Abstract
Study Objectives:
Data have demonstrated adverse health effects of sleep deprivation. We postulate that oxidative stress and systemic inflammation biomarkers will be elevated in relation to short-term and long-term sleep duration reduction.
Methods:
We analyzed data from the baseline examination of a randomized controlled trial involving participants with moderate to severe obstructive sleep apnea (OSA). Baseline polysomnography provided the total sleep time (PSG-TST, primary predictor); self-reported habitual sleep duration (SR-HSD) data was collected. Morning measures of oxidative stress and systemic inflammation included: myeloperoxidase (MPO, pmol/L), oxidized low-density lipoprotein (ox-LDL, U/L), F2-isoprostane (ng/mg), paraoxonase 1 (PON1, nmol·min−1·mL−1), and aryl esterase (μmol·min−1·mL−1). Linear models adjusted for age, sex, race, body mass index (BMI), cardiovascular disease (CVD), smoking, statin/anti-inflammatory medications, and apnea-hypopnea index were utilized (beta estimates and 95% confidence intervals).
Results:
One hundred forty-seven participants comprised the final analytic sample; they were overall middle-aged (51.0 ± 11.7 y), obese (BMI = 37.3 ± 8.1 kg/m2), and 17% had CVD. Multivariable models demonstrated a significant inverse association of PSG-TST and MPO (β [95% CI] = −20.28 [−37.48, −3.08], P = 0.021), i.e., 20.3 pmol/L MPO reduction per hour increase PSG-TST. Alternatively, a significant inverse association with ox-LDL and SR-HSD was observed (β [95% CI] = 0.98 [0.96, 0.99], P = 0.027), i.e., 2% ox-LDL reduction per hour increase SR-HSD.
Conclusions:
Even after consideration of obesity and OSA severity, inverse significant findings were observed such that reduced PSG-TST was associated with elevated MPO levels and SR-HSD with ox-LDL, suggesting differential up-regulation of oxidative stress and pathways of inflammation in acute versus chronic sleep curtailment.
Clinical Trial Registration:
NIH clinical trials registry number NCT00607893.
Citation:
DeMartino T, Ghoul RE, Wang L, Bena J, Hazen SL, Tracy R, Patel SR, Ackley D, Mehra R. Oxidative stress and inflammation differentially elevated in objective versus habitual subjective reduced sleep duration in obstructive sleep apnea. SLEEP 2016;39(7):1361–1369.
Keywords: oxidative stress, sleep deprivation, obstructive sleep apnea, obstructive sleep apnea, oxidized LDL
Significance.
Although data have implicated up-regulation of systemic inflammation and oxidative stress in obstructive sleep apnea (OSA), the extent to which polysomnographically-ascertained reduced sleep duration (PSG-TST) versus subjective chronic habitual reduction of self-reported sleep duration (SR-HSD) relates to these pathways remain unclear. We identify differential linear increases of oxidative stress and systemic inflammation measures—recognized established markers of cardiovascular risk—in relation to reduced sleep duration in moderate to severe OSA. Increases in myeloperoxidase levels were observed with reduction PSG-TST versus increases in oxidized LDL with reduction of SR-HSD after consideration of confounders including obesity and cardiovascular risk. Future investigation should focus on further clarification of the role of reduced sleep in OSA in relation to risk of cardiovascular outcomes.
INTRODUCTION
Both epidemiologic and experimental data have consistently demonstrated adverse health effects of sleep deprivation. This translates into an exceptional degree of population-attributable health burden, particularly as the number of US adults sleeping 6 h or less in a 24-h period has approximately doubled over the past nearly 30 y, from 38.6 million to 70.1 million according to data from the Centers for Disease Control and Prevention.1 The negative health consequences of reduced sleep duration described in epidemiologic studies are numerous and encompass chronic diseases, including objective measures of atherosclerosis2 and cardiovascular disease,3 obesity,4 and increased total mortality5 as highlighted in a joint statement by the American Academy of Sleep Medicine and Sleep Research Society.6 As an increasing proportion of the population experiences sleep restriction, understanding the biology underlying the associated negative health outcomes is becoming readily apparent.
Up-regulation of systemic inflammation has been postulated to represent a key underlying mechanism in sleep deprivation-mediated effects of chronic health disease progression. Differential findings have been described relative to systemic inflammation and subjective chronic habitual reduced sleep duration versus objective polysomnography (PSG)-identified reduced sleep suggesting distinctive mechanistic pathways.7 Overall, the understanding of the patterns of biochemical marker alterations in acute versus chronic sleep loss is limited. Reduced sleep duration objectively ascertained by PSG in individuals with obstructive sleep apnea (OSA) has been understudied. Moreover, relationships with OSA have not uniformly been associated with increased inflammatory cytokines after correcting for confounding influences such as obesity.8 Although reduced sleep is considered to represent a pathologic factor in OSA leading to adverse cardiovascular health sequela, the relationships of acute versus chronic sleep loss in OSA relative to changes in systemic inflammation and oxidative stress is unclear.
Myeloperoxidase (MPO), an enzymatic catalyst for generating reactive oxidants involved in the promotion of lipid peroxidation and a measure of vascular inflammation,9 as well as oxidized low-density lipoproteins (Ox-LDL), a reactive oxidant generated by lipid peroxidation of LDL,10–12 are well-established markers of oxidative stress and inflammation that have been implicated in cardiovascular disease and its progression. Despite the well-established roles of MPO and Ox-LDL, these markers have been relatively understudied in terms of reduced sleep duration in those with OSA. Available data involve small-scale experimental studies that have demonstrated an increase in MPO and MPO-modified LDL levels with polysomnogram-documented sleep restriction in those without OSA.13,14 We therefore conducted an examination to investigate the relationship of oxidative stress markers with a focus on MPO and oxidized LDL relative to sleep duration ascertained via PSG and alternatively by self-reported sleep duration, the latter to gain a sense of habitual sleep duration. We hypothesize that objective PSG-ascertained and subjective self-reported sleep loss in individuals with OSA is associated with increasing levels of oxidative stress with possible differential biomarker elevations.
METHODS
Study Sample
Patients with moderate to severe OSA (apnea-hypopnea index [AHI] ≥ 15) were recruited from Case Western Reserve University affiliated institutions, University Hospitals Case Medical Center (UHCMC), and Metro Health Medical Center sleep programs as part of a randomized controlled trial (Sleep Apnea Stress Study, SASS) aimed to assess the extent to which continuous positive airway pressure (CPAP) and sham CPAP influences a change of oxidative stress levels in patients with OSA (www.clinicaltrials.gov Trial Registration Number: NCT00607893). The current study used the baseline data that were obtained prior to randomization to CPAP versus sham CPAP.
Study Protocol
Participants underwent research-attended PSG and data collection at the University Hospitals Case Medical Center Dahms Clinical Research Unit, during which baseline blood samples and physiological data were collected and recorded. The inclusion criteria were individuals between the ages of 20–75 y, and those with moderate to severe OSA (AHI > 15) established by routine clinical PSG. The exclusion criteria included current or planned use of specific OSA treatments, supplemental oxygen use, primary sleep disorder other than OSA (including shift work sleep disorder), unstable medical conditions, inability to provide informed consent, increased risk for accidents related to sleepiness, alcohol abuse, pregnancy, and potent anti-inflammatory/immunosuppressant medications (i.e. prednisone, azathioprine, etc.). After inclusion and exclusion criteria were applied, 147 participants completed the baseline visit and comprised the final analytic sample. Institutional Review Board approval and full written informed consent were obtained.
Data Collection
Questionnaires and Anthropometric Measurements
The Case Western Reserve University Sleep and Health Questionnaire was used in order to assess symptoms of sleep disorder breathing, medical history, surgical history, social habits (e.g. smoking), and the Epworth Sleepiness Scale (ESS), which is a validated measurement to assess subjective sleepiness symptoms.15 Self-reported habitual sleep duration (SR-HSD) was calculated by a weighted mean of weekday and weekend values (5/7 * weekday sleep duration + 2/7 * weekend sleep duration) collected in response to the following questions: “During the past month, at what time, on average have you gone to bed (closed eyes in attempt to fall asleep) and woken up (after your sleep period)?” A detailed medical and medication history was also obtained including self-reported cardiovascular disease (CVD) as well as any use of statins or anti-inflammatory medications (e.g., nonsteroidal anti-inflammatory medications, aspirin, etc.). Cardiovascular disease was defined as self-reported chest pain, angina, carotid endarterectomy, congestive heart failure (CHF), coronary angioplasty, coronary artery bypass graft surgery, myocardial infarction, or stroke occurrence. Height was measured with a wall-mounted stadiometer to the nearest centimeter and a calibrated scale was used for the measurements of both weight (nearest 0.1 kg) and body mass index (BMI, kg/m2).
Sleep Parameters
An attended 14-channel PSG (Compumedics E series system, Abbotsville, AU) was performed at the baseline visit on all enrolled participants. The recording montage consisted of C3/A2 and C4/A1 electroencephalograms (EEG) (recorded at 128 Hz); bilateral electrooculograms; a bipolar submental electromyo-gram; thoracic and abdominal respiratory inductance plethysmography; airflow (by nasal-oral thermocouple); oximetry (sampling frequency 1 Hz), electrocardiogram (ECG) (recorded at 256 Hz); body position (mercury switch sensor); bilateral leg movements (piezo sensors) and nasal pressure transducers. Sensors were calibrated and signal quality was monitored by a certified technician using standardized techniques. The studies were scored by a trained, registered polysomnologist following the American Academy for Sleep Medicine guidelines.16 The PSG total sleep time (PSG-TST) was ascertained from the overnight baseline PSG. An apnea was measured by using a nasal thermal sensor and defined as a ≥ 90% reduction of airflow for ≥ 10 sec.16 A hypopnea was measured by using nasal pressure transducer and characterized by a ≥ 30% reduction in breathing amplitude, which lasts ≥ 10 sec and associated with ≥ 3% oxygen desaturation.16
Oxidative Stress/Systemic Inflammation Biomarkers
Blood was drawn after overnight fasting (and prior to blood pressure monitoring) in the supine position between 07:00– 08:00 the morning after the PSG. In addition, 24-h urine samples were collected in the home starting the night prior to the overnight Clinical Research Unit visit for F2-isoprostane levels. The samples were then centrifuged and aliquoted according to standard protocols and stored at −80°C until ready for analysis. All individual samples were collected and sent to either the Cleveland HeartLab in Cleveland, OH where MPO, paraoxonase 1 (PON1), aryl esterase, and F2-isoprostane bio-marker levels were analyzed or the University of Vermont Clinical Biochemistry Laboratory in Burlington, Vermont where ox-LDL levels were analyzed. All samples were batched and then sent to the respective laboratories in an attempt to limit the amount of measurement variability that could occur during analysis. Plasma MPO levels were analyzed with an enzyme-linked immunosorbent assay (ELISA)-based assay (CardioMPO Enzyme Immunoassay Reagent Kit) with a total and within-run coefficient of variation of 8.2% and 5.5%, respectively.17 MPO has biphasic kinetics with a short half-life of 2 to 3 h and longer half-life of 8 to 10 h.17 Plasma Ox-LDL levels were measured by the use of competitive ELISA (Mercodia Oxidized LDL Competitive ELISA, Mercodia AB; Uppsala, Sweden) that used monoclonal antibodies. The detectable range of Ox-LDL is 32 to 410 U/L with an approximate normal range of 40 to 100 U/L. The coefficient of variation is less than 10%. F2-isoprostanes levels were analyzed by using an established, stable isotope dilution mass spectrometry-based approach using high-performance liquid chromatography with an on-line electrospray ionization tandem mass spectrometry. Samples were analyzed on an AB SCIEX 5000 triple quadrupole mass spectrometer. The urinary F2-isoprostanes levels were corrected by urinary creatinine to account for differences in urine dilution.18 The PON1 activity was measured (Roche C3 analyzer) by quantifying serum paraoxonase activity using paraoxon as substrate and arylesterase activity using phenyl-acetate as substrate, as previously described.19 The intra- and inter-assay coefficients of variance for the paraoxonase activity were 1.9% and 3.3%, respectively; for aryl esterase activity coefficients of variation were 3.4% and 3.9%, respectively.
Statistical Methods
The predictors in analyses were the PSG-TST (primary) and the self-reported sleep duration (SR-HSD). MPO and Ox-LDL were the pre-specified primary outcome biomarkers considered to reflect oxidative stress and systemic inflammation in those with OSA with varying sleep durations. Continuous and categorical participant characteristics were compared across sleep duration categories using t-tests or Wilcoxon rank sum tests and chi-square tests. Logarithmic transformation was used before analysis for Ox-LDL to satisfy the normal distribution assumption, and transformed back for presentation. The relationship between sleep duration measures and biomarkers were evaluated by Pearson or Spearman correlation coefficients. Linear regression analyses were performed for the primary outcomes, MPO and Ox-LDL. Beta-estimates and 95% confidence intervals are presented. The models were adjusted for potential confounding factors including age, sex, race, BMI, active smoking status, self-reported comorbidities including physician diagnosed hypertension, diabetes mellitus, CVD, use of statins (HMG-CoA reductase inhibitors given anti-inflamma-tory properties) or anti-inflammatory medications (i.e., aspirin or nonsteroidal anti-inflammatory medications), ESS score, and AHI. PSG-TST and SR-HSD were considered as both as continuous measures to maximize efficiency and alternatively as categorical variables, the latter for ease of clinical interpretation and P values for linear trends were calculated. Two-sided P values were presented, with P < 0.05 considered statistically significant. All analyses were performed using JMP version Pro 10 and SAS software version 9.4 (SAS Inc., Cary, NC).
RESULTS
Participant Characteristics by Sleep Duration
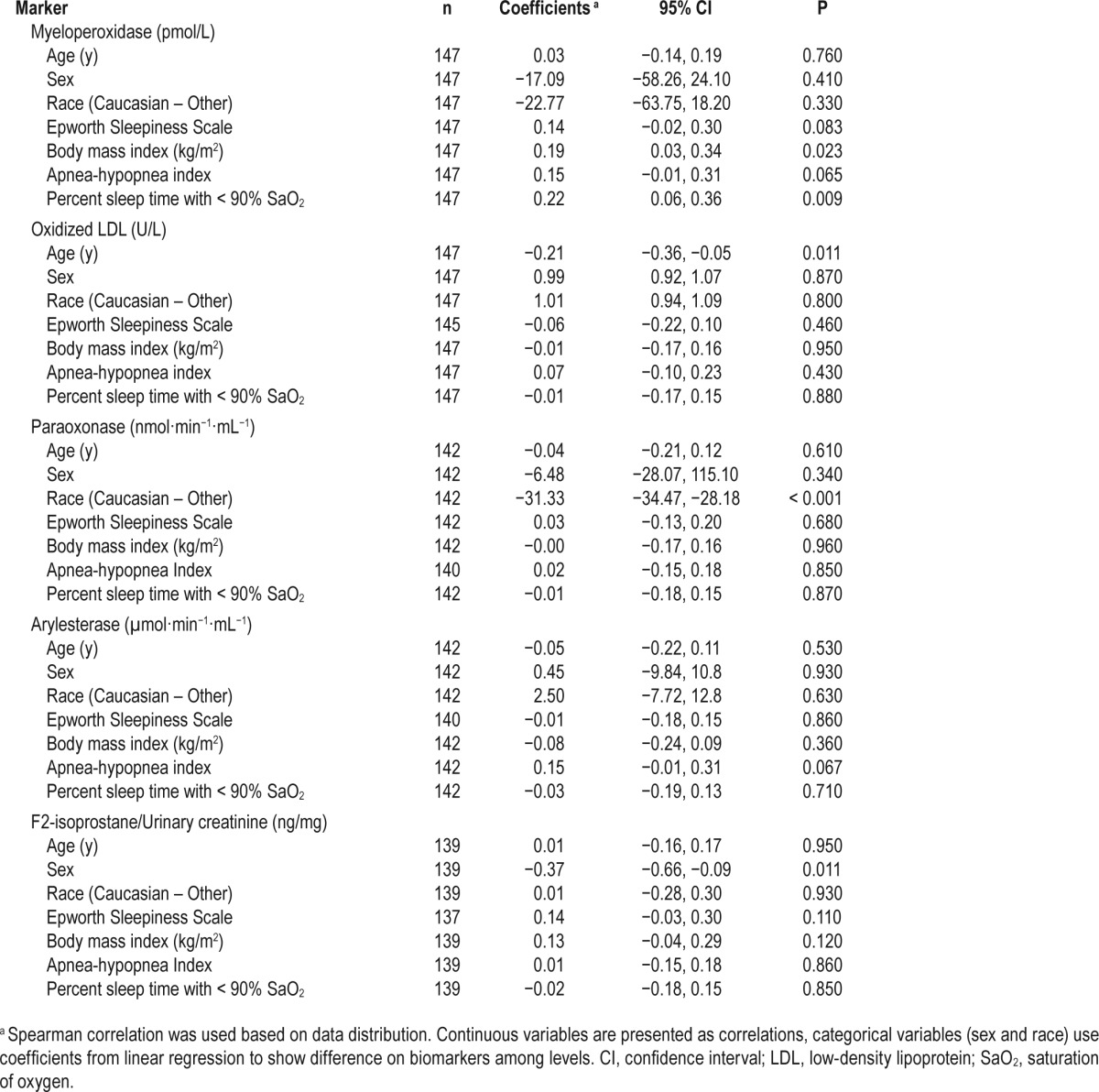
Overall, the analytic sample was middle-aged (51.0 ± 11.7 years) with evenly distributed Caucasian and African American race categories, had balanced sex distribution, was morbidly obese (BMI = 37.3 ± 8.1), and was without overall subjective excessive daytime sleepiness. As expected, given the inclusion of those with moderate to severe OSA, there was a high percentage of cardiovascular risk (diabetes mellitus: 20% and hypertension: 64%) and cardiovascular disease (17%). Across median sleep duration categories, those with lower PSG-TST tended to be older, have more severe OSA, and were more likely to be taking statin medications; those with a lower SR-HSD were also more likely to be taking statin medications (Table 1). The examination of biomarkers relative to subject characteristics revealed a significant correlation of MPO with BMI and hypoxia (Table 2). The Pearson correlation coefficient of PSG-TST and SR-HSD was 0.21, P = 0.013, and absolute difference was 1.92 ± 2.74 h. The PSG TST was 6.1 ± 1.4 h and the self-reported sleep duration reflective of the PSG night was 6.2 ± 1.6 h, r2 = 0.49, P < 0.001.
Table 1.
Participant characteristics by sleep time.
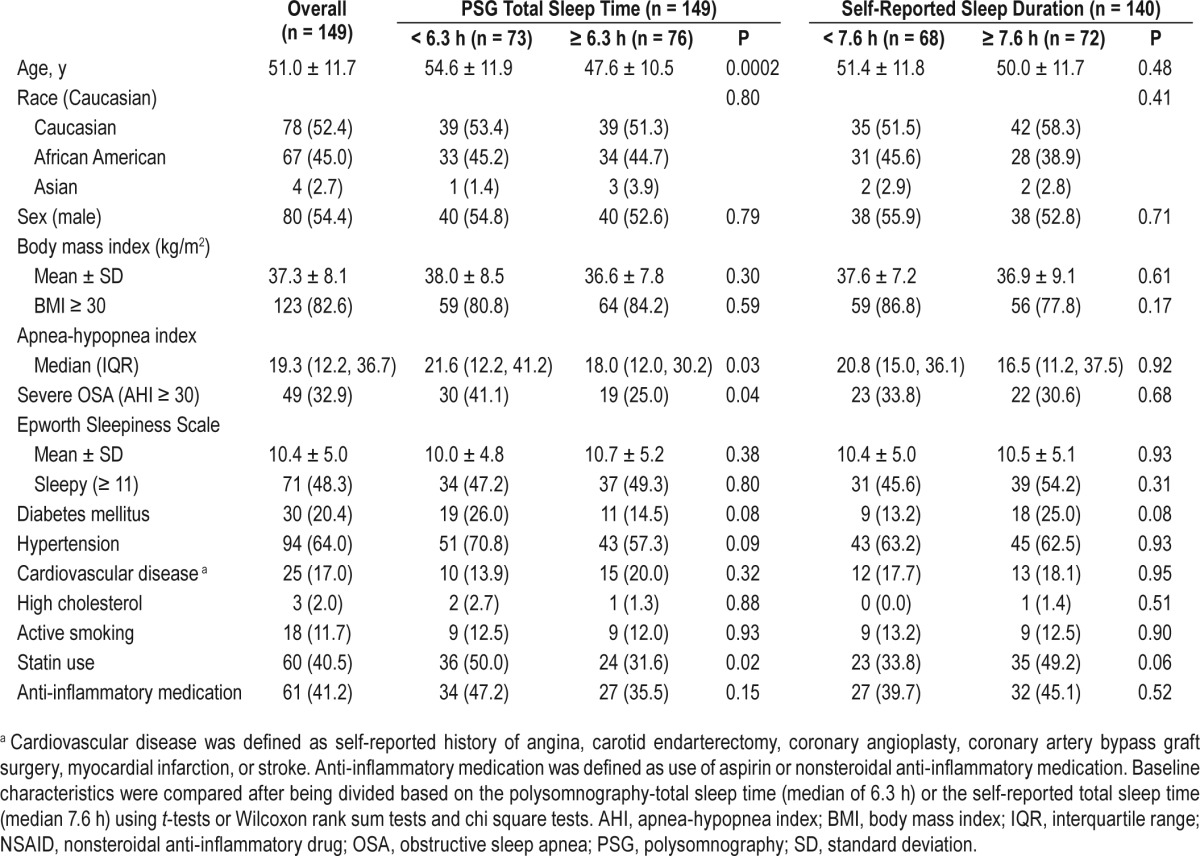
Table 2.
Summary of correlations of biomarkers and subject characteristics.
Sleep Duration Measures and Biomarkers of Oxidative Stress and Systemic Inflammation
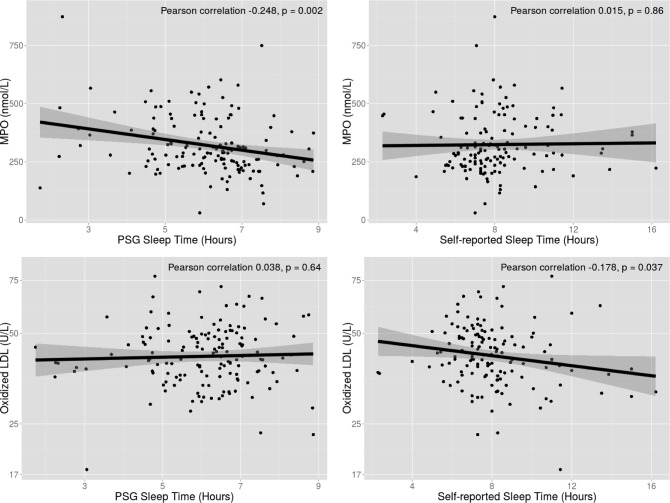
Correlation analysis of the biomarkers demonstrated a very low correlation between the five oxidative stress biomarkers (r2 < 0.2), suggesting reflection of different intrinsic biologic pathways. When considering plausible associations among biomarkers and sleep duration, a significant inverse association between PSG-TST and MPO (but not Ox-LDL) was observed, which persisted after adjustment for age and BMI (P = 0.007) (Table 3, Figure 1). In unadjusted linear regression analysis, a significant association of PSG-TST and MPO was observed that persisted in the multivariable model even after consideration of a host of confounders including BMI, cardiovascular risk factors, CVD, and OSA severity defined by AHI (Table 4). When PSG-TST increased 1 h, MPO decreased by 20.3 pmol/L if covariates are held constant (−20.28, −37.48 to −3.08, P = 0.021). Conversely, there was no statistically signifi-cant relationship of SR-HSD and MPO.
Table 3.
Sleep duration and oxidative stress and systemic inflammation biomarkers.

Figure 1.
Correlation of sleep duration measures relative to myeloperoxidase (top panels) and oxidized low-density lipoprotein (LDL; bottom panels). *Oxidized LDL was logarithmic transformed before analysis and transformed back for presentation. MPO, myeloperoxidase; PSG, polysomnography.
Table 4.
Linear regression of sleep duration measures relative to myeloperoxidase and oxidized LDL.
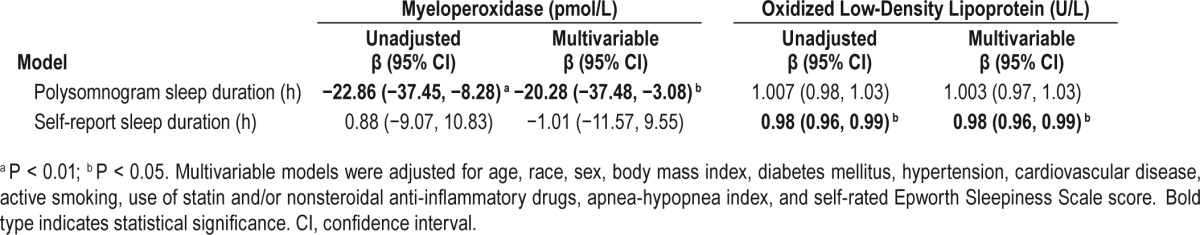
A significant inverse association was also observed between Ox-LDL and SR-HSD even after adjustment for age and BMI (P = 0.028) (Table 3, Figure 1). Multivariable linear regression analysis was conducted to examine the association of SR-HSD and Ox-LDL which remained statistically significant even in the multivariable model. Ox-LDL would decrease 2% when SR-HSD increased 1 h if covariates held constant (0.98, 0.96–0.99, P = 0.027). However, there was no significant relationship observed with PSG-TST and Ox-LDL. There were no other significant correlations observed between PSG-TST and SR-HSD and the other biomarkers.
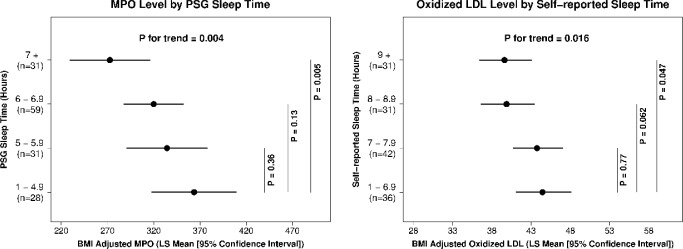
Alternatively, based on previously used cut-offs used in prior large, population-based studies and distributional characteristics,20,21 we examined PSG-TST and SR-HSD as categorical variables (Figure 2), which demonstrated a significant linear trend of increased levels of MPO and Ox-LDL across progressive reduction in sleep duration categories (P value for trend = 0.004 and 0.016 respectively).
Figure 2.
Myeloperoxidase and oxidized low-density lipoprotein (LDL) biomarkers across sleep duration categories. BMI, body mass index; MPO, myeloperoxidase; PSG, polysomnography.
DISCUSSION
In this cohort of moderate to severe OSA, we observed increases in oxidative stress and systemic inflammation unique to the type of sleep duration measure considered. Specifically, even after consideration of obesity, sleep apnea severity and background cardiovascular risk, a significant association was observed of objective reduced sleep ascertained by PSG and increasing MPO levels. These results were consistent whether examining MPO as a continuous outcome and also when examining levels across decreasing PSG-TST category with analyses demonstrating significant linear relationships. These findings are not consistent with the well-recognized U-shaped association of sleep duration with cardiovascular outcomes, rendering those with both short and long sleep at increased cardiovascular risk.3,5 However, these findings are consistent with prior observations identifying an association of increased cytokines in short and not long sleepers, suggesting different culprit mechanisms in short and long sleep.7 Alternatively, reduction of sleep duration based on self-report, theoretically a reflection of chronic, habitual sleep duration, was associated with increased Ox-LDL levels, findings that were most apparent when considering Ox-LDL and sleep duration as continuous variables, but also observed across sleep duration categories.
Markers of systemic inflammation and oxidative stress, of which sleep deprivation appears to represent a relevant promoter,22–24 have long been implicated in adverse health outcomes and development of a whole host of disease entities including CVD. Alterations in the oxidative environment and up-regulation of systemic inflammation result in micro-circulatory incompetence and vascular remodeling including stiffening of the vessel, atherogenesis, and angiogenesis.25 Although there is biologic plausibility to suspect that biomarkers of inflammation and oxidative stress implicated in atherosclerotic disease such as MPO, oxidized LDL, F2-isoprostanes, paraoxonase-1, and aryl esterase are dysregulated in the setting of curtailed sleep, only MPO and oxidized LDL were significantly associated with objectively and subjectively reduced sleep, respectively.
MPO, an integral member of the heme peroxidase-cyclooxygenase superfamily, is expressed by circulating neutrophils, monocytes and tissue macrophages and when released from phagocytes can result in tissue damage in large part due to inflammation and MPO-derived oxidants.9 MPO therefore promotes both inflammation and oxidative stress. Specifically, MPO plays a role in cytokine secretion and up-regulation of messenger RNA cytokine transcripts26 and catalyzes the formation of oxidizing agents, e.g., MPO-dependent oxidation of LDL and high-density lipoprotein.27 In clinic-based studies, increased MPO has been identified to be associated with objectively identified coronary artery disease on coronary angiogram28 and is predictive of subsequent major adverse cardiovascular events in those with angina or acute coronary syndrome.29,30 The relationship of MPO-modification of proteins in relation to human experimental sleep restriction has been described. In a small but rigorously conducted study involving male participants undergoing 11 days and nights of continuous EEG and PSG monitoring, MPO-modified LDL levels increased during sleep restriction and peaked after the first recovery night.13 These findings are corroborated by results from another human sleep restriction experimental study also demonstrating that neutrophils were the most sensitive leukocyte subtype to be increased in sleep restriction and higher MPO levels were observed subsequent to sleep restriction.13,14 The results of our study add to this body of knowledge by extending these findings to demonstrate that reduction of TST during a single night of PSG in a controlled setting was associated with increased morning MPO levels in those with moderate to severe OSA in a diverse sample. Therefore, there is evidence to support that objectively ascertained acute sleep loss or sleep reduction in OSA appears to increase levels of MPO, an established marker of cardiovascular risk. Clinical significance is underscored by MPO levels in those with PSG-time < 5 h and 5- to 6-h categories, i.e., in the range of levels identified to portend increased risk of acute coronary syndrome, i.e. > 350 ug/L.29
Data have amassed in terms of elucidating the effects of increased levels of LDL-cholesterol on vascular biology and the pathogenesis of atherosclerosis with the oxidatively modified form considered to be a more potent instigator of atherogenesis than LDL. Ox-LDL augments the expression of proinflammatory genes, resulting in monocyte recruitment into the vessel wall and thereby promoting vascular dysfunction and generates free radicals exerting endothelial cell cytotoxicity,10–12 and its presence has been described in atheromatous lesions.32 Oxidized LDL is also considered a marker of not only oxidation, but also inflammation, in part by enhancing proinflammatory responses in macrophages and also activates nuclear factor-kappa B transcription factor.33 Although there are limited and conflicting data relating oxidized LDL to OSA,34,35 existing sparse data perhaps are even more unclear relative to sleep restriction. As discussed, MPO-modified oxidized LDL has been observed to be increased with acute sleep restriction.13 However, in the current work, we identify a significant relationship of reduction in self-reported sleep duration relative to increasing Ox-LDL levels suggesting that chronic curtailment of sleep leads to enhanced oxidative stress and may represent one of the pathophysiologic pathways by which reduced sleep increases adverse cardiovascular outcomes in OSA.
It has been well established in multiple large-scale epidemiologic studies that sleep curtailment is associated with increased risk of weight gain,4 cardiovascular outcomes,3 and mortality.5 As collection of objective sleep measures via physiologic monitoring is cost-prohibitive and often not feasible, these studies have for the most part defined sleep duration based on self-reported questionnaire data that may reflect chronic, habitual sleep duration. Inherent limitations of collection of self-reported sleep duration data include inability to differentiate time in bed versus time asleep in bed and lack of characterization of napping patterns. Arguably, a single measure of exposure may not effectively capture the long-term effects of reduced sleep; however, compared to single-night PSG, self-reported sleep duration may offer insights into chronic sleep duration interrelationships, i.e., as previously described, these measures may represent unique biologic constructs.7 Although objective sleep testing has been shown to have a high correlation with subjective self-reported sleep duration measures,36,37 our study suggests that different information may be gleaned from objective PSG versus self-reported sleep duration in terms of relationship to underlying biochemical mechanisms. Furthermore, albeit we have posited that PSG-TST versus SRHSD potentially represent acute versus chronic exposure of sleep duration, it is possible differences in associations with oxidative stress markers may represent residual confounding given variation in accuracy of self-reported sleep duration by individual characteristics such as age, sex, and/or race.
In summary, our results show inverse, disparate relationships of biologically plausible measures of oxidative stress/ systemic inflammation relative to acute versus chronic reduction in sleep duration characterized by elevation of MPO with progressive reduction in PSG sleep time versus increased in Ox-LDL with reduction in chronic, habitual sleep duration. These relationships persisted after consideration of OSA severity and obesity with no mitigation in point estimates, suggesting independence from the confounding influences of these variables. Results are generalizable to an obese group with high OSA burden composed of balanced sex representation and over half of whom were African Americans. The graded linear relationships observed in the face of consistent literature demonstrating both short and long sleep confer adverse cardiovascular sequelae, i.e., the U-shaped distribution, suggest that up-regulation of oxidative stress and systemic inflammation may represent a more potent mechanistic pathway leading to deleterious health outcomes in short versus long sleep. These findings set the stage for future studies to assess reproducibility of these findings, particularly in non-obese populations and those with lesser degree OSA severity, as well as investigation of biomarkers that may serve as prevention or therapeutic targets in acute versus chronic sleep deprivation.
DISCLOSURE STATEMENT
This was not an industry supported study. Supported by National Heart Lung Blood Institute K23HL079114 (RM), NIH HL079114 (RM), NIH HL109493 (RM) and NIH HL108226 (RM), Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. SLH was supported by a NIH grant P01 HL076491. Mass spectrometry studies were performed on instruments housed within a core partially supported by an AB SCIEX Center of Innovation. Dr. Mehra has received NIH funding for which she has served as Principal Investigator (NHLBI RO1 1 R01 HL 109493, R21 HL108226). Her institution has received positive airway pressure machines and equipment from Philips Respironics for use in NIH-funded research. She has received honorarium from the American Academy of Sleep Medicine for speaking. She serves as the Associate Editor for the journal CHEST. She has received royalties from Up to Date. Dr. Hazen has been named as co-inventor on issued and pending patents held by the Cleveland Clinic relating to cardiovascular and inflammation diagnostics. Dr. Hazen has received consultancy fees from Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., P&G, and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Astra Zeneca, Cleveland Heart Lab, Liposcience Inc., P&G, Pfizer Inc. and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Heart Lab, Siemens, Esperion, and Frantz Biomarkers, LLC. Dr. Patel has received funding from the Resmed Foundation. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Ford ES, Cunningham TJ, Croft JB. Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep. 2015;38:829–32. doi: 10.5665/sleep.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe T, Aoki T, Yata S, Okada M. Sleep duration is significantly associated with carotid artery atherosclerosis incidence in a Japanese population. Atherosclerosis. 2011;217:509–13. doi: 10.1016/j.atherosclerosis.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11:931–52. doi: 10.5664/jcsm.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9:1003–12. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–11. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 10.Cominacini L, Pasini AF, Garbin U, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–8. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–95. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Mehta JL. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res. 2005;68:353–4. doi: 10.1016/j.cardiores.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Boudjeltia KZ, Faraut B, Esposito MJ, et al. Temporal dissociation between myeloperoxidase (MPO)-modified LDL and MPO elevations during chronic sleep restriction and recovery in healthy young men. PLoS One. 2011;6:e28230. doi: 10.1371/journal.pone.0028230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraut B, Boudjeltia KZ, Dyzma M, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2012;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 16.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerlich A, Hammel M, Nigon F, Chapman MJ, Schaur RJ. Kinetics of tryptophan oxidation in plasma lipoproteins by myeloperoxidase-generated HOCl. Eur J Biochem. 2000;267:4137–43. doi: 10.1046/j.1432-1327.2000.01449.x. [DOI] [PubMed] [Google Scholar]
- 18.Wedes SH, Khatri SB, Zhang R, et al. Noninvasive markers of airway inflammation in asthma. Clin Transl Sci. 2009;2:112–7. doi: 10.1111/j.1752-8062.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang WH, Hartiala J, Fan Y, et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler Thromb Vasc Biol. 2012;32:2803–12. doi: 10.1161/ATVBAHA.112.253930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13:1261–70. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 23.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 25.Staiculescu MC, Foote C, Meininger GA, Martinez-Lemus LA. The role of reactive oxygen species in microvascular remodeling. Int J Mol Sci. 2014;15:23792–835. doi: 10.3390/ijms151223792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefkowitz DL, Roberts E, Grattendick K, et al. The endothelium and cytokine secretion: the role of peroxidases as immunoregulators. Cell Immunol. 2000;202:23–30. doi: 10.1006/cimm.2000.1638. [DOI] [PubMed] [Google Scholar]
- 27.Schindhelm RK, van der Zwan LP, Teerlink T, Scheffer PG. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem. 2009;55:1462–70. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 28.Brennan ML, Anderson MM, Shih DM, et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–30. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–5. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 30.Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 31.Faraut B, Boudjeltia KZ, Dyzma M, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Tertov VV, Kaplun VV, Sobenin IA, Orekhov AN. Low-density lipoprotein modification occurring in human plasma possible mechanism of in vivo lipoprotein desialylation as a primary step of atherogenic modification. Atherosclerosis. 1998;138:183–95. doi: 10.1016/s0021-9150(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 33.van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis. 2011;214:345–9. doi: 10.1016/j.atherosclerosis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Hackenhaar FS, Martinez D, Medeiros TM, et al. Oxidized-LDL and paraoxonase-1 as biomarkers of coronary artery disease in patients with sleep-disordered breathing. Curr Med Chem. 2012;19:4359–66. doi: 10.2174/092986712802884312. [DOI] [PubMed] [Google Scholar]
- 35.Svatikova A, Wolk R, Lerman LO, et al. Oxidative stress in obstructive sleep apnoea. Eur Heart J. 2005;26:2435–9. doi: 10.1093/eurheartj/ehi440. [DOI] [PubMed] [Google Scholar]
- 36.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 37.Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005;76:1058–63. [PubMed] [Google Scholar]