Abstract
Study Objectives:
Growing literature suggests that patients with restless legs syndrome (RLS) may be at increased risk for hypertension, heart disease, and stroke. Cerebral small vessel disease (SVD) is a known risk factor for clinical stroke. This study evaluated silent cerebral SVD by MRI in patients with RLS, in the absence of a history of previous clinical stroke or known stroke risk factors and taking into account disease duration.
Methods:
Fifty-three patients with RLS < 10 y were prospectively recruited along with 44 with RLS > 10 y and 74 normal controls. A magnetic resonance imaging study was obtained from all subjects and scans were analyzed for area and volume of SVD.
Results:
There was a significant increase in SVD area in the entire group of RLS patients compared to controls (P = 0.036); this was almost entirely driven by the group with RLS > 10 y. SVD area and volume were significantly increased in patients with RLS > 10 y with respect to both controls (P < 0.0001 and P < 0.0014, respectively) and RLS < 10 y (P < 0.00022 and P < 0.003, respectively). Age, duration of RLS, and the interaction of age and duration of RLS were independent predictors of SVD disease. Duration of RLS was an independent predictor of the burden of cerebral SVD (area P < 0.00012 and volume P < 0.0025), whereas sex and insomnia were not.
Conclusion:
RLS duration should be taken into account when analyzing the association between RLS and cerebrovascular disease; our data support the hypothesis that a long-lasting RLS and its accompanying periodic limb movements in sleep are a risk factor for silent SVD and perhaps for the development of clinical stroke.
Citation:
Ferri R, Cosentino FI, Moussouttas M, Lanuzza B, Aricò D, Bagai K, Wang L, McLaughlin B, Walters AS. Silent cerebral small vessel disease in restless legs syndrome. SLEEP 2016;39(7):1371–1377.
Keywords: cerebral small vessel disease, MRI, restless legs syndrome, stroke, Willis-Ekbom disease
Significance.
Restless legs syndrome (RLS) has been reported to lead to cerebrovascular disease by some authors, to be an effect of stroke by others and to have no relationship by another group of authors. This study demonstrates that patients with long-lasting RLS are significantly more frequently affected by cerebral small vessel disease than those with short-duration RLS or controls, that cannot be explained by the presence of other known risk factors. This is the first study that demonstrates an increased frequency of cerebral small vessel disease in RLS which might represent a risk factor for the development of clinical stroke later during the chronic disease course.
INTRODUCTION
The relationships between stroke and restless legs syndrome (RLS) as well as between stroke and periodic leg movements during sleep (PLMS) have been little studied. To date, evidence indicates that RLS and PLMS may lead to clinical stroke1–3 as well as the reverse, i.e., that clinical stroke may lead to RLS/PLMS,4 with a third group of studies showing no relationship.5 In our latest prospective epidemiology study, 72,916 female registered nurses were followed for 6 y and RLS was significantly associated with an increased incidence of stroke that was dependent on the severity of RLS for participants with RLS symptoms > 15 times per month; the association was particularly strong for ischemic stroke alone (adjusted hazard ratio 3.52, P = 0.01).1 Cerebral small vessel disease (SVD) in the absence of clinical stroke is a risk factor for future stroke.6 In addition, in one of our previous studies we showed that heart disease is more likely to develop in the individuals with RLS of longer duration.7 Because of these considerations we conducted a study where we a priori divided patients with RLS into those with shorter duration and longer duration of disease versus a control group to examine the burden of cerebral SVD. In addition, in another of our previous studies with a design similar to the current study there was a nonstatistically significant trend toward the presence of silent cerebral vascular disease in RLS by magnetic resonance imaging (MRI) but the data required statistical correction for additional stroke risk factors.8 We therefore have now examined these relationships in the absence of known risk factors (such as hypertension, diabetes, hypercholesterolemia, previous stroke, previous heart disease, sleep apnea, etc.) and taking into account sleep architecture and the eventual role of PLMS. To our knowledge this is the first MRI study aiming to establish the presence of silent cerebral SVD in RLS subjects in comparison to controls.
METHODS
Patient and Control Selection
The recruitment of patients was carried out in two centers; 83 consecutive patients were selected, following the inclusion/ exclusion criteria detailed in the next paragraph, among 273 patients with a diagnosis of RLS admitted at the Sleep Research Centre, Department of Neurology I.C., Oasi Research Institute, Troina (Italy) between the years 2010 and 2014. The same center recruited 65 normal controls. Two other groups of 14 RLS patients and 9 controls were recruited, following the same inclusion/exclusion criteria by the Department of Neurology at the Vanderbilt University Medical Center, Nashville, TN (USA).
A total group of 171 subjects was thus recruited following the inclusion/exclusion criteria: 74 normal controls (53 females and 21 males, mean age 53.3 y, 13.36 standard deviation [SD]) and 97 RLS patients (72 females and 25 males, mean age 54.2 y, 12.95 SD). The male/female sex composition of the two groups was not significantly different (chi-square 0.14, not significant). All patients had a history and physical (including neurological) examination by a physician experienced in the diagnosis of RLS who confirmed the diagnosis.
Inclusion criteria were: (1) subjects age 25–80 y, with or without RLS; (2) RLS patients meeting the minimum diagnostic criteria developed by the International RLS Study Group (IRLSSG) excluding false positives (mimics)9 and IRLS Severity Scale10 higher than 15; (3) controls without RLS and without a first-degree relative (mother, father, brother sister, child) with RLS. Exclusion criteria were: (1) inability to give consent because of mental retardation, dementia, or inability to understand the consent due to a language barrier; (2) inability to undergo MRI scan because of metal implants from previous surgery in the chest or brain; (3) history of any risk factors for stroke such as high cholesterol (≥ 250 mg/dL), diabetes, heart disease or hypertension (blood pressure > 150/100 mm Hg in any of the available measurements) or use of medications for any of these conditions; (4) previous history of stroke or transient ischemic attack, cigarette smoking or obesity with a body mass index > 40; (5) sleep apnea, as documented by an apnea/hypopnea index > 15/h or a history of snoring or cessation of breathing; (6) other severe medical or neurological disease (kidney disease, liver disease, Parkinson disease, multiple sclerosis, neuroleptic-induced akathisia or other movement disorder, Alzheimer disease or uncontrolled epilepsy, severe head trauma, rheumatoid arthritis, peripheral neuropathy or radiculopathy) or severe psychiatric disease (severe depression, anxiety, schizophrenia); (7) pregnancy, determined from the time of the last menstrual period. Based on the frequent difficulty for persons with a long RLS history to recall the exact date of the disease start and in order to test our initial hypothesis on the disease duration, patients were subdivided into two disease duration subgroups: those with a history of RLS for less than 10 y (RLS < 10 y; n = 53, 42 females and 11 males, mean age 53.7 y, 11.77 SD) and those with a history of RLS for at least 10 y (RLS > 10 y; n = 44, 30 females and 14 males, mean age 54.8 y, 14.36 SD). With this simple but practical classification, all patients found it easy to indicate their status with regard to their disease duration.
Experimental Design
In addition to the demographic information available for all patients and controls, the following data were collected for all RLS patients: IRLS Severity Scale,10 Epworth Sleepiness Scale,11 Insomnia Severity Index,12 blood glucose (fasting), total cholesterol, high-density lipoprotein cholesterol, blood iron, and ferritin.
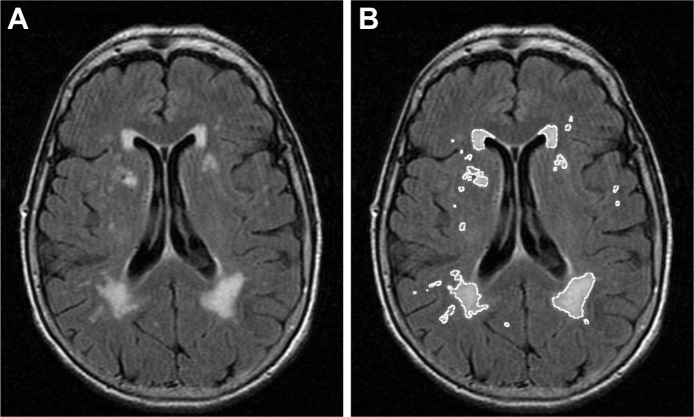
MRI was obtained on all patients and controls. All cerebral MRI scans were analyzed by an investigator (M.M.) masked to the presence/absence or duration of RLS in patients and controls, using a computerized digital image analysis program (ImageJ, v1.37; National Institutes of Health, Bethesda, MD, USA). Individual axial fluid-attenuated inversion recovery images were reviewed for identification and quantification of microvascular ischemic changes in the subcortical fiber tracts and deep nuclear regions of the hemispheres, as well as in the pons of the posterior fossa. Prior to image processing, the scale for image analysis was formatted to that used in the production of the MRI scan. Individual images containing microvascular lesions were selected and processed by manipulation of contrast and intensity functions so as to delineate and isolate the ischemic areas, and to exclude and eliminate nonischemic normal regions (Figure 1). Individual lesion areas were calculated by automated program analysis, lesion volumes were approximated by simple multiplication of lesion area by slice tomography dimension, and final/total lesion volume was determined additively.
Figure 1.
Image processing. Example of an original image (A) in which small vessel disease areas have been delineated by manipulation of contrast and intensity (B).
Complete polysomnographic sleep studies were obtained in 64 RLS patients (31 with RLS < 10 y and 33 with RLS > 10 y), which included the electromyographic recording from the tibialis anterior muscles. Polysomnographic recordings were scored following standard criteria for both sleep staging13 and periodic leg movements analysis and the presence or absence of sleep apneas and hypopneas.14
The study protocol received prior approval by both Institutional Review Boards (Troina and Nashville) and written informed consent was obtained from each subject, according to the Declaration of Helsinki.
Statistical Analysis
The Student t-test was used to compare controls and RLS patients or the two RLS patient subgroups whereas the one-way analysis of variance was used for the comparison between controls and the two RLS subgroups, followed by post hoc least significant difference test for the comparison between the different group pairs, when analysis of variance was significant. Moreover, in order to better detail the association between SVD area and volume with selected parameters of interest (sex, age, insomnia severity, RLS duration and its interaction with age) a multivariate regression analysis was performed. Finally, the correlations (Pearson correlation) between SVD area (or volume) and age of the subjects were assessed for each of the three groups of subjects separately and the estimated slopes of the regression lines were compared.
Of the 97 RLS subjects, 27 remained on RLS medication (13 in the RLS < 10 y and 14 in the RLS > 10 y), which included dopamine agonists, opioids, anticonvulsants, benzodiazepines, or combinations thereof and a subanalysis was done repeating this same regression but excluding those subjects on RLS medication from the comparison, in order to control for the potentially confounding effect of medication on the results. For the comparison of differences in frequencies, the chi-square test was used.
RESULTS
Table 1 reports the comparison between all parameters obtained in controls and in the two individual patient groups considered in this study. Age was very similar in the three groups and not statistically different at the chi-square test. IRLS Severity Scale was very similar between the two RLS patient groups and not statistically different. Epworth Sleepiness Scale was slightly increased in both RLS groups, with statistically significant difference between controls and RLS < 10 y. However, there was no statistically significant difference between the RLS groups in the Epworth Sleepiness Scale. The Insomnia Severity Index was significantly increased in both RLS groups versus controls, without statistically significant difference between the two RLS groups.
Table 1.
Comparison of all parameters obtained in the three subgroups of subjects recruited for this study.
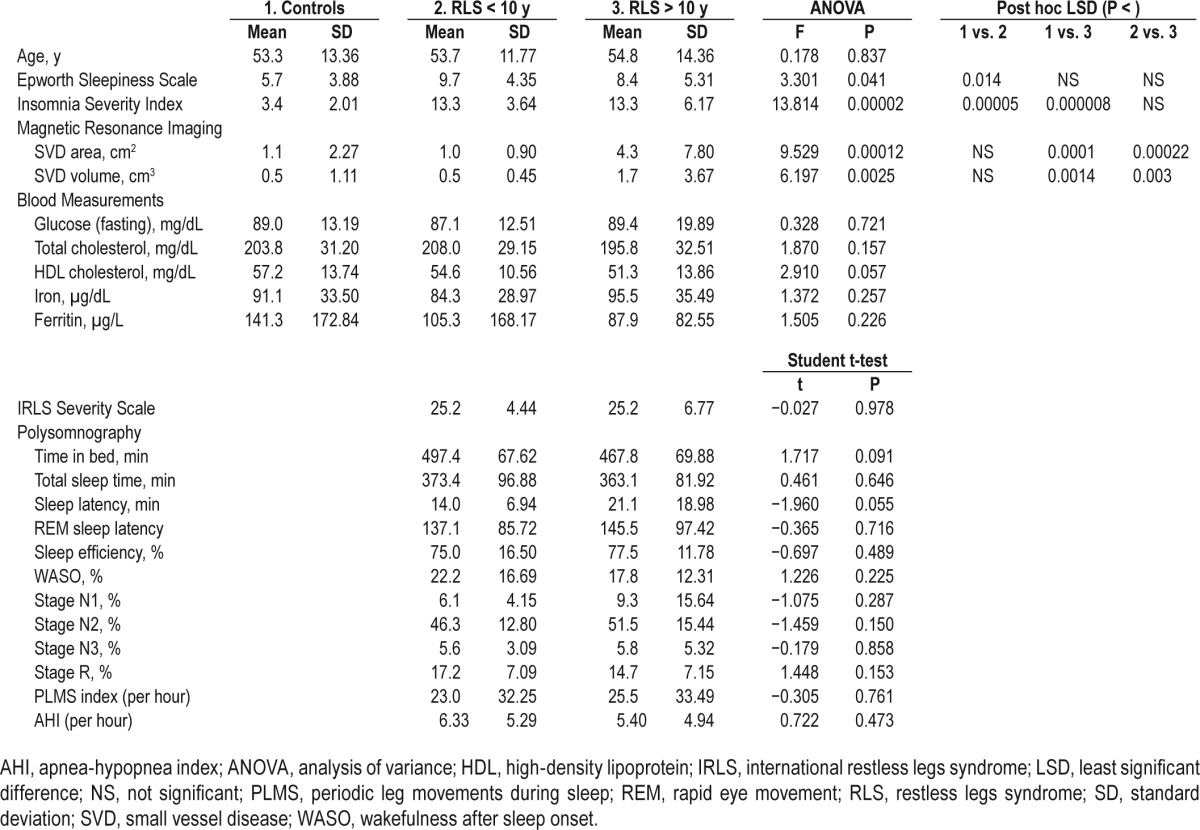
None of the blood parameters (glucose, total and high-density lipoprotein cholesterol, iron, and ferritin) showed statistically significant differences between the groups; however, ferritin showed a non-significant decreasing trend compatible with the iron deficiency hypothesis for RLS: (controls) > (RLS < 10 y) > (RLS > 10 y).1
On polysomnography there were no statistically significant differences in sleep architecture including the percentages of the different stages of total sleep time (Table 1) between the two RLS patient subgroups and also the PLMS index was not different. There was only a tendency for the RLS > 10 y group to have slightly shorter time in bed and slightly longer sleep latency than patients with RLS < 10 y. As specified in the Methods section, none of the subjects included in this study had a history suggestive of clinically significant apnea and this was confirmed by these results.
Finally, there was a significant increase in SVD area (but not volume) in the group of RLS patients as a whole versus controls (RLS mean area 2.5 cm2, 5.51 SD versus controls mean area 1.1 cm2, 2.27 SD; t = 2.115, P = 0.036). This was driven almost entirely by the RLS > 10 y group as can be seen in Table 1, which shows that both SVD measurements (area and volume) are significantly increased in patients with RLS > 10 y when compared separately to either the RLS < 10 y group or the controls, without a statistically significant difference between controls and the RLS < 10 y group.
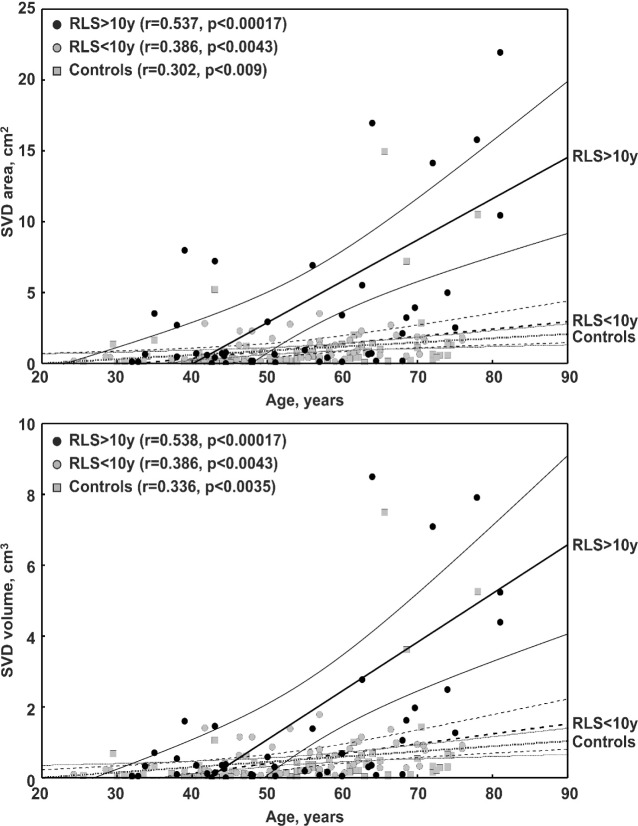
The top panel of Figure 2 shows the effect of age on the SVD area measurement in all groups of subjects. As expected, the correlation is positive and statistically significant in all groups; however, the slope of the regression line found in the RLS > 10 y group is significantly higher than that found in controls (t = 2.328, P < 0.022) and in RLS < 10 y patients (t = 3.329, P < 0.0012), whereas the slope of the regression lines of controls and RLS < 10 y patients does not differ (t = 0.307, not significant). The bottom panel of Figure 2 shows the effect of age on the MRI SVD volume measurement in all groups of subjects. Again, the correlation is positive and statistically significant in all groups and the slope of the regression line in the RLS > 10 y group is steeper than that found in controls or in the RLS < 10 y group but, in this case, the differences do not reach statistical significance. As some data looked like outliers at the simple visual inspection of the graphs, a formal detection of outliers was applied, based on the Grubbs test,15 and only one patient in the RLS > 10 y group showed SVD area and volume measurements that were identified as outliers; however, the elimination of this patient from the analysis did not cause significant changes of the correlation results (r = 0.536 instead of 0.537 for area and r = 0.585 instead of 0.538 for volume).
Figure 2.
Correlation between cerebral small vessel disease (SVD) area (top panel) or volume (bottom panel) and age in the three subgroups of subjects recruited for this study. RLS, restless legs syndrome.
Table 2 shows, in detail, the multivariate regression analysis of SVD area and volume with selected parameters obtained in the subjects recruited for this study. Both age and disease duration correlate positively with SVD area and volume and also their interaction is statistically significant. RLS duration alone, however, is also an independent predictor of SVD area and volume disease (SVD area P < 0.00012 and volume P < 0.0025). Similarly, to the t tests performed, RLS status does show an effect upon SVD area (P < 0.036) in the regression analysis. On the contrary, sex, daytime sleepiness, and insomnia severity do not exert a significant effect.
Table 2.
Multiple regression analysis of cerebral small vessel disease area and volume with selected parameters obtained in the subjects recruited for this study.
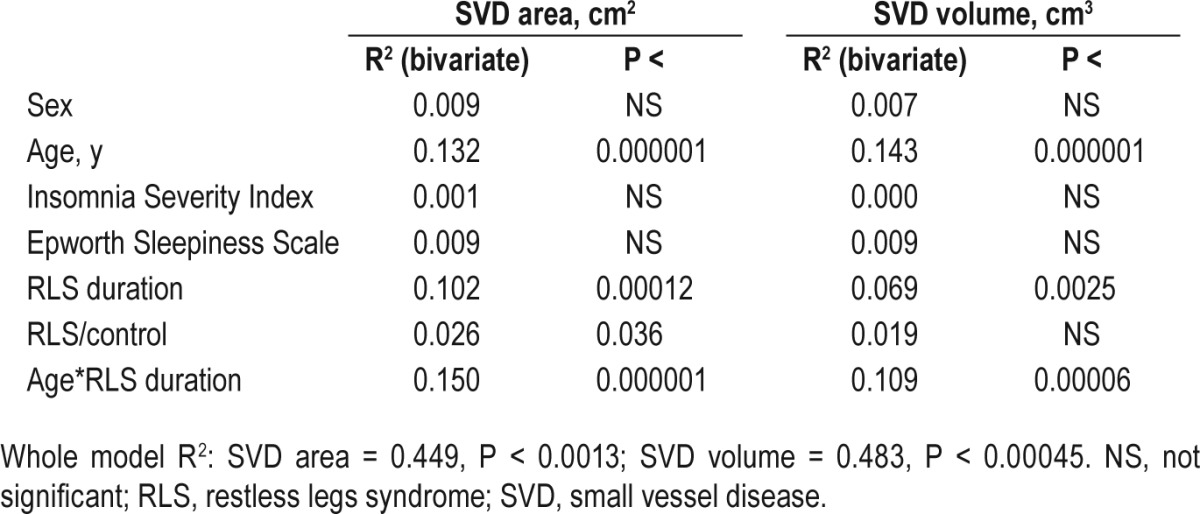
The same significant correlations and interaction are found when patients under drug treatment are not included in the analysis, in order to exclude a possible effect of this variable.
DISCUSSION
The first key finding of our study is that for the entire group of RLS patients there is a significantly increased SVD area compared to controls. Second, patients with RLS > 10 y have more silent cerebrovascular disease than patients with RLS < 10 y or controls. These results are independent of other cardiovascular risk factors such as a history of stroke, cardiovascular disease, hypertension, diabetes mellitus, high cholesterol, sleep apnea, cigarette smoking, obesity, etc., as we excluded such individuals from participation in the study. In addition, laboratory-measured blood pressure, fasting cholesterol, and blood sugar confirm the absence of such risk factors. This result is also independent of sex and the level of daytime sleepiness, nighttime insomnia, and RLS medication. In a regression analysis, age and the interaction of age and duration of RLS are statistically significant predictors of the burden of SVD but the duration of RLS is also a statistically significant independent predictor of cerebral SVD.
Neither the PLMS index nor RLS severity as determined by the IRLS were statistically different between the two RLS subgroups. The PLMS index was determined on a single night of study and RLS severity was determined over the week prior to administration of the scale; in other words, both measurements were done at a relatively stationary point in time. However, one of the main results of our paper is that patients with RLS > 10 y have more silent SVD than patients with RLS < 10 y despite a similar severity of RLS and despite a similar number of PLMS per hour. Given this result, it suggests that the statistically significant difference in the burden of cerebral SVD between subjects with RLS < 10 y and subjects with RLS > 10 y is driven by the cumulative effect of RLS, PLMS, or both over an extended period of time rather than the severity as measured over a brief period of time. From the point of view of PLMS, this can be expressed as PLMS per hour × more hours in > 10 y than hours in < 10 y = more PLMS and more silent SVD.
The results of our study confirm our initial hypothesis that RLS duration is an essential factor to be taken into account when analyzing the association between RLS and silent cerebral SVD, which itself is a known risk factor for the development of clinical stroke.6 These results are in agreement with a previous study similarly indicating that the duration of RLS has an effect on the development of heart disease but our study is unique in that it is the first study pointing out such a relationship in silent cerebral SVD.7 These results are also compatible with one of our other previous studies that showed that frank cerebrovascular disease in RLS is also independent of other known risk factors for the development of the cerebrovascular disease.16 Detecting a significant association between two morbidities is not enough to indicate causality and/or directionality of the association but discussing the possible physiopathological bases of such an association might provide important cues. If RLS is a predictor of the development of cerebrovascular and cardiovascular disease in the absence of the usual risk factors for stroke, as suggested by some of the current literature and by this study, the question remains as to how that risk gets translated into increased cerebrovascular and cardiovascular disease. One intriguing possibility is that nocturnal hypertension may confer increased risk. PLMS are very frequent in RLS9,17 and are accompanied by significant transient rises in heart rate18 and blood pressure, which can constitute the basis for cardiovascular and cerebrovascular sequelae, constituting a sort of intermittent nocturnal hypertensive condition.19,20 Other evidence for this nocturnal hypertension in RLS is that RLS patients have a nondipping nocturnal blood pressure profile which by itself is a known risk factor for cardiovascular and cerebrovascular disease.21 Hypertension, in turn, represents a major risk factor for lipohyalinotic and microatheromatous changes of the small penetrating cerebral vessels,22 which lead to the development of lacunar and leukoaraiotic subcortical ischemic changes characteristic of microvascular disease,23 as evidenced in numerous prior investigations.24,25
However, the search of a mechanism that directly connects PLMS to changes in brain hemodynamics has still received little attention, probably because of the technical difficulties connected with this type of investigation. Nevertheless, an interesting study on a limited number of patients carried out by means of near-infrared spectroscopy disclosed that PLMS are followed by a constant pattern of hemodynamic changes constituted by a first small hemodynamic activation pattern (hyperoxygenation), followed by a pronounced deactivation pattern (hypooxygenation), then followed by another small activation.26 Thus, it can be hypothesized that, as a second mechanism for the development of stroke in RLS, each PLMS is accompanied by a transient predominant brain hypoxia. The repeated transient hypoxia, in turn, might alter the biology of the penetrating arterioles, given their limited caliber,27 and might assume a clinical importance, especially in aged individuals.
Our data are compatible with the hypothesis that nocturnal hypertension or nocturnal hypoxia associated with the PLMS could be the driver of the increased burden of cerebral SVD seen in RLS in this study. Although we did not directly measure the increases in blood pressure around the time of PLMS in this study as Pennestri et al.28 and Siddiqui et al.20 have done previously, our hypothesis is given further strength in that previous studies show that virtually every PLM is accompanied by a rise in blood pressure.20,28,29
This study has some limitations: (1) we could not establish the exact duration of RLS in all patients, especially in those with RLS that had been troubling them for decades and only an arbitrary subdivision of patients into two disease duration subgroups was possible. However, we believe that this was the most accurate way to determine age of onset since the ages of onset were determined retrospectively and trying to determine more accurate ages of onset would be less reliable and subject to recall bias; (2) we were not able to obtain a polysomnographic study for all patients and controls; however, this was available in more than two-thirds of patients and allowed us to exclude the presence of gross sleep architecture or PLMS differences between the two subgroups; and (3) we did not recruit clearly hypertensive patients and this did not allow us to perform a correlation analysis between blood pressure and SVD. However, the strength of the methodology we have chosen is that patients were selected to not have any other risk factors for stroke other than RLS/PLMS. This allowed us to determine the main outcome of the study, which was the relationship of RLS/PLMS to silent cerebral SVD without the need of statistical correction for confounders, such as hypertension.
There is literature to suggest that clinical stroke may lead to new-onset RLS.30 However, it is also true that there is literature suggesting that RLS may lead to hypertension and cardiovascular disease.19,31,32 We cannot exclude for certain the possibility that silent cerebral small vessel disease leads to longer duration of RLS but, given our experimental design, this seems less likely than the possibility that longer duration RLS leads to a greater burden of silent cerebral small vessel disease. Our experimental design was to preselect patients with RLS < 10 y and RLS > 10 y and determine the potential effect on silent SVD.
In conclusion, our findings support the hypothesis that RLS duration is an essential factor to be taken into account when analyzing the association between RLS and cerebrovascular disease and that a long-lasting RLS might be a risk factor for cerebral SVD and potentially for clinical stroke.6 In fact, SVD has been associated with recurrent cerebral ischemia,33 concurrent macrovascular atherothrombotic disease,34 intracerebral hemorrhage,35 disability,36 dementia,37 and mortality.38 Moreover, they indicate the need of accurate epidemiological investigations to evaluate the eventual risk of stroke in this disease and of studies directed at clarifying the eventual patho-physiology of this association.
DISCLOSURE STATEMENT
This was an investigator-initiated study partially supported by UCB Pharma Inc. This work was performed at the Sleep Research Centre, Department of Neurology IC, Oasi Institute (IRCCS), Troina, Italy and at the Vanderbilt University School of Medicine, Nashville, TN, USA. Drs. Ferri, Cosentino, Moussouttas, Bagai, Wang, McLaughlin, and Walters, report grant funding and/or personal fees from UCB Inc., during the conduct of the study. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the help of research assistant Wendi Welch in the completion of this study.
REFERENCES
- 1.Wong J, Li Y, Rexrode K, et al. Restless legs syndrome is associated with subsequent development of stroke: a prospective study of the Nurses Health Study II cohort. Sleep. 2015;38:A311. (Abstract Suppl) [Google Scholar]
- 2.Elwood P, Hack M, Pickering J, Hughes J, Gallacher J. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Community Health. 2006;60:69–73. doi: 10.1136/jech.2005.039057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeiros CA, de Bruin PF, Paiva TR, Coutinho WM, Ponte RP, de Bruin VM. Clinical outcome after acute ischaemic stroke: the influence of restless legs syndrome. Eur J Neurol. 2011;18:144–9. doi: 10.1111/j.1468-1331.2010.03099.x. [DOI] [PubMed] [Google Scholar]
- 4.Benbir G, Karadeniz D. Periodic leg movements in sleep in patients with supratentorial cerebral infarction. Acta Neurol Belg. 2012;112:27–32. doi: 10.1007/s13760-011-0002-0. [DOI] [PubMed] [Google Scholar]
- 5.Winter AC, Schurks M, Glynn RJ, et al. Restless legs syndrome and risk of incident cardiovascular disease in women and men: prospective cohort study. BMJ Open. 2012;2:e000866. doi: 10.1136/bmjopen-2012-000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ntaios G, Lip GY, Lambrou D, et al. Leukoaraiosis and stroke recurrence risk in patients with and without atrial fibrillation. Neurology. 2015;84:1213–9. doi: 10.1212/WNL.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126:1689–94. doi: 10.1161/CIRCULATIONAHA.112.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters AS, Moussouttas M, Siddiqui F, et al. Prevalence of stroke in restless legs syndrome: initial results point to the need for more sophisticated studies. Open Neurol J. 2010;4:73–7. doi: 10.2174/1874205X01004010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria - history, rationale, description, and significance. Sleep Med. 2014;15:859–72. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 11.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 12.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 13.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV. Darien, IL: American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. www.aasmnet.org. [Google Scholar]
- 14.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Grubbs F. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 16.Cosentino FI, Arico D, Lanuzza B, et al. Absence of cardiovascular disease risk factors in restless legs syndrome. Acta Neurol Scand. 2012;125:319–25. doi: 10.1111/j.1600-0404.2011.01563.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferri R, Zucconi M, Manconi M, et al. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 18.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Walters AS, Rye DB. Evidence continues to mount on the relationship of restless legs syndrome/ periodic limb movements in sleep to hypertension, cardiovascular disease, and stroke. Sleep. 2010;33:287. doi: 10.1093/sleep/33.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Erden EC, Erden I, Turker Y, Sivri N, Dikici S, Ozsahin M. Incremental effects of restless legs syndrome on nocturnal blood pressure in hypertensive patients and normotensive individuals. Blood Press Monit. 2012;17:231–4. doi: 10.1097/MBP.0b013e32835b5a39. [DOI] [PubMed] [Google Scholar]
- 22.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 23.Bezerra DC, Sharrett AR, Matsushita K, et al. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2012;78:102–8. doi: 10.1212/WNL.0b013e31823efc42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–72. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 25.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–61. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 26.Pizza F, Biallas M, Wolf M, Valko PO, Bassetti CL. Periodic leg movements during sleep and cerebral hemodynamic changes detected by NIRS. Clin Neurophysiol. 2009;120:1329–34. doi: 10.1016/j.clinph.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura N, Schaffer CB. Big effects from tiny vessels: imaging the impact of microvascular clots and hemorrhages on the brain. Stroke. 2013;44:S90–2. doi: 10.1161/STROKEAHA.112.679621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 29.Cassel W, Kesper K, Bauer A, et al. Significant association between systolic and diastolic blood pressure elevations and periodic limb movements in patients with idiopathic restless legs syndrome. Sleep Med. 2016;17:109–20. doi: 10.1016/j.sleep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Kim JS, Song IU, An JY, Kim YI, Lee KS. Poststroke restless legs syndrome and lesion location: anatomical considerations. Mov Disord. 2009;24:77–84. doi: 10.1002/mds.22303. [DOI] [PubMed] [Google Scholar]
- 31.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol. 2014;261:1051–68. doi: 10.1007/s00415-013-7065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim GM, Park KY, Avery R, et al. Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke. 2014;45:479–85. doi: 10.1161/STROKEAHA.113.003004. [DOI] [PubMed] [Google Scholar]
- 34.De Silva DA, Liew G, Wong MC, et al. Retinal vascular caliber and extracranial carotid disease in patients with acute ischemic stroke: the Multi-Centre Retinal Stroke (MCRS) study. Stroke. 2009;40:3695–9. doi: 10.1161/STROKEAHA.109.559435. [DOI] [PubMed] [Google Scholar]
- 35.Inzitari D, Giordano GP, Ancona AL, Pracucci G, Mascalchi M, Amaducci L. Leukoaraiosis, intracerebral hemorrhage, and arterial hypertension. Stroke. 1990;21:1419–23. doi: 10.1161/01.str.21.10.1419. [DOI] [PubMed] [Google Scholar]
- 36.Leonards CO, Ipsen N, Malzahn U, Fiebach JB, Endres M, Ebinger M. White matter lesion severity in mild acute ischemic stroke patients and functional outcome after 1 year. Stroke. 2012;43:3046–51. doi: 10.1161/STROKEAHA.111.646554. [DOI] [PubMed] [Google Scholar]
- 37.Jokinen H, Kalska H, Ylikoski R, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study. Cerebrovasc Dis. 2009;27:384–91. doi: 10.1159/000207442. [DOI] [PubMed] [Google Scholar]
- 38.Conijn MM, Kloppenborg RP, Algra A, et al. Cerebral small vessel disease and risk of death, ischemic stroke, and cardiac complications in patients with atherosclerotic disease: the Second Manifestations of ARTerial disease-Magnetic Resonance (SMART-MR) study. Stroke. 2011;42:3105–9. doi: 10.1161/STROKEAHA.110.594853. [DOI] [PubMed] [Google Scholar]