Abstract
Study Objectives:
To evaluate the efficacy and safety of oral JZP-110, a second-generation wake-promoting agent with dopaminergic and noradrenergic activity, for treatment of impaired wakefulness and excessive sleepiness in adults with narcolepsy.
Methods:
This was a phase 2b, randomized, double-blind, placebo-controlled, parallel-group trial conducted at 28 centers in the United States. Patients were adults with narcolepsy who had baseline scores ≥ 10 on the Epworth Sleepiness Scale (ESS) and baseline sleep latency ≤ 10 min on the Maintenance of Wakefulness Test (MWT). Patients received a daily placebo (n = 49) or JZP-110 (n = 44) 150 mg/day weeks 1–4 and 300 mg/day weeks 5–12. Primary efficacy endpoints were change from baseline in average MWT sleep latency, and the Clinical Global Impression-Change (CGI-C); secondary endpoints were change from baseline in ESS score and Patient Global Impression-Change.
Results:
Improvements were significantly greater with JZP-110 versus placebo on mean MWT sleep latency (4 w, 9.5 versus 1.4 min, P < 0.0001; 12 w, 12.8 versus 2.1 min, P < 0.0001), percentage of patients with CGI-C improvement (4 w, 80% versus 51%, P = 0.0066; 12 w, 86% versus 38%, P < 0.0001), and mean change in ESS (4 w, −5.6 versus −2.4, P = 0.0038; 12 w, −8.5 versus −2.5, P < 0.0001). Three JZP-110-treated patients (6.8%) discontinued due to adverse events (AEs). The most common AEs with JZP-110 versus placebo were insomnia (23% versus 8%), headache (16% versus 10%), nausea (14% versus 6%), diarrhea (11% versus 6%), decreased appetite (14% versus 0%), and anxiety (11% versus 0%).
Conclusions:
At doses of 150–300 mg/day, JZP-110 was well tolerated and significantly improved the ability to stay awake and subjective symptoms of excessive sleepiness in adults with narcolepsy.
Clinical Trials Registration:
Clinicaltrials.gov identifier NCT01681121.
Citation:
Ruoff C, Swick TJ, Doekel R, Emsellem HA, Feldman NT, Rosenberg R, Bream G, Khayrallah MA, Lu Y, Black J. Effect of oral JZP-110 (ADX-N05) on wakefulness and sleepiness in adults with narcolepsy: a phase 2b study. SLEEP 2016;39(7):1379–1387.
Keywords: excessive sleepiness, JZP-110, narcolepsy, wakefulness
Significance.
Current treatments for excessive sleepiness, which is one of the symptoms present in all patients with narcolepsy, are associated with several limitations and there remains a need for additional effective therapies to promote wakefulness. One positive result in this phase 2 study found that patients treated with JZP-110 had a significant increase in staying awake on each of the five Maintenance of Wakefulness Tests at weeks 4 and 12. In addition, most patients treated with JZP-110 had significant improvement based on the Clinical Global Impression-Change, Patient Global Impression-Change, and Epworth Sleepiness Scale scores. Further research is warranted to evaluate JZP-110 for the treatment of excessive sleepiness.
INTRODUCTION
Excessive sleepiness and the inability to stay awake (i.e., sleep attacks) are clinical hallmarks of narcolepsy, a chronic neurologic disorder with no known cure. Excessive sleepiness is present in all patients with narcolepsy and is the diagnostic criterion required for both of the currently published diagnostic guidelines, the third edition of the International Classification of Sleep Disorders (ICSD-3) and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.1,2 In addition to excessive sleepiness, other symptoms include cataplexy, sleep paralysis, hypnagogic hallucinations, and disrupted nighttime sleep. Narcolepsy is associated with a substantial burden of illness including reductions in patient function as well as signifi-cant healthcare resource utilization and costs.3–5
Treatment goals of decreasing excessive sleepiness and increasing patients' ability to stay awake throughout the day are the primary management strategies in treating narcolepsy. Although the clinical manifestation of excessive sleepiness and impaired wakefulness may appear to overlap, they appear to represent somewhat independent concepts. In support of that idea, studies have reported poor correlations between excessive sleepiness or sleep propensity and impaired wakefulness or the ability to stay awake when assessed by their respective outcome measures of sleepiness scales (e.g., Epworth Sleepiness Scale [ESS], Karolinska Sleepiness Scale, Multiple Sleep Latency Test) and wakefulness tests (e.g., Maintenance of Wakefulness Test [MWT], Sustained Attention to Response test).6–9
Published recommendations for the treatment of narcolepsy suggest use of a variety of medications.10–12 These recommended medications include those that have been approved by the United States Food and Drug Administration for narcolepsy either as a general condition (i.e., stimulants such as Dexedrine [D-amphetamine] and Ritalin [methylphenidate]), to improve wakefulness in adult patients with excessive sleepiness associated with narcolepsy (i.e., wake-promoting agents such as Provigil [modafinil] and Nuvigil [armodafinil]), or specifically for the treatment of excessive daytime sleepiness and cataplexy in narcolepsy (Xyrem [sodium oxybate]). However, each of these medications is associated with limitations for some patients such as narrow therapeutic windows, issues of tolerability, complex medication regimens, increased tolerance over time, abuse potential, and suboptimal response.13 As such, there remains a need for additional effective and tolerable therapies.
JZP-110 ([R]-2-amino-3-phenylpropylcarbamate hydro-chloride; formerly known as ADX-N05) is a phenylalanine-derived, second-generation wake-promoting agent. JZP-110 is a dopamine and norepinephrine reuptake inhibitor. The mechanism of action of JZP-110 differs from modafinil in that it inhibits reuptake at dopamine and norepinephrine transporters14 and differs from traditional stimulants such as D-amphetamine in that JZP-110 does not promote the release of norepinephrine.15 In nonclinical studies, JZP-110 had robust wake-promoting effects in the absence of collateral effects (e.g., pronounced hyperactivity, stereotypic and dys-kinetic movements) observed with traditional stimulants such as D-amphetamine.16,17 In preclinical studies, animals treated with JZP-110 exhibited recovery of rapid eye movement (REM) and non-REM sleep without rebound hypersomnia, whereas animals treated with D-amphetamine exhibited overcompensation of non-REM and REM sleep (i.e., rebound hypersomnia).16,17
A phase 2a double-blind, crossover study with 2 w of JZP-110 (150 mg/day during the first week increased to 300 mg/day for the second week) in 33 patients with narcolepsy demonstrated not only a favorable safety profile but also a significant improvement from baseline at 2 w in mean sleep latency on a four-period MWT relative to placebo (12.7 versus 0.9 min; P = 0.0002).18 Furthermore, as measured by the ESS, JZP-110 significantly improved patient-reported excessive sleepiness (P < 0.0001). These promising preliminary results suggested that further evaluation was warranted. Therefore, the objective of this study was to evaluate the efficacy and safety of JZP-110 for improvement of wakefulness and excessive sleepiness in adults with narcolepsy with or without cataplexy over a longer treatment duration and on a five-period MWT.
METHODS
Study Design
This was a phase 2, double-blind, placebo-controlled, parallel-group study conducted at 28 centers in the United States (Figure 1). The study was designed to evaluate the safety and efficacy of JZP-110 over a 12-w treatment duration in adults with narcolepsy. The study was performed in accordance with the principles of the Declaration of Helsinki, and all patients provided written informed consent prior to participation (clinicaltrials.gov identifier NCT01681121).
Figure 1.
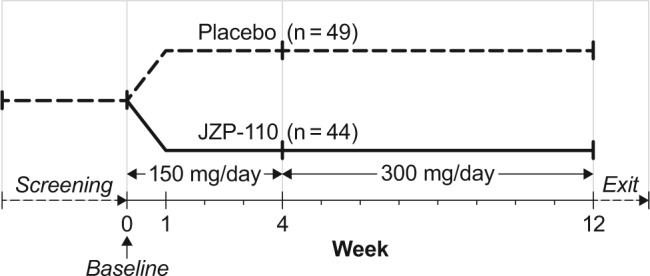
Study design.
Patients
For inclusion, patients had to be adults between 18 and 65 y of age with a confirmed diagnosis of narcolepsy with or without cataplexy based on the second edition of the International Classification of Sleep Disorders (ICSD-2)19; the ICSD-3 had not been published at the time of the study. Additional inclusion criteria were a baseline score of ≥ 10 on the ESS20 and a baseline sleep latency ≤ 10 min for the average of the first four periods of a five-period MWT,21 which was performed the day following an overnight stay, without polysomnography, at the study site.
Exclusion criteria were a history of a medical disorder, other than narcolepsy, that was associated with excessive sleepiness; significant cardiovascular disease; a history of phenylketonuria or hypersensitivity to phenylalanine-derived products; a body mass index > 34; excessive caffeine use 1 w prior to study; a nicotine dependence that has an effect on sleep; a history of alcohol or drug abuse within the past 2 y; use of any product with stimulating or sedating properties; selective serotonin reuptake inhibitors or anticonvulsant agents within 14 days prior to dosing; or any investigational drug use within 30 days prior to dosing. Women who were pregnant or lactating were also excluded. Patients with prior use of medications for the treatment of narcolepsy including any over-the-counter sleep aids or stimulants were allowed to enroll provided their last use was at least five half-lives of the drug(s) in question and they had returned to their baseline level of excessive sleepiness.
Treatments
Patients were randomized in a 1:1 ratio to receive once-daily oral placebo or JZP-110, taken on an empty stomach within 1 h of awakening in the morning. Doses of JZP-110 were 150 mg/day during weeks 1–4 and 300 mg/day during weeks 5–12. At the discretion of the investigator, changes in dosing were permitted based on tolerability and efficacy. Changes in dosing included no dose increase at week 4 in case of adverse events (AEs) or other tolerability issues; increase in dose after week 4 if tolerated; a reduction in dose in patients who could not tolerate the higher dose after week 4 but with a potential re-challenge of the higher dose as tolerated; and discontinuation for any patient who could not tolerate the lower dose.
Outcomes
Efficacy outcomes included objective and subjective (i.e., patient-reported) measures of excessive sleepiness, as well as subjective global assessments of change in disease status. The objective measure of excessive sleepiness was the mean sleep latency derived from the first four periods of the MWT, performed at baseline and at weeks 4 and 12 following an overnight stay at the study site. The MWT consisted of five 40-min periods with a 2-h interval between each period. The 40-min MWT was used because this version has less of a ceiling effect and is recommended for obtaining objective data on the patients' ability to remain awake.21 Sleep latency was defined as the time from lights out to the first of three consecutive epochs of stage N1 or one epoch of N2, N3, or REM sleep, as measured by electroencephalogram. The patient-reported measure of excessive sleepiness was the ESS,20 assessed at baseline and at 1, 2, 4, 6, 8, and 12 w of treatment.
Overall changes in disease status were assessed subjectively from both the clinician and patient perspectives using the Clinical Global Impression of Change (CGI-C) and Patient Global Impression of Change (PGI-C),22 respectively, at 1, 2, 4, 6, 8, and 12 w. These measures are scored using a seven-point Likert-type scale from 1 = “Very much improved” to 7 = “Very much worse.” Primary and secondary analyses compared the percentages of patients who were improved on the CGI-C and PGI-C, respectively, between JZP-110 and placebo at 4 and 12 w.
There were two primary efficacy endpoints: change from baseline to 12-w assessment in the mean sleep latency on the first four MWT periods and the percentage of patients who were rated as improved on the CGI-C at the last assessment, defined as a clinician rating of “very much improved,” “much improved,” or “minimally improved.” Secondary efficacy endpoints were change from baseline at weeks 4 and 12 on the ESS and over treatment duration; change from baseline in sleep latency (average of the first four MWT periods) at 4 w; percentage of patients who achieved CGI-C improvement at 4 w; and the percentage of patients who reported improvement on the PGI-C. The fifth period on the MWT was also evaluated post hoc. As an exploratory endpoint, the change from baseline in the median number of cataplectic attacks per week was evaluated for the subset of patients with cataplexy.
Safety was assessed over the study duration based on incidence of AEs, laboratory assessments including blood chemistry, hematology, and urinalysis (baseline and weeks 4 and 12), and vital signs and electrocardiograms (baseline and weeks 1, 2, 4, 6, and 12). All AEs were graded for severity and relatedness to treatment. The risk of suicidality was also evaluated at each study visit using the Columbia-Suicide Severity Rating Scale (C-SSRS).23
Statistics
Sample size calculation was based on the difference in the mean change from baseline at 12 w in the mean sleep latency (in minutes) from the first four periods of the five MWT periods comparing JZP-110 to placebo. A minimum sample size of 41 patients per treatment group was considered to be sufficient to detect a difference in mean change from baseline in sleep latency times of 3.8 min given a pooled standard deviation (SD) of 6.0 min, a power of 80%, and a significance level of 0.05 using a two-sample t-test. Derivation of the 3.8-min difference was based on modafinil and armodafinil studies in which average differences of 2.9 to 4.5 min in sleep latency on the MWT were observed between active treatment and control.24–27 To allow for missing data of approximately 10%, the sample size was increased to 45 patients per treatment group.
A sample size calculation was also considered for the other primary endpoint of percentage of patients achieving at least minimal improvement on the CGI-C. Results from a previous crossover study of JZP-110 suggested that approximately 75% of the active treatment group and approximately 40% of the placebo group would experience at least some improvement.18 Based on these assumed values, 37 patients per treatment group would be required to achieve 80% power using Fisher exact test. Because the two primary endpoints are expected to be correlated, no additional adjustment was made to the sample size for multiple comparisons.
All analyses were based on the intent-to-treat population, defined as all patients who were randomized, received at least one dose of study drug, and had at least one post-baseline efficacy assessment. For the co-primary endpoints at 12 w, missing data were imputed using last-observation-carried forward; results for assessments at other time points during treatment are presented as observed. Comparisons between treatment groups were evaluated using two-sided t-tests except for percentages of patients (CGI-C and PGI-C), which were evaluated using Fisher exact test. An α-level was maintained at 0.05 for the analyses of both primary endpoints, and no adjustments were made for multiple comparisons in testing other endpoints. An analysis of covariance was performed as a sensitivity analysis for the primary efficacy endpoint of MWT to confirm treatment differences and to evaluate potential site or treatment-by-site interactions. Estimation of the effect size of the mean MWT sleep latency change from baseline was performed post hoc based on the least squares mean divided by the SD. Statistical analyses were performed using SAS Version 9.2 or later (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Population
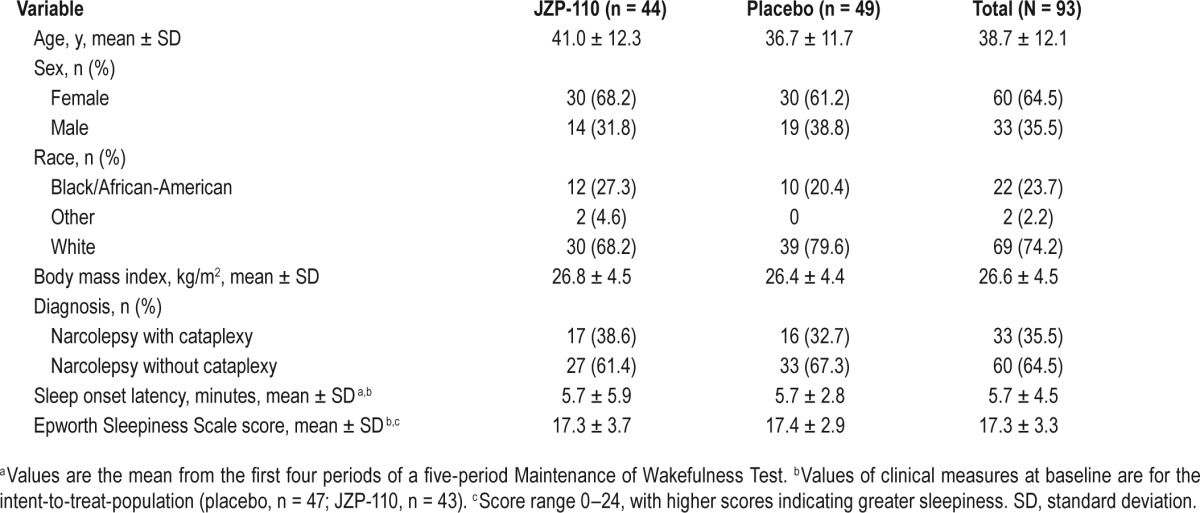
Of 213 patients who were screened, 93 were enrolled and randomized, 44 to JZP-110 and 49 to placebo (Figure 2). Demographic and clinical characteristics were similar between the two treatment groups (Table 1). The majority of patients were female (64.5%) and white (74.2%), with a mean (± SD) age of 38.7 ± 12.1 years and a body mass index of 26.6 ± 4.5 kg/m2. The mean ESS score of 17.3 ± 3.3 indicated pathological levels of excessive sleepiness, and slightly more than one-third of patients (35.5%) had cataplexy (Table 1), with a mean of 19.2 ± 45.3 attacks per week and a median of 4.0 attacks per week among the 31 patients who had narcolepsy with cataplexy and who reported these data at baseline.
Figure 2.
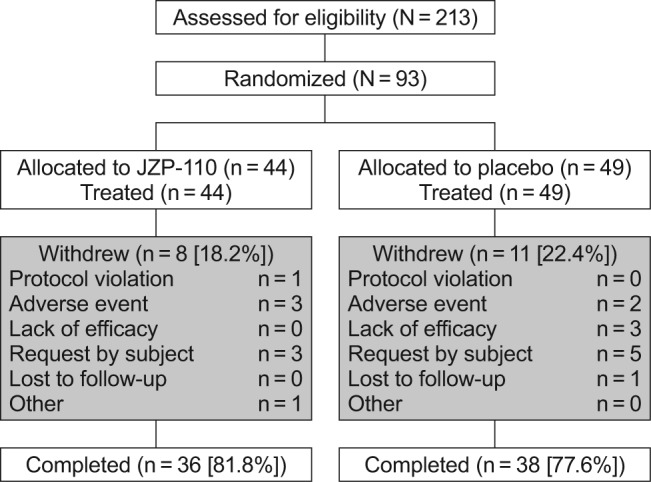
Patient disposition.
Table 1.
Demographic and clinical characteristics of the population at baseline.
As shown in Figure 2, which presents patient disposition, 81.8% and 77.6% of patients in the JZP-110 and placebo groups, respectively, completed the study. Withdrawals in the active treatment group were mainly for AEs (n = 3; conversion disorder, acute cholecystitis, and exacerbation of anxiety) and patient request (n = 3), whereas placebo withdrawals were primarily due to patient request (n = 5), lack of efficacy (n = 3), and AEs (n = 2). The safety population consisted of all 93 patients, but 3 patients (2 placebo and 1 JZP-110) had no post-baseline efficacy measurements, and thus the intent-to-treat population consisted of 90 patients with evaluable efficacy data.
Adherence and Dosing
Adherence to therapy, defined as when the dose taken was equal to the dose dispensed, was similarly high in both treatment groups, 99.4% and 99.3% for JZP-110 and placebo, respectively. At week 4, all patients who had been randomized to receive JZP-110 were taking 150 mg/day, and among the 36 patients who had been randomized to receive JZP-110 and who completed the study at week 12, 86.1% (31/36) were taking the 300 mg/day dose as of the final visit. The dose was reduced to 150 mg/day as of the week 12 visit in five patients in response to AEs (n = 1 for each: anxiety; tachyphrenia and feeling abnormal; decreased appetite; insomnia; tension headache). Five patients in the JZP-110 group who discontinued the study were on 150 mg/day as their last dose.
Efficacy
For both of the primary endpoints, JZP-110 resulted in improvements at week 12 that were significantly greater than those observed with placebo (Figure 3). The mean (standard error) change from baseline in average sleep latency on the first four periods of the MWT was 12.8 (1.6) min with JZP-110 versus 2.1 (1.2) min with placebo (P < 0.0001; Figure 3A). On the CGI-C, 86.0% of the patients treated with JZP-110 were assessed as improved, compared with 38.3% of placebo patients (P < 0.0001; Figure 3B). The sensitivity analysis confirmed the results of the primary analysis for MWT; the least squares mean difference between treatments of 11.1 min, favoring JZP-110, was statistically significant (P < 0.0001), and no effects were observed by site or for treatment-by-site interaction. This difference resulted in an effect size (Cohen d) of 1.0 at week 4 and 1.2 at week 12 based on MWT.
Figure 3.
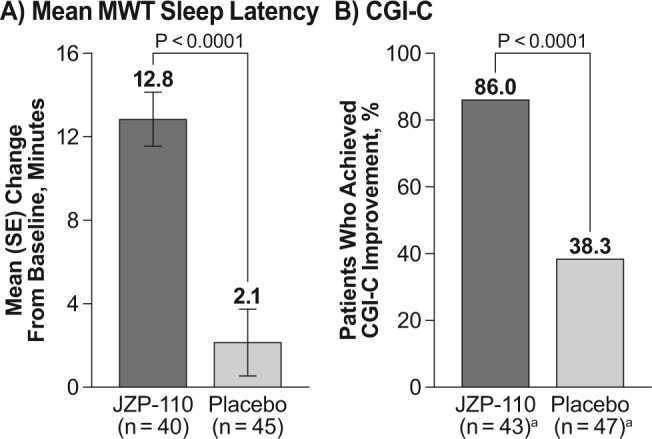
Results of the two primary efficacy endpoints at 12 w. (A) Change from baseline in mean sleep latency on the Maintenance of Wakefulness Test (MWT; average of first four periods). (B) Percentage of patients who were improved on the Clinical Global Impression of Change (CGI-C), defined as scores of “very much improved,” “much improved,” or “minimally improved.” aWeek 12 is an endpoint analysis representing last postbaseline observation carried forward; baseline and week 12: JZP-110 (n = 43); placebo (n = 47). SE, standard error.
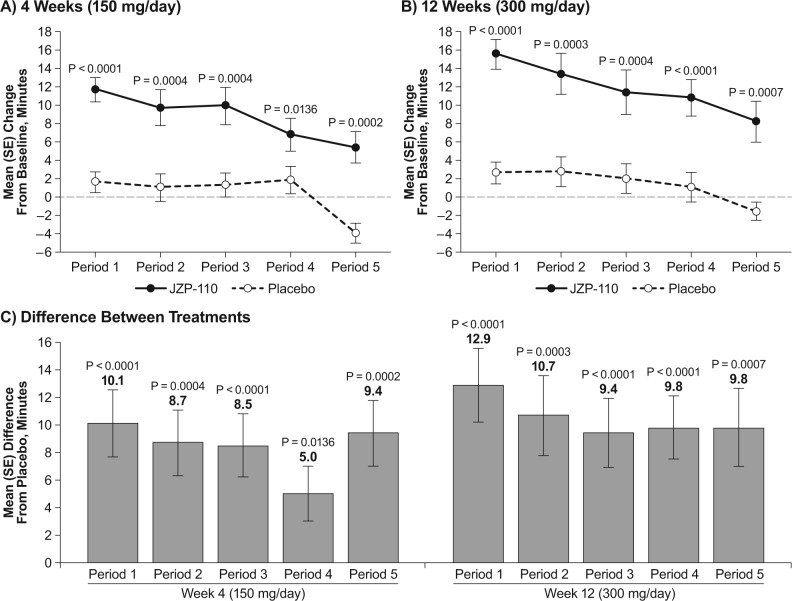
As shown in Figure 4, the mean change from baseline in sleep latency was significantly greater with JZP-110 than placebo for each of the MWT periods both at week 4 (Figure 4A) and at week 12 (Figure 4B). Changes from baseline at week 4 ranged from 11.7 min (period 1) to 5.4 min (period 5) with JZP-110, and 1.6 min to −4.0 min (periods 1 and 5, respectively) with placebo. Changes from baseline at week 12 ranged from 15.5 to 8.2 min with JZP-110 and 2.6 to −1.6 min with placebo (Figures 4A and 4B). At week 4 the mean (standard error) change from baseline in average sleep latency for the first four periods of the MWT was 9.5 (1.3) with JZP-110 and 1.4 (1.1) with placebo (P < 0.0001; data not shown).
Figure 4.
Mean change from baseline in sleep latency on each of the individual periods in the Maintenance of Wakefulness Test (MWT) at weeks 4 (A) and 12 (B), and mean difference in change from baseline between JZP-110 and placebo (C). SE, standard error.
Baseline scores on the ESS, slightly over 17 points in both treatment groups (i.e., placebo and JZP-110), indicated pathological levels of excessive sleepiness. Although scores at weeks 4 and 12 were lower in both treatment groups, scores were significantly lower with JZP-110 relative to placebo at both time points. In addition, the mean ESS score in the JZP-110 group at week 12 was within the normal range (i.e., < 10; Figure 5A). In contrast, there were no further reductions in the placebo group at week 12 relative to week 4, and these scores remained within the range that is indicative of excessive sleepiness (Figure 5A). A significant difference in ESS scores between the groups was observed as early as 1 w after the start of dosing (P < 0.0001) and was maintained through week 4 with the lower dose of JZP-110 (Figure 5B). After titration to 300 mg/day at week 4, further improvement was observed at week 6 and was maintained through the end of treatment, with all differences from placebo showing significance (P < 0.005; Figure 5B).
Figure 5.
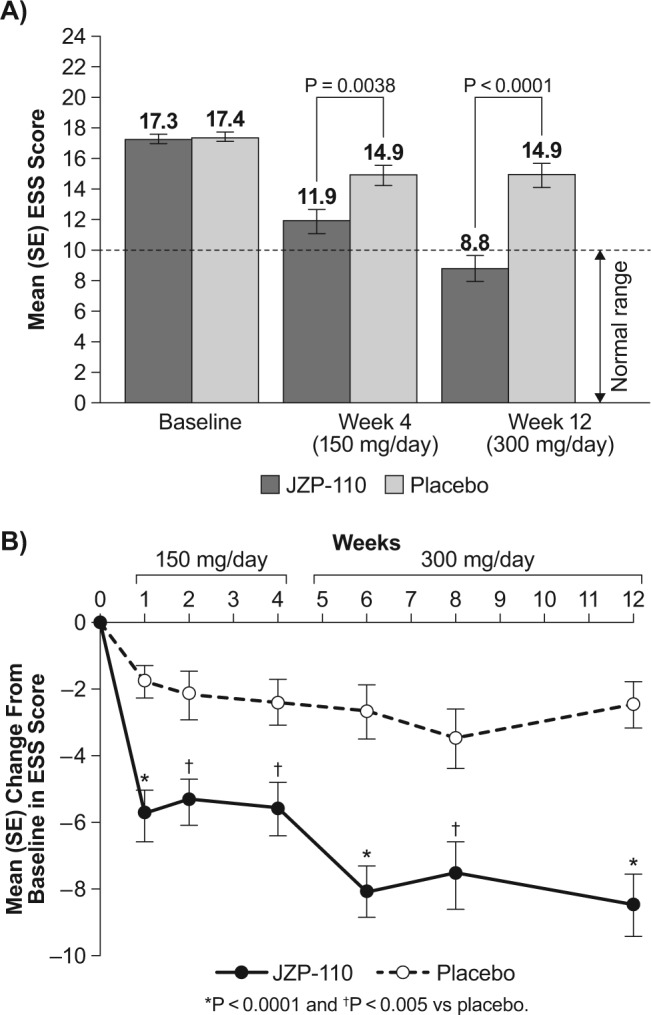
Change in Epworth Sleepiness Scale (ESS) scores. (A) Absolute ESS scores at weeks 4 and 12 relative to baseline. The horizontal broken line in A represents the threshold for normal ESS score. (B) Change in score over study duration. SE, standard error.
JZP-110 consistently resulted in a significantly greater percentage of patients who improved on both the PGI-C (Figure 6A) and the CGI-C (Figure 6B) relative to placebo at the evaluated time points of 1, 4, and 12 w (P < 0.05 for all comparisons). The improvements with JZP-110 were observed as early as week 1 at the 150 mg/day dose (83.7% for both PGI-C and CGI-C) and maintained through the end of treatment at the 300 mg/day dose (93% and 86% for PGI-C and CGI-C, respectively). In contrast, the percentages with placebo decreased at each subsequent assessment, from 53.2% to 44.4% to 38.3% on the PGI-C, and 55.3% to 51.1% to 38.3% on the CGI-C. Additionally, percentages of patients who achieved improvement were similar on both subjective measures, and in a post hoc analysis, the correlation between PGI-C and CGI-C at week 12 was strong and statistically significant (Spearman r = 0.868; P < 0.0001).
Figure 6.
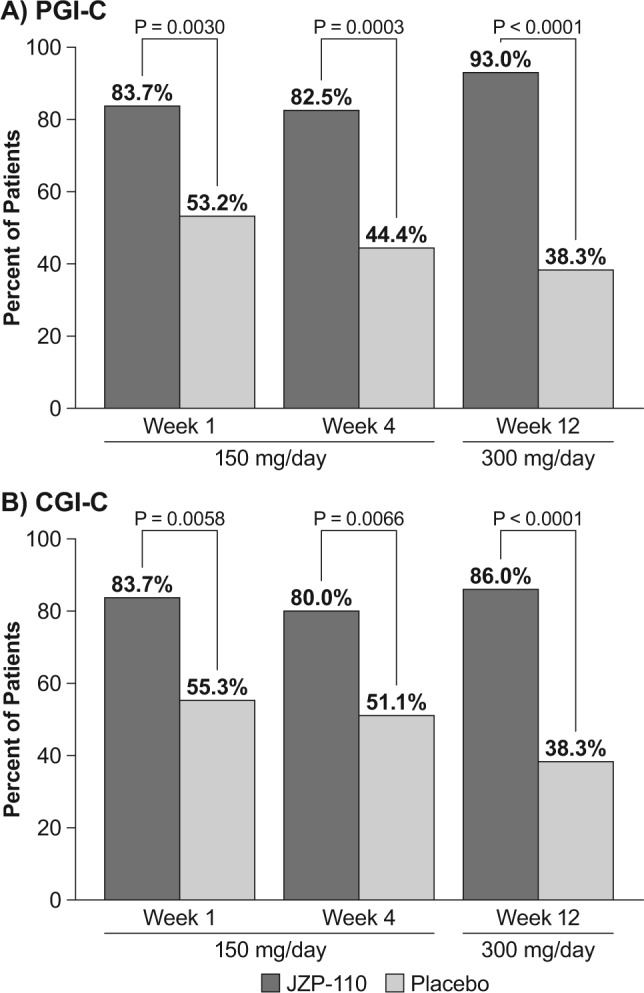
Percentages of patients who achieved improvement on global impression of change measures at weeks 1, 4, and 12. (A) Patient Global Impression of Change (PGI-C). (B) Clinician Global Impression of Change (CGI-C).
There was no significant difference between groups in the median change from baseline in number of weekly cataplexy attacks (JZP-110 −1.0; placebo, 0) in the 33 patients with cataplexy (n = 17, JZP-110; n = 16, placebo). Additionally, in the subset of patients with cataplexy, results were similar to those observed in the overall intent-to-treat population for change from baseline in mean MWT sleep latency; JZP-110 resulted in increases in mean MWT sleep latency that were significantly greater than placebo (P < 0.05) on the mean of the first four periods of the MWT, as well as the five individual MWT periods at both week 4 and week 12 (data not shown).
Safety
The overall incidence of AEs was higher with JZP-110 (81.8%) than with placebo (59.2%; Table 2). Based on the dose period, the incidence of AEs was 61.4% at the lower dose (first 4 weeks) and 79.5% at the higher dose (subsequent 8 w). Nearly all AEs were of mild or moderate severity (JZP-110, 93%; placebo, 94%). No deaths occurred in the study, and there were only two serious AEs, both in the JZP-110 treatment group and resulting in discontinuation; one case of conversion disorder (150 mg/day dose) and one case of acute cholecystitis (300 mg/day), neither of which was deemed by the investigator to be related to study medication.
Table 2.
Adverse events.
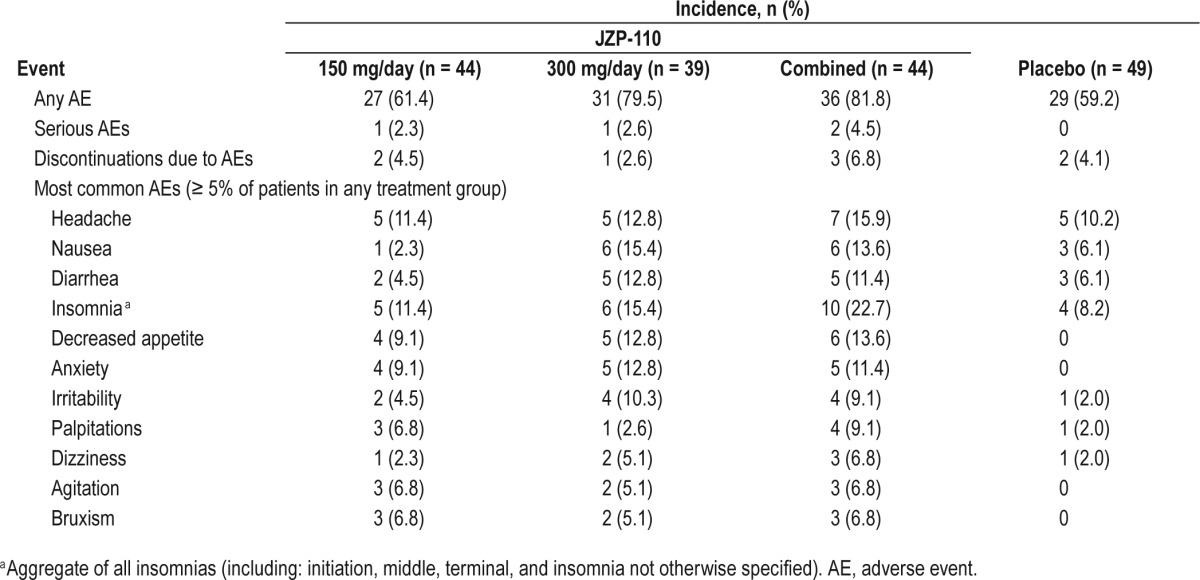
The most frequently reported AEs, defined as occurring in ≥ 5% of patients in any treatment group, were observed with a higher incidence in the JZP-110 group, and generally at the higher dose (Table 2). In the JZP-110 group, the most common of these AEs was insomnia (22.7%), followed by headache (15.9%), nausea (13.6%), decreased appetite (13.6%), and diarrhea (11.4%; Table 2). There were no findings of suicidality on the C-SSRS during the study, none of the reported AEs was suggestive of euphoria, and none of the reported AEs was indicative of abuse or addiction.
Clinical laboratory evaluations did not suggest any drug-related effects. At week 4, mean (SD) increases from baseline in systolic and diastolic blood pressure with placebo were 0.69 (8.11) for systolic and 0.95 (5.21) for diastolic, and for JZP-110 150 mg/day these were 0.41 (7.51) and 0.85 (5.03), respectively. At week 12, mean (SD) increases from baseline in systolic blood pressure were 2.55 (7.71) mm Hg and 1.90 (6.97) mm Hg for placebo and JZP-110 300 mg/day dose, respectively; increases from baseline in diastolic blood pressure were 1.92 (5.25) and 2.13 (4.45) mm Hg, respectively. Both treatment groups exhibited increases from baseline in heart rate at 4 w, 2.1 (5.0) beats/ min and 2.5 (7.2) beats/min with placebo and JZP-110 150 mg/ day, respectively, and at 12 w, 1.8 (4.9) beats/min with placebo and 2.9 (7.1) beats/min with JZP-110 300 mg/day. None of the differences between treatment groups in change from baseline for blood pressure or heart rate was statistically significant. Both heart rate and blood pressure returned to placebo levels 24 hours after dosing. There were no effects on quantitative electrocardiogram parameters of PR, QRS, and corrected QT using Bazett's (QTcB) and Friderica's (QTcF) formulas.
DISCUSSION
This study, the first 12-w, randomized, placebo-controlled clinical trial to evaluate JZP-110 for the treatment of patients with narcolepsy, showed that once-daily oral administration of JZP-110 resulted in significant improvements relative to placebo in decreasing excessive sleepiness as measured by the ESS and in increasing the ability to remain awake as measured by the MWT.
Notably, JZP-110 was significantly superior to placebo on all primary and secondary measures of efficacy including objective as well as patient-reported outcomes. These data confirm and extend the results of a small, double-blind crossover study with 2 w of JZP-110 treatment,18 and demonstrate that the effects of JZP-110 are maintained across 12 w of treatment.
Improvements in the mean MWT sleep latency and on each of the five individual periods of the MWT, an objective measure of a subject's ability to maintain wakefulness, were substantial and significantly better than placebo at both 4 and 12 w. The magnitude of the response was consistent with that reported in the small crossover study,18 and the effect size (Cohen d) of 1.0 at week 4 and 1.2 at week 12 demonstrated the robustness of the treatment effect. Additionally, there were significantly greater changes from baseline in sleep latency on each of the five individual MWT periods, especially at week 12, which ranged from 8.2 to 15.5 min with JZP-110, relative to changes with placebo that ranged from −1.6 to 2.6 min. The difference in effect remained highly statistically significant for period 5 both at week 4 (P = 0.0002) and week 12 (P = 0.0007), suggesting that the increase in wakefulness associated with JZP-110 was maintained throughout the 9-h period following dosing. These results are in contrast to what has been observed in studies of modafinil and armodafinil, which showed that the effects of these drugs were diminished by the fifth period of the MWT.27,28
Although statistically significant increases in sleep latency on all five periods of the MWT relative to placebo were observed after 4 w of treatment, the greatest effects on sleep latency were observed at 12 w, which was 8 w after titration to the 300-mg dose. In addition, significantly greater benefits of JZP-110 relative to placebo were observed on the ESS, CGI-C, and PGI-C as early as 1 w, which was the first assessment after patients began taking the lower dose of 150 mg/day. These benefits were maintained over the study duration for the global assessments, with the percentages of JZP-110-treated patients who improved on both the CGI-C and PGI-C remaining similarly high at 4 and 12 w. Furthermore, the percentages on the PGI-C paralleled those on the CGI-C, demonstrating concordance between patient- and clinician-reported perspectives of global improvement. Of additional clinical relevance are the incremental improvements observed on the ESS following titration to the higher dose, resulting in an absolute score of 8.8 at week 12, which is within the range considered normal (≤ 10).29
The relative lack of an effect on cataplexy might be surprising considering JZP-110 is a dopamine and norepinephrine reuptake inhibitor. However, cataplexy was an exploratory endpoint and this study was not designed to evaluate the effects of treatment on cataplexy; a minority of patients had cataplexy and cataplexy did not occur very frequently in this population (median of four attacks per week at baseline). In contrast, the robust effects of JZP-110 on promoting wakefulness and decreasing excessive sleepiness are likely to be related to its ability to inhibit dopamine reuptake. Reuptake inhibitors of serotonin and/or norepinephrine that lack activity at the dopamine transporter are not potent wake-promoting drugs.30 Conversely, affinity for the dopamine transporter is highly correlated with potency to produce wake-promoting effects in narcoleptic dogs.30 It is possible that the combination of dopamine and norepinephrine reuptake inhibition that JZP-110 offers might be responsible for the robust wake-promoting effects that have been observed here and in a previous study of JZP-110.18
The safety profile of JZP-110 was consistent with the previous phase 2a study in narcolepsy patients and the phase 2 studies in major depressive disorder.18,31 The AEs observed across these studies are consistent with a wake-promoting profile of effects (insomnia, decreased appetite, anxiety, arousal). Importantly, only five patients required dose reduction and adherence to treatment was high (99%), with no evidence of suicidal ideation or behavior or abuse during the study or follow-up period. Small changes in blood pressure and heart rate were noted relative to placebo in both the proof-of-concept and the current studies. These were transient, not statistically significant between treatments, and not considered clinically significant. The increases with JZP-110 relative to placebo were less than the differences from placebo reported for stimulants,32 and similar to or less than the range reported for armodafinil.33 No abnormalities were noted on electrocardiography.
Study limitations include that the enrolled population may be biased as a result of selection that potentially favored patients who were resistant to traditional stimulants or wake-promoting agents. The patients in this study were also predominantly female, and narcolepsy has been reported to affect both sexes with a slight preponderance of males.1 Additionally, although substantial effects were observed at the lower dose, the study design did not allow for direct comparison between the doses for efficacy or tolerability because the effects of 300 mg/day were confounded by additional time on drug. Another limitation of the study design is that it does not allow for an adequate assessment of JZP-110 on cataplexy.
In conclusion, JZP-110 is a second-generation wake-promoting agent with a distinct mechanism of action that involves dopamine and norepinephrine reuptake inhibition. However, the safety profile appears to be consistent with other wake-promoting agents in adults with narcolepsy. At doses of 150 to 300 mg/day, JZP-110 significantly improved both the ability to stay awake and subjective symptoms of excessive sleepiness in these patients. The magnitude of these effects was large and clinically significant, and treatment with JZP-110 resulted in a mean ESS score within the normal range. These results warrant further evaluation of JZP-110 as a new therapeutic option for the treatment of excessive sleepiness.
DISCLOSURE STATEMENT
Jazz Pharmaceuticals, Inc., has licensed JZP-110 from Aerial BioPharma. Under the direction of the authors, E. Jay Bienen, PhD, of The Curry Rockefeller Group, LLC (CRG), provided editorial assistance for the development of this manuscript. Editorial assistance in formatting, proofreading, copy editing, and fact checking was also provided by CRG. Jazz Pharmaceuticals, Inc., provided funding to CRG for all editorial support. This research was funded by Aerial BioPharma, LLC. Dr. Ruoff is an employee of Stanford University and has served as an advisory board member and unpaid consultant for Jazz Pharmaceuticals, Inc.; Stanford University received grant funding from Aerial BioPharma, LLC. Dr. Swick is an employee of Neurology and Sleep Medicine Consultants; has received consultancy fees and/or honoraria from Jazz Pharmaceuticals, Inc., Vanda Pharmaceuticals, XenoPort Pharmaceuticals, UCB Pharma, Merck Pharma, Aerial BioPharma, LLC; has received research funding from Jazz Pharmaceuticals, Inc., Aerial BioPharma, LLC, GSK Pharmaceuticals, Otsuka Pharmaceuticals, Teva Pharmaceuticals, Vanda Pharmaceuticals; and is on the speakers bureau for Jazz Pharmaceuticals, Inc., XenoPort Pharmaceuticals, UCB Pharma. Dr. Doekel has received research funding from Aerial BioPharma, LLC. Dr. Emsellem has received consultancy fees from and/or has been a Speakers Bureau member for GLG Research, Jazz Pharmaceuticals, Inc., and Vanda Pharmaceuticals; and has received research funding from Aerial BioPharma, LLC, ApniCure, Jazz Pharmaceuticals, Inc. Dr. Feldman has received research funding from Aerial BioPharma, LLC, Jazz Pharmaceuticals, Inc., Cephalon; and is on the speakers bureau for Jazz Pharmaceuticals, Inc. Dr. Rosenberg has served as a member of the speakers bureau or given a presentation for Jazz Pharmaceuticals, Inc., and Purdue Pharmaceuticals; Dr Rosenberg has also received research grants or funding from Merck, Aerial BioPharma, LLC, Teva Pharmaceuticals, GlaxoSmithKline, Philips-Respironics, and Cerêve. Dr. Bream was an employee of Aerial BioPharma, LLC, with stock options, while this study was conducted; he has no current affiliation. Dr. Khayrallah was an employee of Aerial BioPharma, LLC, with stock options, while this study was conducted; his current affiliation is QuatroBio, LLC, Raleigh, NC. Ms. Lu is an employee of Jazz Pharmaceuticals, Inc., who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Dr. Black is a part-time employee of Jazz Pharmaceuticals, Inc., and holds stock in Jazz Pharmaceuticals plc.
REFERENCES
- 1.American Academy of Sleep Medicine. The International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 3.Dodel R, Peter H, Spottke A, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007;8:733–41. doi: 10.1016/j.sleep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Jennum P, Ibsen R, Petersen ER, Knudsen S, Kjellberg J. Health, social, and economic consequences of narcolepsy: a controlled national study evaluating the societal effect on patients and their partners. Sleep Med. 2012;13:1086–93. doi: 10.1016/j.sleep.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Black J, Reaven NL, Funk S, et al. The Burden of Narcolepsy Disease (BOND) Study: Healthcare Utilization and Cost Findings. Sleep Med. 2014;15:522–9. doi: 10.1016/j.sleep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Sangal RB, Thomas L, Mitler MM. Maintenance of wakefulness test and multiple sleep latency test. Measurement of different abilities in patients with sleep disorders. Chest. 1992;101:898–902. doi: 10.1378/chest.101.4.898. [DOI] [PubMed] [Google Scholar]
- 7.Sangal RB, Mitler MM, Sangal JM. Subjective sleepiness ratings (Epworth sleepiness scale) do not reflect the same parameter of sleepiness as objective sleepiness (maintenance of wakefulness test) in patients with narcolepsy. Clin Neurophysiol. 1999;110:2131–5. doi: 10.1016/s1388-2457(99)00167-4. [DOI] [PubMed] [Google Scholar]
- 8.Fronczek R, Middelkoop HA, van Dijk JG, Lammers GJ. Focusing on vigilance instead of sleepiness in the assessment of narcolepsy: high sensitivity of the Sustained Attention to Response Task (SART) Sleep. 2006;29:187–91. [PubMed] [Google Scholar]
- 9.Sadeghniiat-Haghighi K, Moller HJ, Saraei M, Aminian O, Khajeh-Mehrizi A. The Epworth Sleepiness Scale for screening of the drowsy driving: comparison with the maintenance of wakefulness test in an Iranian sample of commercial drivers. Acta Med Iran. 2014;52:125–9. [PubMed] [Google Scholar]
- 10.Billiard M, Bassetti C, Dauvilliers Y, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13:1035–48. doi: 10.1111/j.1468-1331.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- 11.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keam S, Walker MC. Therapies for narcolepsy with or without cataplexy: evidence-based review. Curr Opin Neurol. 2007;20:699–703. doi: 10.1097/WCO.0b013e3282f22ad9. [DOI] [PubMed] [Google Scholar]
- 13.Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic managment of narcolepsy. Sleep Med. 2015;16:9–18. doi: 10.1016/j.sleep.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Wisor J. Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions. Front Neurol. 2013;4:139. doi: 10.3389/fneur.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman RB, Baumann MH, Dersch CM, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Hasan S, Pradervand S, Ahnaou A, Drinkenburg W, Tafti M, Franken P. How to keep the brain awake? The complex molecular pharmacogenetics of wake promotion. Neuropsychopharmacology. 2009;34:1625–40. doi: 10.1038/npp.2009.3. [DOI] [PubMed] [Google Scholar]
- 17.Gordon R, Bernard P, Pack E, Lee K, Choi YM. Pharmacological profile of YKP10A: a novel antidepressant [abstract] Soc Neurosci. 1998;583:24. (part 2):Abstract 583.9. [Google Scholar]
- 18.Bogan R, Feldman N, Emsellem HA, et al. Effect of oral JZP-110 (ADX-N05) treatment on wakefulness and sleepiness in adults with narcolepsy. Sleep Med. 2015;16:1102–8. doi: 10.1016/j.sleep.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 22.Guy W. Rockville, MD: National Institute of Mental Health; 1976. ECDEU assessment manual for psychopharmacology, revised. US Department of Health, Education, and Welfare publication (ADM 76-338) [Google Scholar]
- 23.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broughton RJ, Fleming JA, George CF, et al. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology. 1997;49:444–51. doi: 10.1212/wnl.49.2.444. [DOI] [PubMed] [Google Scholar]
- 25.Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. US Modafinil in Narcolepsy Multicenter Study Group. Ann Neurol. 1998;43:88–97. doi: 10.1002/ana.410430115. [DOI] [PubMed] [Google Scholar]
- 26.Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy: US Modafinil in Narcolepsy Multicenter Study Group. Neurology. 2000;54:1166–75. doi: 10.1212/wnl.54.5.1166. [DOI] [PubMed] [Google Scholar]
- 27.Harsh JR, Hayduk R, Rosenberg R, et al. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr Med Res Opin. 2006;22:761–74. doi: 10.1185/030079906X100050. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz JR, Feldman NT, Bogan RK, Nelson MT, Hughes RJ. Dosing regimen effects of modafinil for improving daytime wakefulness in patients with narcolepsy. Clin Neuropharmacol. 2003;26:252–7. doi: 10.1097/00002826-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–9. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 30.Nishino S, Okura M, Mignot E. Narcolepsy: genetic predisposition and neuropharmacological mechanisms. Sleep Med Rev. 2000;4:57–99. doi: 10.1053/smrv.1999.0069. [DOI] [PubMed] [Google Scholar]
- 31.Amsterdam JD, Brunswick DJ, Hundert M. A single-site, double-blind, placebo-controlled, dose-ranging study of YKP10A--a putative, new antidepressant. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1333–8. doi: 10.1016/s0278-5846(02)00298-1. [DOI] [PubMed] [Google Scholar]
- 32.Mick E, McManus DD, Goldberg RJ. Meta-analysis of increased heart rate and blood pressure associated with CNS stimulant treatment of ADHD in adults. Eur Neuropsychopharmacol. 2013;23:534–41. doi: 10.1016/j.euroneuro.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuvigil (armodafinil) prescribing information. Frazer, PA: Teva Pharmaceuticals, 2015. Available at www.nuvigil.com.