and preclinical studies have highlighted the importance of the WS gene GTF2I, which encodes for general transcription factor IIi (GTF2I) implicated in transcriptional regulation of a wide range of genes. For instance, in one case study, the microdeletion of this region was partial and spared GTF2I. Unlike the social profile typically seen in WS, this individual did not exhibit hyper-sociability, and instead was rated by her parents as much less likely to approach strangers compared to individuals with the full WS microdeletion (4, 5). A case study for a different individual in which partial deletion spared GTF2I and the gene encoding general transcription factor IIi repeat domain-containing protein 1 (GTF2IRD1) revealed that this individual was categorized as a typically developing control based on multivariate pattern classification analysis with gray matter structure, implicating these genes more broadly in gray matter neuroanatomical abnormalities in WS (6).
Recent research has extended the characterization of GTF2I to the general population by examining the association between common single nucleotide polymorphisms (SNPs) in this region and variation in social and cognitive phenotypes. Specifically, two GTF2I SNPs in high linkage disequilibrium, rs13227433 and rs4717907, have been associated with individual differences on a composite WS profile score, including reduced communication abilities as rated on the Autism Spectrum Quotient scale, and also reduced social anxiety (7). Moreover, GTF2I-deficient mice evidence increased social interaction but not alterations in learning and memory or general anxiety, suggesting that deletion of GTF2I may play a specific role in the social phenotype of WS (8).
Here we employ an imaging genetics strategy to examine the effect of common variation in imputed GTF2I genotypes on a systems-level neural phenotype, threat-related amygdala reactivity, associated with socioemotional behavior broadly and the hyper-sociability observed in WS specifically (9-11). We further test if this neural phenotype mediates associations between GTF2I genotype and extraversion, a personality trait that encompasses increased sociability (12). One previous study reported a positive correlation between extraversion and amygdala reactivity to happy but not threat-related (i.e., angry and fearful) facial expressions (13). However, this study was limited by a small sample size that likely reduced power to detect significant effects and prevented the examination of potential moderators such as sex. We expect that lower threat-related amygdala reactivity to fearful and angry facial expressions will predict higher extraversion for several reasons. First, WS is consistently associated with reduced amygdala reactivity to threat-related facial expressions (9, 10). Second, consistent with its role in hyper-sociability, decreased amygdala reactivity to fearful facial expressions predicts individual differences in social approach towards strangers in individuals with WS (11). Third, mirroring the findings in WS, social anxiety, which is negatively correlated with extraversion (12, 14), is consistently associated with relatively increased threat-related amygdala reactivity (15). Based on this evidence, we hypothesized that the GTF2I rs13227433 A allele, which has been linked to reduced social anxiety, would be associated with relatively decreased threat-related amygdala reactivity. Moreover, we hypothesized that such genotype-related differences in threat-related amygdala reactivity would indirectly link rs13227433 genotype to variability in extraversion.
Methods and Materials
Participants
Participants included 808 young adult university students aged 18-22 years old who completed the ongoing Duke Neurogenetics Study (DNS) as of March 2, 2015 (Table 1). All procedures were approved by the Duke University Medical Center and participants provided informed consent before participating in the study. Recruitment and exclusion criteria have been described in detail elsewhere (16-18). Diagnosis of any past or current DSM-IV Axis I disorder or select Axis II disorders (antisocial personality disorder and borderline personality disorder), assessed with the electronic Mini International Neuropsychiatric Interview (19) and Structured Clinical Interview for the DSM-IV subtests (20) was not an exclusion, as the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology. Consistent with epidemiological data and the dimensional nature of psychopathology, 157 participants (19%) in the final sample reported here met criteria for at least one current or past Axis I disorder, including 101 with substance use disorders, 39 with major depressive disorder, 7 with bipolar disorder, 13 with bipolar disorder-not otherwise specified, and 36 with anxiety disorders (see Supplementary Table 1 for full breakdown of diagnoses). Analyses were restricted to non-Hispanic Caucasian (n=427), African American (n=110), and Asian (n=271) participants, as these were the largest racial/ethnic sub-groups available within the DNS (see Supplemental Results for analyses by sub-group).
Table 1.
Participant characteristics as a function of GTF2I rs13227433 genotype and race
| C Carrier Mean (SD) | AA Mean (SD) | Group difference | |
|---|---|---|---|
| Caucasian | n=162 | n=265 | |
| Age | 19.8 (1.3) | 19.7 (1.2) | t(425)=.15, p=.88 |
| Sex (% female) | 54% | 52% | χ2(1)=.20, p=.65 |
| Diagnosis | 23% | 22% | χ2(1)=.22, p=.64 |
| African American | n=28 | n=82 | |
| Age | 19.6 (1.1) | 19.7 (1.2) | t(108)=−.48, p=.63 |
| Sex (% female) | 61% | 74% | χ2(1)=1.89, p=.17 |
| Diagnosis | 21% | 21% | χ2(1)=.01, p=.94 |
| Asian | n=57 | n=214 | |
| Age | 19.3 (1.2) | 19.7 (1.3) | t(269)=−1.96, p=.05 |
| Sex (% female) | 42% | 59% | χ2(1)=.5.12, p=.02 |
| Diagnosis | 16% | 14% | χ2(1)=.12, p=.74 |
| Total | n=247 | n=561 | |
| Age | 19.6 (1.3) | 19.7 (1.3) | t(806)=−.86, p=.39 |
| Sex (% female) | 52% | 58% | χ2(1)=2.27, p=.13 |
| Diagnosis | 21% | 19% | χ2(1)=.93, p=.33 |
Note: Diagnosis is the percentage of participants in each group meeting criteria for at least one current or past psychiatric diagnosis.
Amygdala reactivity paradigm
Amygdala reactivity to threat was assessed using an emotional face matching challenge paradigm shown to consistently elicit robust amygdala reactivity in this and in previous samples (17, 18). The paradigm version used in the DNS consists of four blocks of a face-processing task interleaved with five blocks of a sensorimotor control task. During task blocks, participants view a trio of faces and match 1 of 2 faces (bottom) identical to a target face (top). Each trial in the face matching blocks lasts for 4 seconds with a variable interstimulus interval (ISI) of 2 to 6 seconds (M=4 seconds), for a total block length of 48 seconds. In the control blocks, each of the six shape trios is presented for 4 seconds with a fixed ISI of 2 seconds for a total block length of 36 seconds. Total task time is 390 seconds.
BOLD fMRI data acquisition, preprocessing, and quality assurance
Participants were scanned using a research-dedicated GE MR750 3T scanner at the Duke-UNC Brain Imaging and Analysis Center. A series of 34 interleaved axial functional slices aligned with the anterior commissure-posterior commissure (AC-PC) plane were acquired for full-brain coverage using an inverse-spiral pulse sequence to reduce susceptibility artifact (TR = 2,000 ms; TE = 30 ms; flip angle = 60; FOV = 240 mm; 3.75 × 3.75 × 4 mm voxels; interslice skip = 0).
Functional MRI data were processed in SPM8 using the standard pre-processing stream used in previously published research from the DNS (17, 18), including realigning images to the first volume in the time series to correct for head motion, spatially normalizing images into a standard stereotactic space (Montreal Neurological Institute (MNI) template) using a 12-parameter affine model (final resolution of functional images = 2 mm isotropic voxels), and smoothing, set at 6-mm full-width at half-maximum.
Data quality assurance (QA)
Artifact detection software (http://www.nitrc.org/projects/artifact_detect) was used to create regressors of no interest for: 1) volumes exhibiting significant mean-volume signal intensity variation (i.e., within volume mean signal greater or less than 4 standard deviations of mean signal of all volumes in time series), and 2) individual volumes where scan-to-scan movement exceeded 2 mm translation or 2° rotation in any direction. Quality control criteria for inclusion of a participant's imaging data were: <5% volumes exceed artifact detection criteria for motion or signal intensity outliers, ≥ 90% coverage of signal within the anatomically-defined bilateral amygdala region of interest, and accuracy ≥75% on the matching task performed during scanning. Within the current sample of 808 genotyped participants, 734 had fMRI data meeting QA criteria.
BOLD fMRI data analysis
The general linear model of SPM8 was used to conduct fMRI data analyses. Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate main effects of expression for each individual. Individual contrast images were then entered in second-level random effects models to determine mean condition-specific regional responses using one-sample t-tests. We extracted parameter estimates from functional clusters within anatomically defined amygdala regions of interest (Automated Anatomical Labeling Atlas) at p<.05 family-wise error (FWE) corrected across the search volumes, for the contrast of Angry and Fearful blocks > Control blocks. We did not have a priori hypotheses regarding laterality as WS and symptoms of hyper-sociability have been associated with both altered activity in the left (11) and right amygdala (10), and a meta-analysis found evidence for heightened left amygala reactivity to facial expressions in individuals with social anxiety disorder relative to controls but reduced right amygdala reactivity in individuals with WS relative to controls (15). We therefore tested each separately and corrected for multiple comparisons using a false discovery rate (FDR) correction (i.e., the Benjamini-Hochberg procedure).
Genotyping
Genotyping was conducted by 23andMe, Inc. Genomic DNA from all participants was isolated from buccal cells derived from Oragene DNA self-collection kits (DNA Genotek, Inc., Kanata, Canada) customized for 23andMe. DNA extraction and genotyping were performed at the National Genetics Institute, a CLIA-certified clinical laboratory and subsidiary of Laboratory Corporation of America. One of two different Illumina arrays with custom content was used to provide genome-wide SNP data, the HumanOmniExpress or HumanOmniExpress-24 (21-23). As neither SNP (rs4717907 nor rs13227433) previously linked to social behavior was present on these arrays, genotypes at each locus were imputed using available SNP data.
Genotype imputation was performed on all DNS participants with genome-wide chip data using the pre-phasing/imputation stepwise approach implemented in SHAPEIT / IMPUTE2 (24, 25). Imputation was run separately for participants genotyped on the Illumina HumanOmniExpress (n=728) and the Illumina HumanOmniExpress-24 (n=246) arrays using biallelic SNPs only, the default value for effective size of the population (20,000), and chunk sizes of 3Mb and 5Mb for the respective arrays. Within each array batch, genotyped SNPs used for imputation were required to have missingness < 0.02, Hardy-Weinberg equilibrium P > 10−6, and MAF > 0.01. The imputation reference set consisted of 2,504 phased haplotypes from the full 1000 Genomes Project Phase 3 dataset (May 2013, over 70 million variants, release “v5a”). Imputed SNPs were retained if they had high imputation quality (INFO >0.9), low missingness (<5%), and MAF > 0.01.
Imputed SNP data were available for 808 participants for rs13227433 and for 805 participants for rs4717907 (data for 3 participants did not meet QA criteria for this SNP). Data for these imputed SNPs were perfectly correlated given that they are in high linkage disequilibrium; to maximize participants included, analyses were conducted with rs13227433. Of note, results remain as reported when excluding the 3 participants with low imputation quality for rs4717907. The imputation quality for rs13227433 was 0.992 (HumanOmniExpress) and 0.981 (HumanOmniExpress-24). Moreover, we observed high levels of LD (R2=0.98) between imputed rs13227433 genotype and a proxy SNP (rs6964833; HapMap 3: CEU+TSI, JPT+CHB, GIH R2=1.00, YRI R2=0.90) that was genotyped in a portion of the sample (n=617). We further tested the reliability of results obtained using imputed SNP data by analyzing data using the genotyped proxy SNP rs6964833; results remain as reported with the imputed SNP data from the full sample (see Supplementary Analyses).
Genotype distribution did not deviate from Hardy-Weinberg equilibrium across our entire sample (χ2(1)=.89 p=.345), or within Caucasian (χ2(1)=.39, p=.532), African American (χ2(1)=.001, p=.975), or Asian (χ2(1)=.04, p=.841) sub-groups. There were a small number of participants homozygous for the minor C allele (Caucasian n=22, African American n=2, Asian n=3), therefore we created a binary variable grouping participants as A allele homozygotes or C allele carriers (see also Supplementary Analyses supporting this grouping). Genotype was dummy-coded so that the AA genotype (previously associated with a higher WS profile score and lower social anxiety (7)) was the group of interest (C allele carriers = 0; AA = 1). Population stratification reflecting genetic heterogeneity associated with ancestry was examined using identity by state (IBS) analysis in PLINK of the whole genome SNPs, extracting the first four multidimensional scaling (MDS) components for inclusion as covariates in all genotype analyses (26).
Extraversion
As a component of the DNS all participants completed the Revised NEO Personality Inventory (NEO-PI-R; 27, 28). Here we focus on the Extraversion scale because it encompasses several pro-social behaviors including warmth, gregariousness, assertiveness, activity, excitement seeking, and positive emotions.
Covariates
As described above, 4 MDS components were derived from IBS analysis and included as covariates in the path from rs13227433 genotype to amygdala reactivity to control for possible genetic heterogeneity associated with ancestry. In addition, age and sex were included as covariates on all paths. In preliminary analyses, we found that past or current DSM-IV diagnosis (dummy-coded: 0=no diagnosis, 1=past or current diagnosis) did not moderate the effects of interest (all p's>.05); therefore, we included diagnosis as a binary covariate on all paths but did not separate the groups for our main analyses. Finally, to test whether amygdala reactivity specifically predicted extraversion above and beyond broader anxiety, we also included as a covariate on the brain to behavior path scores on the trait version of the State Trait Anxiety Inventory (29).
Statistical Analyses
Our main aim was to construct a mediation model to test whether threat-related amygdala reactivity mediates the association between rs13227433 genotype and extraversion. Before this, we performed analyses to determine the best-fitting parameters for each path. For the gene to brain path (GTF2I to amygdala reactivity), we tested the effect of rs13227433 genotype on left and right amygdala reactivity controlling for age, sex, diagnosis, and the MDS components. Next, given observed sex differences in extraversion (30), we further examined whether sex moderated links between GTF2I and amygdala reactivity (excluding sex as a covariate). Moderation was assessed using χ2 difference tests; if constraining parameter estimates to be equal across groups leads to a significant decrease in model fit, then one can conclude the effect is significantly moderated by sex. For the brain to behavior path (amygdala reactivity to extraversion), we conducted regressions examining associations between left and right amygdala reactivity and total NEO-PI-R extraversion scores. We first modeled the effect in the full sample including age, sex, diagnosis, and trait anxiety as covariates. We next conducted a multi-group analysis to examine whether this path varied across sex. Lastly, following observations of a significant association between amygdala reactivity and extraversion, post-hoc testing examined which facets (i.e., warmth, gregariousness, assertiveness, activity, excitement-seeking, positive emotions) were primarily driving this association. All analyses were conducted with Mplus v7 software which utilizes Full Information Maximum Likelihood estimation to provide unbiased estimates in the presence of missing data.
Indirect effects model
Once model parameters were determined for each path, we constructed a mediation model to examine the indirect effect of rs13227433 genotype on extraversion, mediated by threat-related amygdala reactivity. We also modeled the direct effect of genotype on behavior by including it as a predictor in the second path. To provide a measure of general effect size for the indirect effect, we report the product of coefficients αβ statistic (e.g., also called a “Sobel” test). However, given well-documented distributional assumptions that are generally not met in that test, we also provide bootstrapped confidence intervals which use a Monte Carlo simulation (1,000 draws), which do not assume normality of the distribution of indirect effects and provide better power to detect the indirect effect.
Results
Threat-related amygdala reactivity
Consistent with prior research, the Angry and Fearful blocks > Control blocks contrast elicited significant threat-related amygdala reactivity in the left, t(733)=25.7, p<.001, peak coordinates: (−22, −6, −18), and right hemispheres, t(733)=29.5, p<.001, (28, −4, −20).
GTF2I genotype to amygdala reactivity
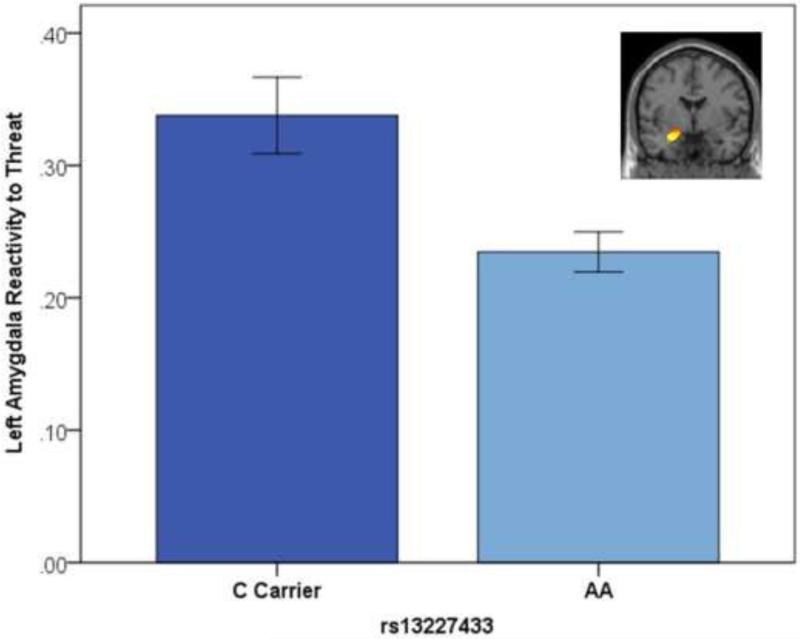
The AA genotype of rs13227433 predicted decreased bilateral amygdala reactivity across the entire sample (Left amygdala: B=−.10, SE=.03, Beta=−.26, p=.005, FDR-corrected p=.01, Δr2=.01, Table S2, Figure 1; Right amygdala: B=−.06, SE=.03, Beta=−.19, p=.029, FDR-corrected p=.029, Δr2=.01). Multi-group analyses indicated that this effect was not moderated by sex for the left, Δχ2(1)=.40, p=.527, or right amygdala, Δχ2(1)=.01, p=.920.
Figure 1. GTF2i rs13227433 genotype predicts left amygdala reactivity to threat.
Parameter estimates for reactivity to threat were extracted from a functional cluster within the anatomically defined left amygdala region of interest for the contrast of Angry and Fearful blocks > Control blocks. Error bars represent 1 standard error.
Amygdala reactivity to extraversion
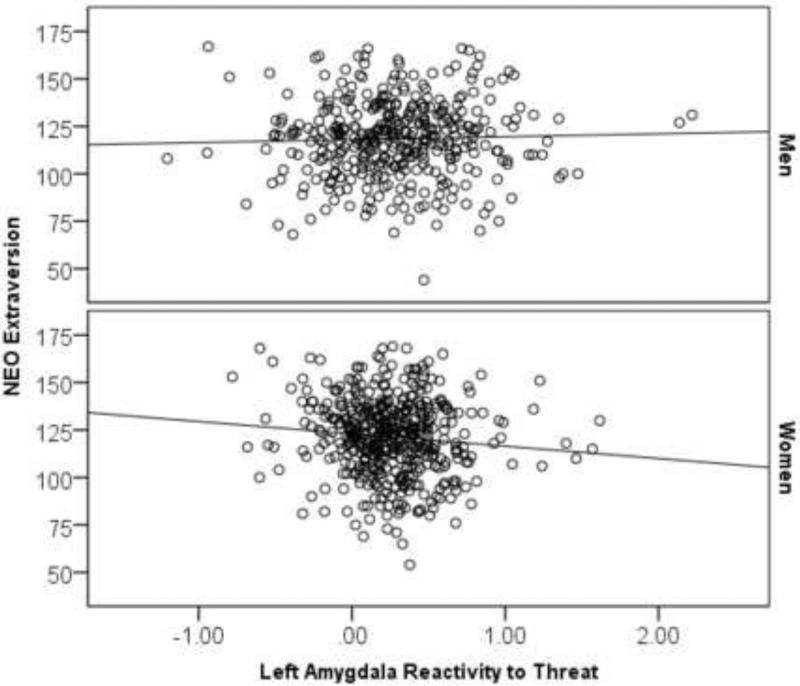
The effect of left amygdala reactivity predicting extraversion was not significant in the full sample, B=−1.36, SE=1.84, Beta=−.03, p=.457, Δr2=.001. However, a multi-group analysis indicated that this effect was moderated by sex, Δχ2(1)=3.83, p=.050. In women, reduced left amygdala reactivity predicted higher extraversion, B=−5.59, SE=2.73, Beta=−.09, p=.041, Δr2=.01 (Table S2; Figure 2), but in men this effect was not significant, B=1.66, SE=2.44, Beta=.04, p=.498, Δr2=.001. Right amygdala reactivity did not significantly predict extraversion in the full sample, B=−.84, SE=2.25, Beta=−.01, p=.708, Δr2=.001, and this effect was not moderated by sex. Finally, we examined whether the effect of left amygdala reactivity in women was specific to extraversion subscales and found specificity for the warmth facet, B=−1.78, SE=.56, Beta=−.13, p=.001, FDR-corrected p=.024, Δr2=.02 (all other facets p's>.05). Notably, this effect survived FDR correction for the 6 subscales × 2 hemispheres × 2 groups (men, women) tested.
Figure 2. Decreased amygdala reactivity to threat predicts higher extraversion in women.
Parameter estimates for reactivity to threat were extracted from a functional cluster within the anatomically defined left amygdala region of interest for the contrast of Angry and Fearful blocks > Control blocks.
Indirect effects model
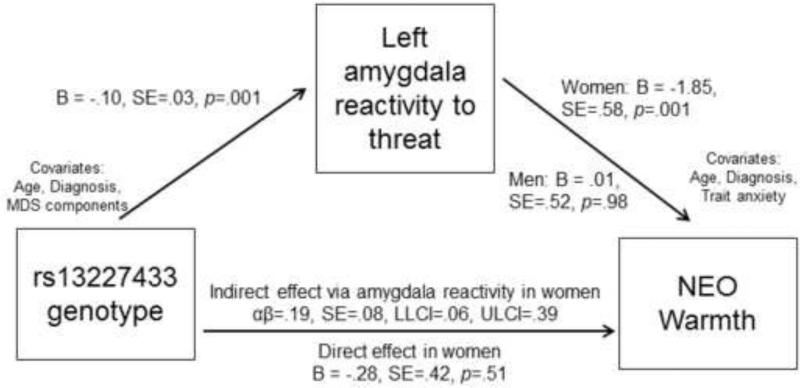
For the final mediation model, we examined the effect of rs13227433 genotype on extraversion, mediated by left amygdala reactivity. The gene to brain parameters were constrained to be equal across sex but the brain to behavior parameters were allowed to vary given evidence for moderation by sex. The model had an excellent fit, χ2(17)=16.84, p=.465, RMSEA=.00, CFI=1.0, SRMR=.016. As reported above, the AA genotype of rs13227433 predicted decreased left amygdala reactivity to threat, B=−.10, SE=.03, p=.001. Moreover, in women decreased left amygdala reactivity predicted higher scores on extraversion, B=−5.69, SE=2.85, p=.046. Finally, in women there was a significant indirect effect of rs13227433 genotype on extraversion, mediated by decreased left amygdala reactivity to threat, αβ=.58, SE=.32, 95% bias-corrected confidence intervals=[.07, 1.32]. Because the association between amygdala reactivity and extraversion was driven by the facet of warmth, we also tested this as an outcome in the mediation model. The indirect effect of rs13227433 on warmth mediated by left amygdala reactivity was also significant in women, αβ=.19, SE=.08, [06, .39] (Figure 3).
Figure 3. Full mediation model testing indirect effect of rs13227433 genotype on warmth, mediated by left amygdala reactivity to threat.
Parameters for the gene to brain path were constrained to be equal for men and women. The direct effect was modeled by including genotype as a predictor in the regression for warmth, in addition to the mediator (threat-related amygdala reactivity) and covariates. αβ = indirect effect, SE=standard error, LLCI=95% lower-limit confidence interval; ULCI=95% upper-limit confidence interval.
Discussion
We provide novel results that common variation in the WS gene GTF2I previously associated with reduced social anxiety predicts threat-related amygdala reactivity. We further demonstrate that decreased threat-related amygdala reactivity partially mediates the association between GTF2I genotype and the personality trait of warmth in women but not men. Collectively, our results suggest that the effect of common variation in GTF2I on sociability may be mediated by reduced amygdala reactivity to threat and may extend dimensionally to normative populations and the personality dimension of warmth.
While our current results along with those of Crespi and Hurd (7) are consistent with the hyper-social phenotype of WS, the molecular mechanisms through which these associations occur is unclear. One intriguing possible molecular pathway through which GTF2I rs13227433 genotype may affect neural and behavioral socioemotional phenotypes is serotonin signaling, which plays an important role in modulating corticolimbic circuit function, including amygdala reactivity (31-33). GTF2I protein regulates the transcription of the ligand-gated ion channel serotonin receptor 3A (HTR3A; 34), which is expressed in the amygdala (35). Thus, the observed neural circuit and behavioral effects of GTF2I rs13227433 may reflect differential transcriptional regulation of serotonin-related fast depolarization of neurons in the amygdala (36). Of course, given the wide range of genes regulated by GTF2I, this is but one of several molecular signaling pathways (e.g., pathways involved in dendritic spine formation and synaptic plasticity) that could be considered in future research. Moreover, given evidence of abnormalities in the oxytocin pathway in WS (37) and the modulatory role of oxytocin on amygdala function (38), future research is warranted to test whether genes associated with the oxytocin signaling pathway may also be regulated by GTF2I.
This is the first study to our knowledge to identify an association between amygdala reactivity to threat-related facial expressions and extraversion in the general population, and as such will require replication in future research. It is notable that the association between threat-related amygdala reactivity and extraversion was only significant for women. Although many factors may have contributed to this finding, prior research has demonstrated sex differences in the functional connectivity of the amygdala, including increased functional connectivity between the left amygdala and several prefrontal regions in women compared to men (39). Sex differences in the association between amygdala reactivity and extraversion may therefore reflect in part sex differences in how the amygdala relays information to prefrontal cortical regions. It is also notable that this association was specific to warmth, given that increased warmth (e.g., hugging of strangers) is part of the characteristic hyper-sociability phenotype of WS, and was less robust for other subscales related to activity or energy levels such as excitement seeking or positive emotions. Individuals with relatively decreased threat-related amygdala reactivity may be less inhibited and more likely to seek out social interactions, explaining their higher levels of extraversion, and specifically, warmth. The specificity of effects to the left amygdala was not hypothesized a priori, but may reflect the finding that the left amygdala evidences more sustained responses to threatening faces whereas the right amygdala habituates more quickly (40), such that the sustained signal in the left amygdala may have produced more robust effects. Prior research indicates that individuals with WS evidence greater amygdala reactivity to happy facial expressions relative to controls (10) and that in the general population greater amygdala reactivity to positive (e.g., happy) faces is also associated with higher levels of extraversion (13). We were unable to test this possibility in the current study because our fMRI paradigm does not include happy facial expressions. Thus, this represents an important future direction for research to further elucidate the neural mechanisms mediating the association between common variation in the GTF2I gene and extraversion.
In addition to the lack of happy facial expressions, the lack of threatening non-social stimuli is a limitation of the current investigation. Individuals with WS evidence low levels of social anxiety, but high levels of non-social anxiety, coupled with heightened amygdala reactivity to non-social, threatening scenes (9). We were unable to examine with the current paradigm whether the effect of GTF2I rs13227433 genotype was specific to threatening faces, or whether this would generalize to amygdala reactivity to threatening, non-social stimuli. Moreover, as neither rs4717907 nor rs13227433 were present on the Illumina arrays, we imputed these genotypes based on available genetic data. However, results were similar when using a genotyped proxy SNP in high linkage disequilibrium with rs4717907 and rs13227433 (see Supplementary Analyses), increasing confidence in our results and the quality of imputation. An additional limitation of the present study is that the DNS battery did not include a measure specific to social anxiety or a measure of social abilities similar to the Autism Spectrum Quotient used in prior research examining these GTF2I genotypes (7), and so we were unable to construct a WS profile similar to that examined by Crespi and Hurd. However, given that extraversion is negatively correlated with social anxiety (12), individuals who reported relatively higher levels of extraversion in the DNS likely also had low social anxiety. Moreover, by controlling for trait anxiety in our model, we were able to demonstrate that amygdala reactivity predicts individual differences in extraversion above and beyond broader anxiety and thus likely reflects socioemotional characteristics. Finally, it is also important to note that although effects were statistically significant, effect sizes were in the modest range for both paths. These effect sizes, however, are consistent with prior imaging genetics research (41, 42), and likely reflect the complex and polygenic nature of variability in both brain and behavior.
In summary, our results help shed light on the role of common variation in GTF2I in the emergence of individual differences in neural circuit function associated with prosocial behaviors, and suggest that a similar neural pathway may be implicated in the distinct social phenotype characteristic of WS, including hyper-sociability and increased approach of strangers. These results also have implications for identifying a potential genetic risk marker for social anxiety disorder, given that low levels of extraversion are associated with high social anxiety (12, 14). As such, our results provide a foundation for pursuing the molecular mechanisms through which variation in GTF2I may impact gene transcription, affect amygdala function, and influence individual differences in socioemotional behavior.
Supplementary Material
Acknowledgments
The Duke Neurogenetics Study is supported by Duke University and NIH grant DA033369. JRS received support through the Center for the Study of Adolescent Risk and Resilience (P30DA023026) and through NIH grant R01AG049789. RB is supported by the Klingenstein Third Generation Foundation and NIH grant AG045231. LWH is supported by NIH grant L40DA036468. ARH is supported by NIH grants R01DA033369 and R01AG049789. The authors would like to thank Dr. Qiang Chen, Ph.D. at the Lieber Institute for Brain Development for assistance with genome-wide SNP imputation and Spenser Radtke, B.S. for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Jarvinen-Pasley A, Bellugi U, Reilly J, Mills DL, Galaburda A, Reiss AL, et al. Defining the social phenotype in Williams syndrome: A model for linking gene, the brain, and behavior. Dev Psychopathol. 2008;20:1–35. doi: 10.1017/S0954579408000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberg DP, Jabbi M, Berman KF. Bridging the gene-behavior divide through neuroimaging deletion syndromes: Velocardiofacial (22q11.2 Deletion) and Williams (7q11.23 Deletion) syndromes. Neuroimage. 2010;53:857–869. doi: 10.1016/j.neuroimage.2010.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: A unique window to genetic influences on cognition and behaviour. Nature Reviews Neuroscience. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- 4.Dai L, Bellugi U, Chen XN, Pulst-Korenberg AM, Jarvinen-Pasley A, Tirosh-Wagner T, et al. Is it Williams syndrome? GTF2IRD1 implicated in visual-spatial construction and GTF2I in sociability revealed by high resoluation arrays. Am J Med Genet A. 2009;149A:302–314. doi: 10.1002/ajmg.a.32652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle TF, Bellugi U, Korenberg JR, Graham J. “Everybody in the world is my friend”: Hypersociability in young children with Williams syndrome. Am J Med Genet A. 2004;124A:263–273. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- 6.Hoeft F, Dai L, Haas BW, Sheau K, Mimura M, Mills D, et al. Mapping genetically controlled neural circuits of social behavior and visuo-motor integration by a preliminary examination of atypical deletions with Williams syndrome. PLOS One. 2014;9:e104088. doi: 10.1371/journal.pone.0104088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crespi BJ, Hurd PL. Cognitive-behavioral phenotypes of Williams syndrome are associated with genetic variation in the GTF2I gene, in a healthy population. BMC Neurosci. 2014;15:127. doi: 10.1186/s12868-014-0127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai T, Dorr NP, Takahashi N, McInnes A, Elder GA, Buxbaum JD. Haploinsufficiency of Gtf2i, a gene deleted in Williams Syndrome, leads to increases in social interactions. Autsim Research. 2011;4:28–39. doi: 10.1002/aur.169. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 10.Haas BW, Mills D, Yam A, Hoeft F, Bellugi U, Reiss A. Genetic influences on sociability: Heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. The Journal of Neuroscience. 2009;29:1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas BW, Hoeft F, Searcy YM, Mills D, Bellugi U, Reiss A. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia. 2010;48:1283–1288. doi: 10.1016/j.neuropsychologia.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naragon-Gainey K, Watson D, Markon KE. Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. J Abnorm Psychol. 2009;118:299–310. doi: 10.1037/a0015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- 14.Spinhoven P, Elzinga BM, van Hemert AM, de Rooij M, Penninx BW. A longitudinal study of facets of extraversion in depression and social anxiety. Personality and Individual Differences. 2014;71:39–44. [Google Scholar]
- 15.Binelli C, Subira S, Batalla A, Muniz A, Sugranyes G, Crippa JA, et al. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: A systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64:205–217. doi: 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Nikolova YS, Koenen KC, Galea S, Wang C, Seney ML, Sibille E, et al. Beyond genotype: Serotonin transporter epigenetic modification predicts human brain function. Nat Neurosci. 2014;17(9):1153–5. doi: 10.1038/nn.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prather AA, Bogdan R, Hariri AR. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom Med. 2013;75:350–358. doi: 10.1097/PSY.0b013e31828ef15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structure diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, Williams JBM. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition. New York State Psychiatric Institute, Biometrics Research Department; New York: 1996. [Google Scholar]
- 21.Eriksson N, Macpherson JM, Tung JY, Hon LS, Naughton B, Saxonov S, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tung JY, Do CB, Hinds DA, Kiefer AK, Macpherson JM, Chowdry AB, et al. Efficient replication of over 180 genetic associations with self-reported medical data. PLoS One. 2011;6:e23473. doi: 10.1371/journal.pone.0023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3. 2011;1:457–470. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2012;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, Thomas LA, Ferreira MA, Bender D, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa PT, Jr., MacCrae RR. Manual for the Revised NEO Personality Inventory (NEO-PIR) and the NEO Five-Factor Inventory (NEO-FFI) Psychological Assessment Resources, Inc.; Odessa, FL: 1992. [Google Scholar]
- 28.McCrae RR, Costa PT. Discriminant validity of NEO-PIR facet scales. Educational and Psychological Measurement. 1992;52:229–237. [Google Scholar]
- 29.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 30.DeBolle M, De Fruyt F, McCrae RR, Lockenhoff CE, Costa PT, Jr., Aguilar-Vafaie ME, et al. The emergence of sex differences in personality traits in early adolescence: A cross-sectional, cross-cultural study. J Pers Soc Psychol. 2015;108:171–185. doi: 10.1037/a0038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Segura-Puimedon M, Borralleras C, Pereze-Jurado LA, Campuzano V. TFII-I regulates target genes in the PI-3K and TGF-B signaling pathways through a novel DNA binding motif. Gene. 2013;527:529–536. doi: 10.1016/j.gene.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 35.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. PNAS. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SM, Williams CL. Contribution of serotonin type 3 receptors in the successful extinction of cued or contextual fear conditioned responses: Interactions with GABAergic signaling. Rev Neurosci. 2012;23:555–569. doi: 10.1515/revneuro-2012-0052. [DOI] [PubMed] [Google Scholar]
- 37.Haas BW, Smith AK. Oxytocin, vasopressin, and Willimas syndrome: epigenetic effects on abnormal social behavior. Frontiers in Genetics. 2015;6:28. doi: 10.3389/fgene.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 39.Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 40.Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- 41.Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolova YS, Singhi EK, Drabant EM, Hariri AR. Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes Brain Behav. 2013;12:516–524. doi: 10.1111/gbb.12035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.