Abstract
Study Objectives:
To evaluate the association between obstructive airway disease (OAD) and sleep apnea in older men.
Methods:
A community-based cross-sectional study of 853 community-dwelling older men (mean age 80.7 ± 4.1 years [range 73 to 90]) across 6 centers in the United States from the Outcomes of Sleep Disorders in Older Men Study. Sleep was objectively measured using full in-home polysomnography and lung function was objectively measured using spirometry. The association of OAD (pre-bronchodilator FEV1/FVC ratio < 0.7 and FEV1 < 80% predicted) and sleep apnea (apnea-hypopnea index [AHI] ≥ 15 events/hour) was assessed using logistic regression.
Results:
OAD and sleep apnea were identified in 111 (13.0%) and 247 (29.0%) men, respectively. In univariate analysis, participants with OAD had a lower AHI (mean ± SD; 8.7 ± 11.7 vs. 12.7 ± 13.8, P = 0.0009) and a lower prevalence of sleep apnea (14.4 vs. 31.1%, P = 0.0003) compared to participants without OAD. OAD remained independently associated with a lower odds of sleep apnea (odds ratio 0.30, 95% CI 0.16 to 0.55, P = 0.0001) after adjustment for demographics, body composition, smoking, and potential mediators (arousal index, time spent in rapid eye movement sleep). Individuals with OAD and sleep apnea (n = 16) had an increased arousal index and lower oxygen saturation level as compared to individuals with OAD alone (P values < 0.05).
Conclusions:
Obstructive airway disease was associated with a lower prevalence of sleep apnea in a cohort of community-dwelling elderly men, and unexplained by differences in adiposity or sleep architecture. Although uncommon in this cohort, coexisting sleep apnea and OAD was associated with increased sleep fragmentation and nocturnal oxygen desaturation compared to OAD alone.
Citation:
Zhao YY, Blackwell T, Ensrud KE, Stone KL, Omachi TA, Redline S, Osteoporotic Fractures in Men (MrOS) Study Group. Sleep apnea and obstructive airway disease in older men: outcomes of sleep disorders in older men study. SLEEP 2016;39(7):1343–1351.
Keywords: obstructive sleep apnea, obstructive airway disease, aging, sleep quality, overlap syndrome
Significance.
Although both sleep apnea and obstructive airway disease are common in community-dwelling elderly men, coexisting sleep apnea and obstructive airway disease is uncommon, and thus this study does not support use of obstructive airway disease as a trigger for initiating specific screening for sleep apnea. Obstructive airway disease is associated with a lower odds of sleep apnea, independent of antropometric characteristics. Future research is needed to identify the mechanisms for a protective association between obstructive airway disease and sleep apnea, which may include lung hyperinflation and altered respiratory arousability.
INTRODUCTION
Sleep apnea and obstructive airway disease (OAD) are both highly prevalent conditions. Recent literature estimates that sleep apnea affects approximately 27% of men and 9% of women, while OAD (both asthma and chronic obstructive pulmonary disease [COPD]) affects approximately 20% of the general population, with increased prevalence of airflow obstruction seen in the elderly and in males.1–5
Several studies have shown a high prevalence of sleep apnea among patients with COPD.6,7 Similarly, an increased prevalence of sleep apnea has been reported among individuals with asthma, with the prevalence of sleep apnea increasing with greater asthma severity.8 In addition, sleep apnea has also been associated with poorly controlled asthma and frequent asthma exacerbations.9 However, these studies were limited by relatively small sample size and were subject to potential selection bias. In contrast, authors from the Sleep Heart Health Study (SHHS) found that the median apnea-hypopnea index (AHI) was lower in participants with OAD, and that these associations were present across all categories of body mass index (BMI).10
OAD and sleep apnea each has been associated with altered sleep architecture, poor sleep quality, and nocturnal hypoxemia.11,12 Thus, patients with both conditions may have more disturbed sleep and more severe nocturnal hypoxemia, and may be at an increased risk for the development of adverse cardiovascular disease such as pulmonary hypertension, heart failure, cardiac arrhythmias, and even death compared to patients with either condition alone.13
Given the high prevalence of both OAD and sleep apnea in the elderly population, and the potential for increased adverse outcomes in individuals with both conditions, further research is needed to help understand the association between OAD and sleep apnea. The objectives of our study are (1) to characterize and quantify the association between OAD and sleep apnea in a large cohort of community-dwelling older men, (2) to determine the prevalence of concomitant sleep apnea and OAD in this population, (3) to characterize and compare sleep architecture and quality in participants with and without OAD, and (4) to explore whether sleep quality mediates the association between OAD and sleep apnea.
METHODS
A detailed description of the methodology is provided in the supplemental material.
Participants and Study Design
The study sample was derived from the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study, an ancillary study of the prospective, multicenter Osteoporotic Fractures in Men Study (MrOS).14,15 Between November 2009 and March 2012, 853 participants from 6 U.S. centers underwent spirometry and polysomnography (PSG) and were included in the current analysis (Figure 1). The mean number of days between spirometry and PSG was 5.5 ± 9.8 days, with 77% of participants completing their spirometry within 1 week of the PSG. Study protocols were approved by the institutional review board at each site, and all participants provided written informed consent.
Figure 1.
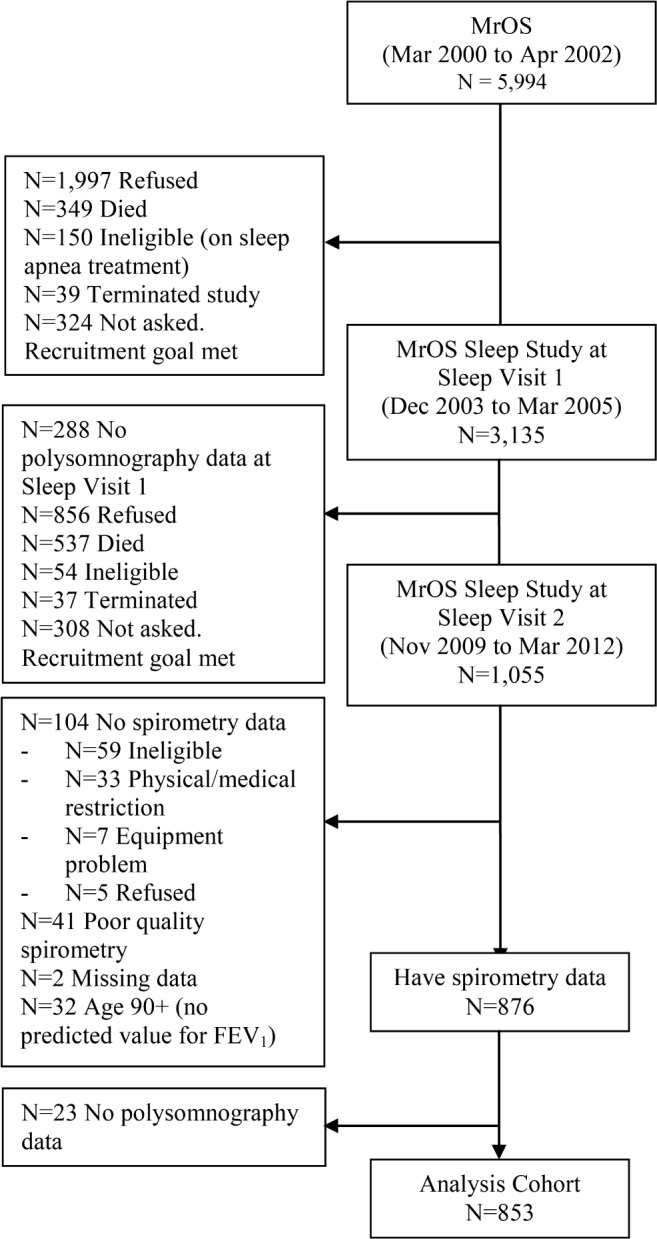
Flow diagram of patient recruitment from the initial Osteoporotic Fractures in Men (MrOS) Study, to the ancillary Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study, to the current study cohort. FEV1, forced expiratory volume in one second.
Polysomnography and Spirometry
Details regarding MrOS PSG methodology have been published.16,17 Briefly, in-home 13-channel portable PSG (Safiro; Compumedics, Inc., Melbourne, Australia) were completed and centrally scored at the Sleep Reading Center (Boston, MA) by trained research polysomnologists. Spirometry was performed using a SensorMedics model 1022 dry-rolling seal volume spirometer (SensorMedics; Yorba Linda, CA) and centrally reviewed at the Reading Center (Boston, MA). Testing and interpretation were performed according to American Thoracic Society recommendations.18 The largest forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) values were selected from an acceptable curve.
OAD Predictor
OAD was defined using the National Clinical Guidance Centre (NICE) criteria (pre-bronchodilator FEV1/FVC ratio < 0.70 and FEV1 < 80% predicted) using National Health and Nutrition Examination Survey (NHANES) reference equations.19,20 Additional analyses were conducted using the “fixed ratio” criteria of FEV1/FVC < 0.70 and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (FEV1/FVC < 0.70: mild if FEV1 ≥ 80% predicted, moderate to very severe if FEV1 < 80% predicted).21 Sensitivity analysis was performed using a ratio of FEV1/FVC < 0.65 given potential misclassification in the elderly when a higher threshold is used.22
Outcome Measures
Sleep apnea was defined by an AHI ≥ 15 events/h, which is the number of all apneas (episodes of absent or nearly absent airflow for ≥ 10 s) and hypopneas (≥ 30% reduction in amplitude of either respiratory effort or airflow with ≥ 4% oxygen desaturation) that occurred per hour of sleep. An AHI cutoff of 15 was chosen as older individuals present an increase in the number of age-related respiratory events during sleep.23 Respiratory events and arousals were scored according to published criteria.24 Baseline demographics including smoking status and clinical history were extracted from self-administered questionnaires. A score > 10 on the Epworth Sleepiness Scale indicated excessive daytime sleepiness,25 and a score > 5 on the Pittsburgh Sleep Quality Index indicated poor sleep.26 BMI was calculated as kg/m2.
Statistical Analysis
Between-group comparisons were made using χ2 tests for categorical variables, and t-tests or Wilcoxon rank-sum tests for continuous variables depending on distribution. Logistic regression models were used to assess the association between OAD and the outcome of sleep apnea, adjusting for age, race, study site, BMI and neck circumference, smoking status, and potential mediators (arousal index and percent of sleep time spent in REM) in successive models. Linear regression models using the same covariates were used to examine the association of OAD and a number of sleep-related outcomes including AHI, REM AHI, and percent of sleep times with SpO2 < 90%. Sensitivity analysis was performed excluding participants with self-reported asthma. Exploratory analyses were also performed to evaluate the association between categories of self-reported OAD and sleep apnea. Odds ratios (ORs) or adjusted means with 95% confidence intervals (CI) are presented. Two-tailed P values < 0.05 were considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Population Characteristics
Approximately 13% of the entire cohort had OAD as defined by criteria from the NICE guideline, 29% of the cohort had sleep apnea as defined by an AHI ≥ 15, and 1.9% of participants had coexisting OAD and sleep apnea (Table 1). Baseline characteristics by GOLD OAD stage is shown in Table S1 (supplemental material). Individuals with and without OAD did not differ by age, race, or BMI. However, those with OAD had larger waist and neck circumferences than those without OAD. Participants with self-reported chronic bronchitis, emphysema, or COPD (but not asthma) were more likely to be past or current smokers.
Table 1.
Baseline characteristics of the study population by presence of obstructive airway disease.
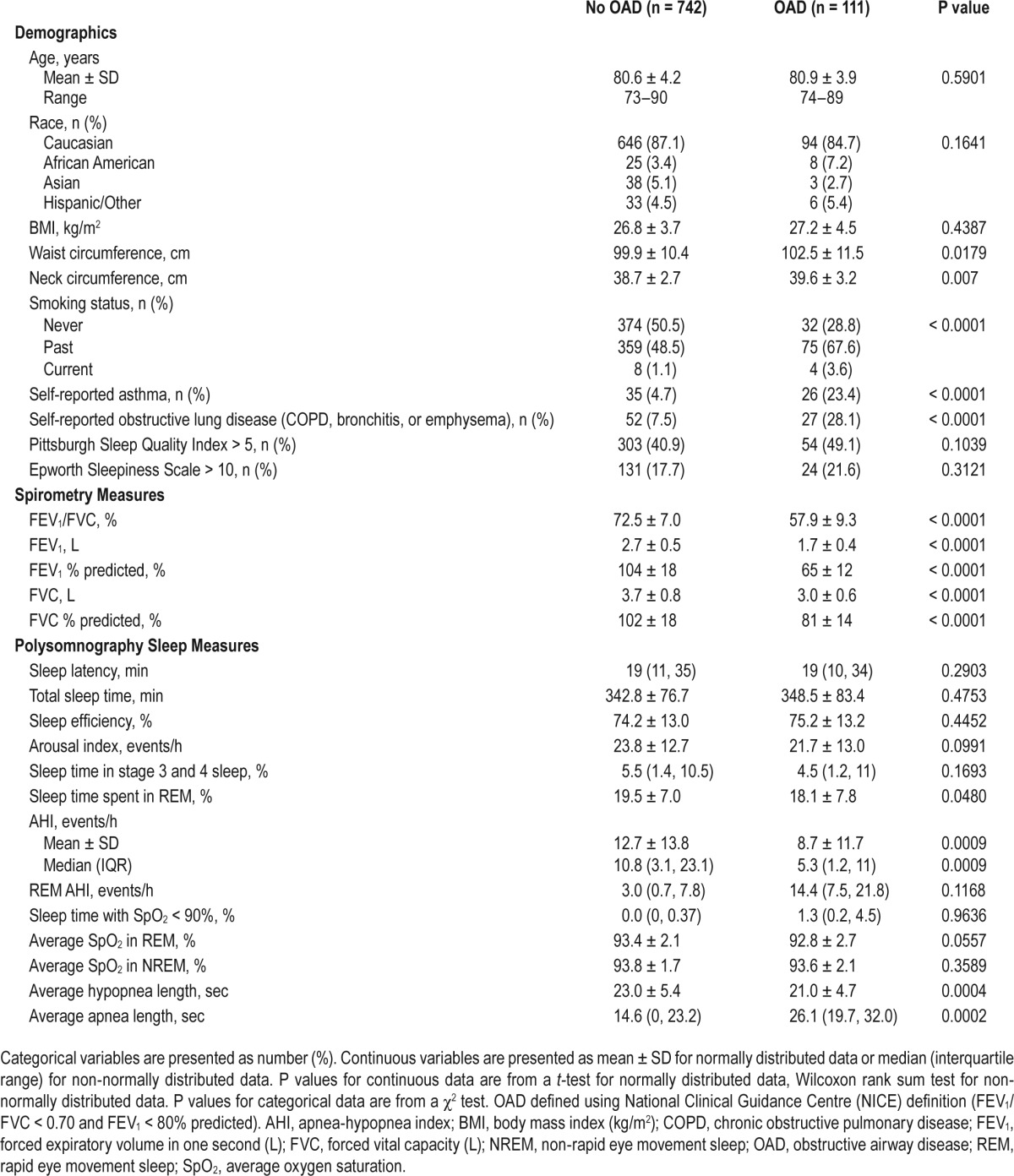
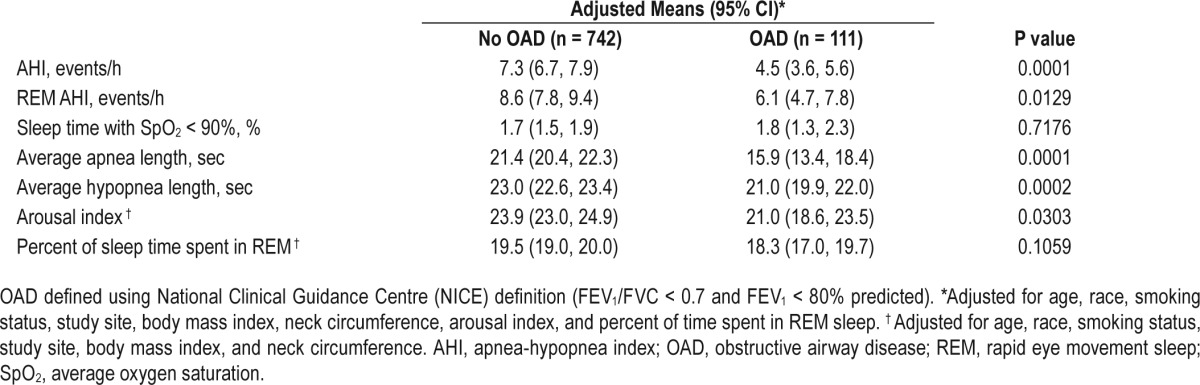
Compared to participants without OAD, those with OAD spent slightly less time in REM sleep. Time spent in other stages of sleep and measures of sleep quality were similar between the two groups (Table 1). A similar proportion of participants in both groups reported poor sleep quality and excessive daytime sleepiness, as assessed by the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale, respectively.
Compared to participants with AHI < 15, those with AHI ≥ 15 had greater adiposity and had generally worse sleep quality with reduced total sleep time, sleep efficiency, slow wave and REM sleep, and increased arousal index (see Table S2, supplemental material). Participants with sleep apnea also had a lower prevalence of never smoking, lower prevalence of self-reported asthma, and a higher FEV1/FVC ratio.
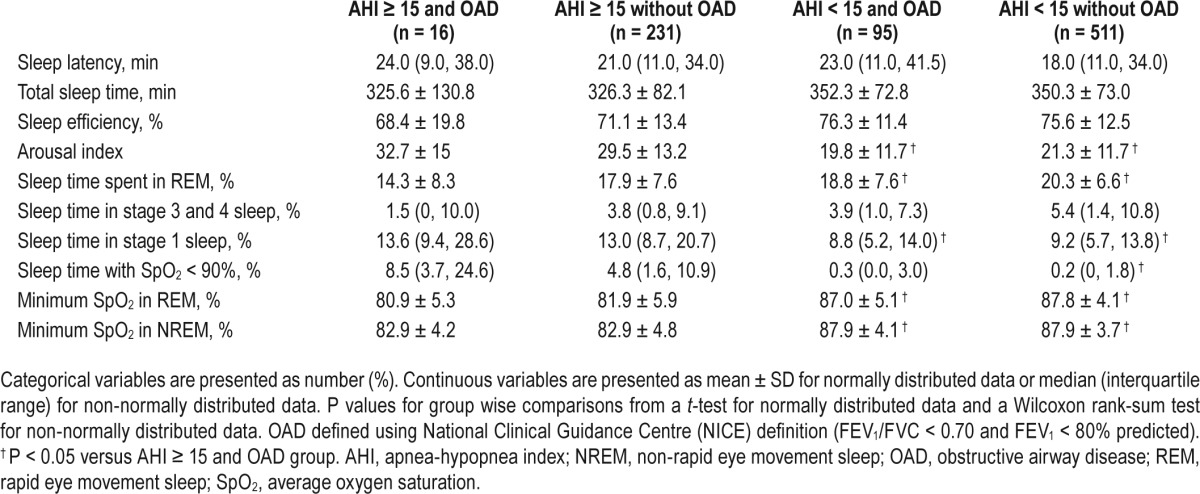
Sleep architecture and nocturnal oxygen saturation (SpO2) were similar between participants with coexisting sleep apnea and OAD and those with only sleep apnea (Table 2). However, compared to participants without sleep apnea (either with or without OAD), participants with both sleep apnea and OAD had significantly higher arousal index, spent more time in stage 1 sleep and less time in REM sleep, and spent a greater proportion of the night with SpO2 less than 90% (Table 2). Similarly, participants with both OAD and sleep apnea (n = 16) had higher arousal index (mean ± SD 32.7 ± 15 vs. 23.4 ± 12.7, P = 0.0038), spent more time in stage 1 sleep (median [IQR] 13.5 [9.4, 28.6] vs. 9.9 [6.4, 15.6], P = 0.0230) and less time in REM sleep (mean ± SD 14.3 ± 8.3 vs. 19.5 ± 7.1, P = 0.004), and spent a greater proportion of the night with SpO2 less than 90% (median [IQR] 8.5 [3.7, 24.6] vs. 0.75 [0.0, 4.2], P < 0.0001) compared to the rest of the participants.
Table 2.
Sleep architecture and oxygen saturation among participants with and without obstructive airway disease.
Using the “fixed-ratio” criterion of FEV1/FVC < 0.7, the prevalence of OAD in our study cohort was 42.2%, with 249 (29.2%), 97 (11.4%), and 14 (1.6%) having mild, moderate, and severe obstructive disease by GOLD criteria, respectively.26,32
Association between OAD and Sleep Apnea
In univariate analysis, mean AHI was significantly lower in participants with OAD than without OAD (Table 1). Participants with self-reported asthma also had a lower mean AHI than those without self-reported asthma (8 vs. 13, P = 0.0026). OAD was associated with a lower unadjusted odds of sleep apnea compared to non-OAD (OR 0.37, 95% CI 0.22 to 0.65) with only 16 (14.4%) participants in the OAD group having an AHI ≥ 15 compared to 231 (31.1%) in the non-OAD group (P = 0.0003). No significant differences in mean REM AHI, percent of sleep time with SpO2 less than 90%, mean SpO2 in REM and NREM sleep were noted between OAD and non-OAD groups. Participants in the OAD group also had shorter mean apnea and hypopnea lengths than those without OAD.
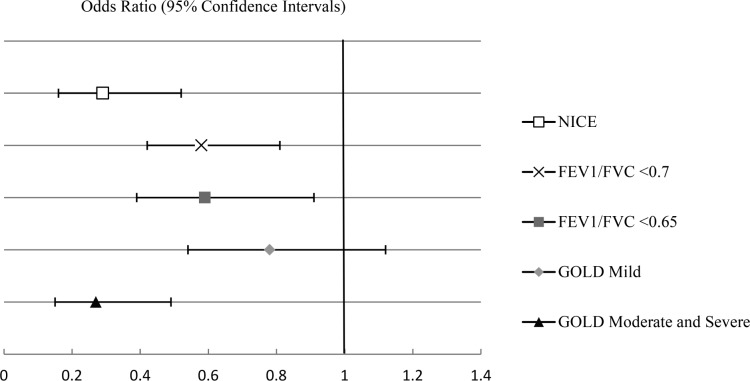
After adjusting for multiple potential confounders including adiposity and sleep architecture, OAD remained independently associated with a lower odds of sleep apnea (OR 0.30, 95% CI, 0.16–0.55, P = 0.0001) (Table 3). Inclusion of the baseline SpO2 as a covariate did not change our results (data not shown). Using two alternative definitions of OAD (FEV1/FVC ratio < 0.7 and GOLD), we found that those with moderate to severe obstructive disease by GOLD criteria had the lowest odds for sleep apnea (Figure 2). Similar association between OAD and sleep apnea was found using a FEV1/FVC ratio < 0.65 as cutoff for OAD. The association between OAD and sleep apnea was also consistent across all categories of self-reported OAD (see Table S3, supplemental material). Sensitivity analyses conducted using extrapolated values of FEV1 % predicted for those 90 years of age or older (n = 32) did not change results (data not shown). Exclusion of participants with self-reported asthma (n = 61) also did not change our main findings (data not shown).
Table 3.
Association of obstructive airway disease and sleep apnea.
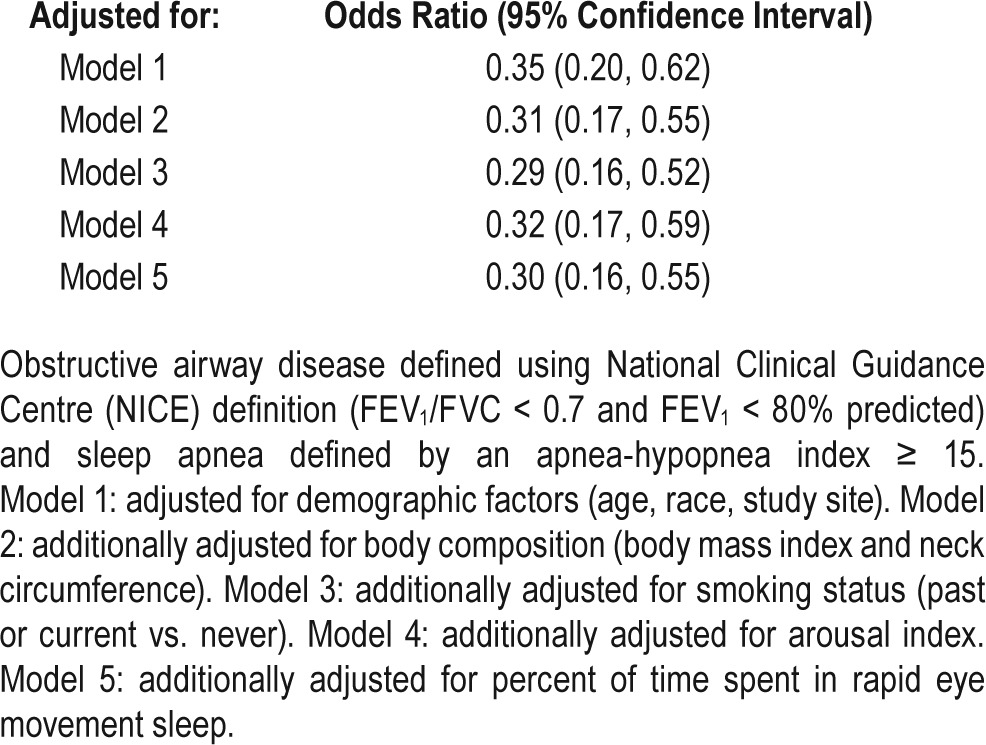
Figure 2.
Association of sleep apnea and obstructive airway disease using three different definitions: National Clinical Guidance Centre (NICE) (FEV1/FVC < 0.7 and FEV1 < 80% predicted), “fixed-ratio” FEV1/FVC < 0.7, Global Initiative for Chronic Obstructive Lung Disease (GOLD) (FEV1/FVC < 0.7: mild if FEV1 ≥ 80% predicted, moderate to severe if FEV1 < 80% predicted). Sensitivity analysis was conducted using a ratio of FEV1/FVC < 0.65. Sleep apnea was defined as an apnea-hypopnea index ≥ 15. Models were adjusted for age, race, study site, body mass index, neck circumference, and smoking status. FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Table 4 shows the association between OAD and several sleep variables. In adjusted models, compared to participants in the non-OAD group, those in the OAD group had a significantly lower mean AHI and REM AHI after adjusting for potential confounders. Participants in the OAD group also had shorter mean apnea and hypopnea lengths, and lower arousal index compared to participants in the non-OAD group. After excluding subjects with self-reported asthma, the adjusted difference in REM AHI between participants with and without OAD was no longer significant (data not shown).
Table 4.
Association of obstructive airway disease with sleep apnea and potential mediators.
DISCUSSION
In this large community-based study of elderly men evaluated with standardized PSG and spirometry, we found that OAD and sleep apnea were both prevalent conditions, but were uncommonly observed together. Approximately 13% had OAD (FEV1/FVC < 0.7 and FEV1 < 80% predicted) and 29% had moderate to severe sleep apnea (AHI ≥ 15). Sleep apnea was observed in 14.4% of participants with OAD, and coexisting sleep apnea and OAD was found in only 1.9% of the entire cohort. OAD was associated with a lower prevalence of sleep apnea in multivariable analysis, and this relationship was consistent using different definitions of OAD and across different types of OAD. Sleep architecture and quality were similar between OAD and non-OAD groups, as were self-reported sleep quality and daytime sleepiness. However, although uncommon in this cohort, participants with both sleep apnea and OAD had worse sleep quality and greater nocturnal oxygen desaturation compared to participants with only OAD or those with neither condition. Our data do not support OAD as a significant risk factor for sleep apnea, but rather indicate that among elderly men, OAD is associated with a reduced prevalence of sleep apnea.
There have been conflicting data in the literature regarding the prevalence of sleep apnea in patients with OAD. Several earlier reports of increased prevalence of sleep apnea in OAD patients may be limited by the small size of the studies, selection bias, and use of self-reported diagnosis in the estimation of prevalence.6–8 Our study found that overlapping OAD and sleep apnea was present in 1.9% of the study population. It is possible that our results were subject to a survivorship bias, in that individuals with coexisting OAD and sleep apnea did not survive to an older age to be included in the study. It is also possible that selection bias influenced participation in the sleep study. However, the observed prevalence rate of both conditions is similar to the 1% reported by Bednarek and colleagues in a younger cohort (mean age 56.6).27 The SHHS reported a 19% prevalence of OAD and an 18% prevalence of sleep apnea (AHI ≥ 15).10 The higher prevalence of sleep apnea in our study is likely due to the older age of our cohort (mean age in OAD group 80.9 vs. 64.5 in the SHHS) and our inclusion of only men. The lower prevalence of OAD seen in our study is likely due to the more specific definition of OAD in the present study (FEV1/FVC < 0.70 and FEV1 < 80% predicted) compared to the SHHS definition (FEV1/FVC < 0.70). In fact, using the “fixed-ratio” criteria of FEV1/FVC < 0.7 identified 42.2% of our cohort with OAD. The prevalence and severity of OAD observed in our cohort is comparable to those observed for males and for older adults from recently published NHANES data.4,5,28 In SHHS, subjects with OAD had lower mean AHI values than those without OAD, and the AHI tended to be lower as the FEV1/FVC ratio decreased.10 The significance of those findings was not clear and concerns about residual confounding by obesity were noted. The results suggested by the SHHS are now confirmed in the present study, whereby OAD was shown to be associated with a lower odds of sleep apnea in multivariable analyses. Providing further strength to our finding, we demonstrated similar results using different definitions of OAD and across different self-reported categories of OAD.
Our analyses also provide some support for an inverse “dose-response” relationship between severity of OAD and prevalence of sleep apnea. Individuals with moderate to severe airflow obstruction by GOLD criteria had the lowest odds for sleep apnea compared to a referent group without OAD, while those with only mild OAD did not have a decreased odds of sleep apnea. Resta et al. also noted patients with overlap syndrome showed less severe obstructive impairment than patients with COPD alone.29
Prior research from the Wisconsin Sleep Cohort Study has shown that a self-reported diagnosis of asthma was associated with an increased risk of new-onset obstructive sleep apnea,30 Although we did not have specific information to accurately characterize asthma in this elderly population, we found that the association between self-reported asthma and sleep apnea to be similar to our main finding. Compared to those without self-reported asthma, those with self-reported asthma in our study (n = 61) had a lower mean AHI (13. vs. 8 events/h, P = 0.0026) and fewer participants had an AHI > 15 (30% vs. 13%). Participants with self-reported asthma also had a lower odds of sleep apnea compared to participants without self-reported asthma (OR 0.34, 95% CI, 0.15–0.74). These findings may reflect differences in asthma phenotypes in older versus younger people, or due to differences in asthma-COPD overlap in the elderly. Study participants from the Wisconsin study were younger (mean age 50.4 vs. 80.7 years in our cohort) and 52% were female. Additionally, participants with OAD in our study had significantly worse lung function compared to the subset of participants with asthma and spirometry data (n = 33) in the Wisconsin study (mean FEV1/FVC ratio 0.58 vs. 0.77 and FEV1 % predicted 65% vs. 92%).
Although the exact mechanism underlying the association between OAD and sleep apnea is unknown, we propose a number of pathophysiological mechanisms that may explain these findings. One such mechanism is that lung hyperinflation secondary to OAD is associated with decreased upper airway resistance and collapsibility and increased upper airway lumen dimensions.31–33 Additionally, it is plausible that reduced diaphragmatic efficiency, resulting from respiratory muscle dysfunction, altered respiratory muscle interactions, and metabolic derangements seen in OAD, may reduce the negative pressures exerted in the upper airways, protecting against upper airway collapse during sleep.34 Studies in subjects with both OAD and sleep apnea are needed to further explore this possible mechanistic link. Alternatively, OAD subjects may have altered body mass and body fat distribution, and that the effects of obesity on sleep apnea in these patients differ than in control subjects. Although we noted significant differences in anthropometric characteristics between OAD and non-OAD groups, the higher waist and neck circumferences in the OAD group would be expected to increase rather than decrease risk of sleep apnea. One study found that BMI was a stronger predictor of sleep apnea severity in control subjects compared to COPD subjects, and that REM-related events during sleep were not dependent on overall AHI or obesity in COPD subjects.35 Another possibility is that OAD may be associated with lighter sleep and greater arousability, resulting in a greater propensity for apneas to be terminated in response to pharyngeal obstruction, facilitating resumption of airflow. Although we did not observe significant differences in sleep architecture between participants with and without OAD, the finding of shorter apnea and hypopnea lengths in the OAD group supports such a mechanism. In addition, OAD has been associated with reduced REM sleep, a period of the night particularly vulnerable to upper airway collapse and obstructive sleep apnea. In our study, participants with OAD spent less time in REM sleep than those without OAD, although percent of sleep time spent in REM was not an important predictor in multivariable analysis. Lastly, nicotine may also play a role in modulating the interaction between OAD and sleep apnea. Nicotine has been shown in animal models to increase ventilation, stimulate upper airway muscles, and reduce upper airway resistance.36 Gothe et al. reported a reduction in apneas in eight male patients with sleep apnea syndrome treated with nicotine gum.37 In our cohort, only four individuals with OAD were current smokers, making it unlikely that current nicotine exposure explained the observed findings.
We did not observe significant differences in sleep quality between participants with and without OAD, which may be due to the relatively mild degree of airflow obstruction seen in OAD participants. Reflecting this, no significant differences in mean or minimum SpO2 were noted between the OAD and non-OAD groups during REM and NREM sleep. However, we did observe increased nocturnal oxygen desaturation and worse sleep quality among participants with both OAD and sleep apnea, including increased arousal index and stage 1 sleep, and reduced REM sleep, compared to participants with only OAD and no sleep apnea. These findings suggest that coexisting sleep apnea may be of clinical significance in patients with OAD.
Our study directly evaluated the association between OAD and sleep apnea in a relatively healthy sample of community-based older men. The use of standardized spirometry and PSG allowed for accurate and objective assessment of OAD and sleep. In addition, comprehensive and detailed collection of covariate data with standardized scoring approaches allowed alternative definitions of OAD to be evaluated. Limitations of our study include the availability of only a single night of PSG data. However, the night-to-night variability for the AHI determined using home polysomnography is low.38 We also did not have data on airway responsiveness or imaging data, precluding further characterization of OAD phenotypes. By convention, we used a FEV1/FVC ratio < 0.70 and reduced FEV1 to define OAD. Recognizing that this definition may overestimate OAD in this elderly cohort, we performed a sensitivity analysis using FEV1/FVC ratio < 0.65 and noted a similar association between OAD and sleep apnea. The cross-sectional design of our study precludes inferring causality. In addition, the study results may not be generalizable to groups other than predominantly white, community-dwelling older men. Given the inconsistencies in the literature, further work, including longitudinal research and potentially interventional studies, is required to understand the causal relationships, including potential effect modification by age, gender, and OAD phenotype (asthma vs. COPD). We note, however, that the observed cross-sectional relationship between sleep apnea and OAD appears less likely to be due to a protective effect of sleep apnea against OAD, given the lack of biologic mechanisms to support such causality. Finally, the low prevalence of severe OAD limits evaluation of sleep in this subset of participants.
CONCLUSIONS
OAD is associated with a lower odds of sleep apnea in a large cohort of community-dwelling elderly men. Future studies are needed to investigate the mechanisms underlying this association, including the roles of OAD phenotype, lung volume, and arousability in patients with both OAD and sleep apnea. We also observed a low prevalence of coexisting sleep apnea and OAD, which may be clinically significant as it leads to increased sleep fragmentation and nocturnal oxygen desaturation compared to OAD alone.
DISCLOSURE STATEMENT
This was not an industry sponsored study. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Drs. Redline is the site PI for a phase III trial sponsored by Jazz Pharmaceuticals Inc. Dr. Omachi is currently an employee of Genentech. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the investigators in the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep): Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): K. L. Stone (Principal Investigator), D.C. Bauer (co-Investigator), S.R. Cummings (co-Investigator), N. Goldschlager (co-Investigator), G. Tranah (co-Investigator), P. Varosy (co-Investigator), K. Yaffe (co-Investigator), P.M. Cawthon (co-Investigator), R. Fullman (Project Director), R. Benard, T. Blackwell, L. Concepcion, J. Diehl, S. Ewing, C. Fox, M. Jaime-Chavez, E. Kwan, S. Litwack, W. Liu, L.Y. Lui, K. Peters, W. Sauer, J. Schneider, R. Scott, D. Tanaka, J. Ziarno; Administrative Center (Oregon Health & Sciences University): E. Orwoll (Principal Investigator), C. Lee (co-Investigator), C. Pedersen (Project Director), M. Abrahamson, L. Masterfield; University of Alabama, Birmingham: C. E. Lewis (Principal Investigator), J. Shikany (co-Investigator), P. Johnson (Project Director), M. Young, S. House, N. Webb, S. Felder, J. King, T. Johnsey, C. Collier, K. Hardy, J. Smith, H. Dwivedi; University of Minnesota: K. Ensrud (Principal Investigator), S. Diem (co-Investigator), H. Fink (co-Investigator), N. Nelson (Clinic Coordinator), R. Andrews, S. Fillhouer, M. Forseth, K. Jacobson, S. Luthi, K. Moen, M. Paudel, P. Van Coevering, S. Ziesche; Stanford University: M. Stefanick (Principle Investigator), A. Hoffman (co-Investigator), K. Kent, N. Ellsworth, S. Belding, A. Krauss; University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (co-Investigator), M. Danielson (Study Administrator), L. Harper (Project Director), L. Buck (Clinic Coordinator), D. Cusick, M. Gorecki, C. Newman; University of California, San Diego: E. Barrett-Connor (Principal Investigator), S. Ancoli-Israel (co-investigator), T. Dam (co-Investigator), ML Carrion-Petersen (Project Director), D. Claflin, N. Kamantigue, K. Marksbury Jappe, P. Miller, M. Stephens; Brigham and Women's Hospital Sleep Reading Center: S. Redline (Principal Investigator), S. Surovec (Project Administrator), D. Mobley (Chief Polysomonologist), M. Rueschman (Programmer Analyst), M. Morrical (Polysomnologist), J. Arnold (Polysomonologist), R. Nawabit (Polysomnologist).
Author Contributions: Dr. Zhao and Ms. Blackwell had full access to all of the data in the study and are responsible for the integrity of the data and the accuracy of the data analysis; Drs. Zhao, Redline, Stone, Omachi, and Ensrud contributed to the study design. Drs. Ensrud and Stone contributed to the data collection. Ms. Blackwell, Drs. Zhao, Stone and Omachi contributed to the data analysis. Ms. Blackwell, Drs. Zhao, Redline, Stone and Omachi contributed to the interpretation of results. All authors contributed to the preparation of the manuscript.
Ethics Approval: The study was approved by the ethical review board at each of the six participating centers: University of Alabama at Birmingham Institutional Review Board for Human Use, Birmingham, USA (F030725004); Human Research Protection Program at the University of Minnesota, Minnesota, USA (0307M50161); Stanford University, California, USA (13647); University of Pittsburgh, Pennsylvania, USA (IRB980305); Oregon Health & Science University, Oregon, USA (IRB00001296); University of California, San Diego Human Research Protections Program, California, USA (071795).
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- FEV1/FVC
forced expiratory volume in one second to forced vital capacity ratio
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- MrOS
Osteoporotic Fractures in Men Study
- MrOS Sleep
Outcomes of Sleep Disorders in Older Men Study
- NICE
National Clinical Guidance Centre
- NHANES
National Health and Nutrition Examination Survey
- NREM
non-rapid eye movement sleep
- OAD
obstructive airway disease
- OR
odds ratio
- PSG
polysomnography
- REM
rapid eye movement sleep
- REM AHI
apnea-hypopnea index in rapid eye movement sleep
- SHHS
Sleep Heart Health Study
- SpO2
average oxygen saturation
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. [Accessed Feb 5, 2014]. Available from: http://www.ginasthma.org/
- 3.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–32. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 4.Doney B, Hnizdo E, Dillon CF, Paulose-Ram R, Tilert T, Wolz M, Beeckman-Wagner LA. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12:355–65. doi: 10.3109/15412555.2014.948998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using preand post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007-2010. Respir Res. 2013;14:103. doi: 10.1186/1465-9921-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:82–6. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 7.Larsson LG, Lindberg A, Franklin KA, Lundback B. Obstructive sleep apnoea syndrome is common in subjects with chronic bronchitis. Report from the Obstructive Lung Disease in Northern Sweden studies. Respiration. 2001;68:250–5. doi: 10.1159/000050506. [DOI] [PubMed] [Google Scholar]
- 8.Julien JY, Martin JG, Ernst P, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124:371–6. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 9.ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26:812–8. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 10.Sanders MH, Newman AB, Haggerty CL, et al. Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 11.McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17:1119–24. doi: 10.1111/j.1440-1843.2012.02217.x. [DOI] [PubMed] [Google Scholar]
- 12.Luyster FS, Teodorescu M, Bleecker E, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath. 2012;16:1129–37. doi: 10.1007/s11325-011-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mieczkowski B, Ezzie ME. Update on obstructive sleep apnea and its relation to COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:349–62. doi: 10.2147/COPD.S42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlated of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2007;55:1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.NICE Guideline Chronic Obstructive Pulmonary Disease CG101. [Accessed June 2, 2015]. http://www.nice.org.uk/guidance/cg101.
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.Viegi G, Pedreschi M, Pistelli F, et al. Prevalence of airways obstruction in a general population: European Respiratory Society vs American Thoracic Society definition. Chest. 2000;117:339S–345S. doi: 10.1378/chest.117.5_suppl_2.339s. [DOI] [PubMed] [Google Scholar]
- 22.Szanto O, Montnemery P, Elmstahl S. Prevalence of airway obstruction in the elderly: results from a cross-sectional spirometric study of nine age cohorts between the ages of 60 and 93 years. Prim Care Respir J. 2010;19:231–6. doi: 10.4104/pcrj.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young T, Shahar E, Nieto J, et al. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 25.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72:142–9. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988-1994 to 2007-2010. Chest. 2013;143:1395–406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resta O, Foschino Barbaro MP, Brindicci C, Nocerino MC, Caratozzolo G, Carbonara M. Hypercapnia in overlap syndrome: possible determinant factors. Sleep Breath. 2002;6:11–8. doi: 10.1007/s11325-002-0011-6. [DOI] [PubMed] [Google Scholar]
- 30.Teodorescu M, Barnet J, Hagen E, Palta M, Young TB, Peppard PE. Association between asthma and risk of developoing obstructive sleep apnea. JAMA. 2015;313:156–64. doi: 10.1001/jama.2014.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–31. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 32.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130:175–8. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 33.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 34.Decramer M. Hyperinflation and respiratory muscle interaction. Eur Respir J. 1997;10:934–41. [PubMed] [Google Scholar]
- 35.Krieger AC, Patel N, Green D, et al. Respiratory disturbance during sleep in COPD patients without daytime hypoxemia. Int J Chron Obstruct Pulmon Dis. 2007;2:609–15. [PMC free article] [PubMed] [Google Scholar]
- 36.Strohl KP, Gottfried SB, Van de Graaff W, Wood RE, Fouke JM. Effects of sodium cyanide and nicotine on upper airway resistance in anesthetized dogs. Respir Physiol. 1986;63:161–75. doi: 10.1016/0034-5687(86)90111-8. [DOI] [PubMed] [Google Scholar]
- 37.Gothe B, Strohl KP, Levin S, Cherniack NS. Nicotine: a different approach to treatment of obstructive sleep apnea. Chest. 1985;87:11–7. doi: 10.1378/chest.87.1.11. [DOI] [PubMed] [Google Scholar]
- 38.Quan SF, Griswold ME, Iber C, et al. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography—the Sleep Heart Health Study. Sleep. 2002;25:843–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.