Abstract
Study Objectives:
Animal sleep deprivation (SDEP), in contrast to human SDEP, is involuntary and involves repeated exposure to aversive stimuli including the inability of the animal to control the waking stimulus. Therefore, we explored intracranial self-stimulation (ICSS), an operant behavior, as a method for voluntary SDEP in rodents.
Methods:
Male Sprague-Dawley rats were implanted with electroencephalography/electromyography (EEG/EMG) recording electrodes and a unilateral bipolar electrode into the lateral hypothalamus. Rats were allowed to self-stimulate, or underwent gentle handling-induced SDEP (GH-SDEP), during the first 6 h of the light phase, after which they were allowed to sleep. Other rats performed the 6 h ICSS and 1 w later were subjected to 6 h of noncontingent stimulation (NCS). During NCS the individual stimulation patterns recorded during ICSS were replayed.
Results:
After GH-SDEP, ICSS, or NCS, time in nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep increased. Further, in the 24 h after SDEP, rats recovered all of the REM sleep lost during SDEP, but only 75% to 80% of the NREM sleep lost, regardless of the SDEP method. The magnitude of EEG slow wave responses occurring during NREM sleep also increased after SDEP treatments. However, NREM sleep EEG slow wave activity (SWA) responses were attenuated following ICSS, compared to GH-SDEP and NCS.
Conclusions:
We conclude that ICSS and NCS can be used to sleep deprive rats. Changes in rebound NREM sleep EEG SWA occurring after ICSS, NCS, and GH-SDEP suggest that nonspecific effects of the SDEP procedure differentially affect recovery sleep phenotypes.
Citation:
Oonk M, Krueger JM, Davis CJ. Voluntary sleep loss in rats. SLEEP 2016;39(7):1467–1479.
Keywords: delta power, gentle handling, intracranial self-stimulation, slow wave amplitude, theta power, total sleep deprivation
Significance.
Many occurrences of sleep deprivation in humans are self-selected by substituting preferred activities to sleep. Current rodent models of sleep loss consist of obtruded and aversive techniques that have unknown consequences. Here we introduce an effective voluntary rodent sleep deprivation model. Our approach of using protracted intracranial self-simulation for sleep deprivation is novel and provides a testbed to contrast imposed stimulation and contemporary sleep deprivation methods. We report that intracranial self-stimulation affects EEG signals during sleep rebound differently than other methods. An animal sleep deprivation model that better reflects sleep loss in humans is key to making accurate conclusions about any shared physiologies.
INTRODUCTION
Animal research is required to elucidate the neurobiological processes underlying sleep and sleep loss. To decipher sleep mechanisms, sleep deprivation (SDEP) is often used in conjunction with measurements of brain activity and neurochemistry. To date, all animal SDEP methods use repeated aversive stimuli to prevent sleep and this introduces the confound of whether the changes in subsequent brain activity are the result of the aversive stimuli or due to sleep loss per se. For instance, SDEP induced by gentle handling (GH-SDEP) typically entails the experimenter perturbing the animal when behavioral and/or electrophysiological signs of sleep are observed, by tapping the cage, disturbing the bedding, introducing novel objects, and/or touching the animal. Further, animal SDEP methods are forced and inescapable, creating an uncontrollable environment from the animal's perspective. Such SDEP methods fail to model the voluntary sleep loss incurred in human SDEP experiments. Aversive stimuli (also interpreted as stressors) differentially affect sleep.1,2 For instance, exposure to social defeat increases subsequent nonrapid eye movement (NREM) sleep electroencephalogram (EEG) slow wave activity (SWA).3–6 Similarly, following immobilization at light onset, NREM sleep EEG SWA increases whereas rapid eye movement (REM) sleep duration decreases.7 In contrast, brief exposure to ether vapors enhances REM sleep duration without affecting NREM sleep duration or NREM sleep EEG SWA.8 Controllability of the stressor also affects subsequent sleep. Escapable foot shock, for instance, increases REM sleep duration, whereas inescapable foot shock decreases REM sleep and NREM sleep duration.9,10 Because many stressors alter one or more sleep phenotypes, it is not clear if the aversive nature of standard animal SDEP practices, or sleep loss per se drive subsequent outcomes.
In the current study, we use intracranial self-stimulation (ICSS) as a SDEP method devoid of aversive stimuli and giving stimulus control to the animal. Discovered over 65 y ago by Olds and Milner,11 ICSS is a well-studied operant behavior wherein animals are trained to press a lever that triggers brief electrical stimulation of the mesolimbic dopaminergic brain reward system. The primary pathway of the brain reward system consists of the medial forebrain bundle, which originates in the ventral tegmental area and traverses through the lateral hypothalamus to the nucleus accumbens. ICSS is considered to have more incentive value than conventional rewards, e.g., food.12,13 Depending on electrode placement and stimulation parameters, rodents will self-stimulate at high rates for days, with marginal rest periods.14–17 The rewarding property of ICSS is evident from the high frequency of lever presses and the apparent lack of satiation. In addition, humans subjectively report pleasure in response to brief electrical stimulation to brain reward areas.18 Similar to ICSS, noncontingent stimulation (NCS) delivers direct electrical impulses to the brain reward-circuit. In NCS, however, the lever is removed from the chamber and thus the stimulus is not self-administered, but is externally prescribed by the experimenter. Although the stimulation parameters and temporal delivery patterns are identical between ICSS and NCS, animals predominantly choose to self-stimulate when given the option, demonstrating the importance of controllability.19–21
To date, few studies have examined ICSS or NCS in relation to sleep, and primarily focus on how REM sleep affects ICSS.22–24 Herein we show that ICSS-induced wakefulness is an effective self-selected SDEP method capable of maintaining wake for 6 h, followed by NREM sleep and REM sleep rebounds similar to those observed after GH-SDEP. However, differences in NREM sleep EEG SWA rebound, occurring after ICSS-SDEP, compared to NCS-SDEP and GH-SDEP, suggests that the uncontrollable and aversive nature of the SDEP method differentially affects recovery sleep phenotypes. As such, ICSS-SDEP could be useful in dissociating method confounds from animal SDEP.
METHODS
Animals
Male Sprague-Dawley rats (9–12 w; Harlan Laboratories; Kent, WA) were individually housed under standard conditions (21 ± 1°C; 12:12 h light:dark cycle, lights on at Zeitgeber time (ZT) 0). Food and water were available ad libitum in both housing and experimental conditions. The study was approved by Washington State University's Institutional Animal Care and Use Committee and was consistent with National Institutes of Health guidelines.
Rats were anesthetized with intramuscular ketamine-xylazine (87 mg/kg and 13 mg/kg, respectively) and provided EEG electrodes over the left parietal and frontal cortices. An EMG electrode was placed in the dorsal neck muscles, and an electrode affixed over the cerebellum served as a ground. In addition, rats were provided a twisted-pair, polyimide-coated, stainless steel bipolar stimulating electrode (0.150-mm diameter; Plastics One, Roanoke, VA) into the right lateral hypothalamus (anterior/posterior: −3.8 mm, medial/lateral: −1.6 mm, dorsal/ventral: −8.2 mm); the brain area that elicits the highest lever pressing response rates.25,26 The electrodes were secured to the skull with dental cement and rats were given at least 7 days to recover from surgery prior to ICSS training.
ICSS Equipment
Operant chambers (model 80004, Lafayette Instruments, Lafayette, IN), equipped with lights and a lever, were placed in sound attenuating chambers and interfaced with the stimulator (model 3800, A-M Systems, Carlsborg, WA) by a locally-programmed Uno-R3 micro-controller board (Arduino, Jamco Electronics, Belmont, CA). Onboard software was used to program the stimulation parameters. The stimulating electrode was connected to the stimulator through a commutator and a stimulus isolation unit (model 3820, A-M Systems). ABET II software version 2.14 (Lafayette Instruments) was programmed to run the operant chambers, record the lever presses, and trigger the stimulator.
ICSS Training
The stimulator was configured to generate a 200-Hz biphasic square wave (0.25 msec pulse duration) for 500 msec followed by a 500 msec inactive period. Thus, lever presses that occurred during a 500-msec stimulation period, or the subsequent 500 msec, did not result in additional stimulations. All rats were trained to lever press before the SDEP experiments began, first with stimulations delivered by the experimenter when the rat approximated a lever press, and thereafter stimulations were delivered on a 1-sec fixed-interval schedule of reinforcement that was contingent on the rats' lever pressing. In the next two input/output sessions, optimal stimulus current was established in each rat, starting with the minimal current required for responding (20–110 μA). Current was increased by 5 μA every 5 min until the response rate plateaued (± 3 lever presses) for 15 min, or remained below the maximum response rate (± 3 lever presses) for 25 min. For each rat, the current intensity for subsequent training and SDEP sessions was halfway between the lower and upper limits of responding, and ranged from 60 to 215 μA. Training consisted of four 30 min sessions and one 2 h session over a 6-day period, prior to SDEP. In order to minimize sleep disruption, the 30-min training sessions were conducted at the end of the light period (ZT 10–12) and the 2-h session was conducted at the beginning of the dark period (ZT 12–14).
Gentle Handling-Induced Sleep Deprivation
During GH-SDEP, experimenters interacted with rats only when necessary; otherwise, rats were allowed to behave naturally (eat, drink, explore, groom, etc.), but not to sleep. In the event that the rats' vibrissae stopped whisking and were non-ambulatory and inactive and/or large amplitude EEG waves were observed, one of the following perturbations occurred: (1) a soft artisan's paint brush was used to stir up the nest/bedding, (2) fingers were snapped or the cage was lightly tapped or rotated, and (3) if the aforementioned methods were ineffective, rats were stroked with the paint brush, initially on the tail, then body or whiskers as required to keep rats awake. No novel objects were used.
Polysomnographic Recording and Analysis
The EEG and EMG electrodes were connected to the amplifier (Grass, Model 12 Neurodata acquisition system, West Warwick, RI) through recording cables and a six-channel commutator (Plastics One). The EEG signal, recorded over the left parietal cortex, referenced to the left frontal cortex, was amplified (×1,000) and high-pass and low-pass filtered at 0.1 Hz and 100 Hz, respectively, with a 60-Hz notch filter. Similarly, the EMG signal was amplified (×500) and high-pass and low-pass filtered at 30 Hz and 3 kHz, respectively. The amplified and filtered EEG and EMG signals were digitized (128 Hz sampling rate) and recorded using Sleepwave v2.01 (Biosoft Studios, Hershey, PA). The recorded EEG signal was manually scored in 10-sec epochs as wakefulness, NREM sleep, and REM sleep using Sleep Sign software (Kissei Comtec Co., Matsumoto, Japan). Time in wakefulness, NREM sleep and REM sleep, and NREM sleep and REM sleep episode durations and episode frequencies, were analyzed in 6-h bins. An episode was defined as at least one 10-sec epoch. EEG SWA (1–4 Hz), EEG theta activity (6–9 Hz), and power spectra (1–20 Hz) during artifact-free NREM sleep or REM sleep epochs were determined offline with SleepSign by fast Fourier transformation, using a Hanning window, and normalized as previously described.27 Briefly, NREM sleep EEG SWA (1–4 Hz) and REM sleep EEG theta activity (6–9 Hz) were normalized using the average 24-h baseline values, then multiplied by 100 to transform to percent and analyzed in 2-h bins. NREM sleep power spectra during ZT 6–8 and REM sleep power spectra during ZT 12–14 of recovery sleep were normalized using the 24-h state-specific baseline averages of the summed power across all frequencies (1–20 Hz). Last, NREM sleep EEG delta energy and REM sleep EEG theta energy were analyzed in 2-h bins. Energies were defined as the product of the average raw NREM sleep EEG SWA (1–4 Hz) or REM sleep EEG theta activity values (6–9 Hz) and time spent in the respective states.
Experiments
ICSS-1 Versus GH-SDEP (Experiment 1)
Rats (n = 7) were acclimated to the recording chambers and the recording cable tether at least 2 days prior to baseline recording. At least 24 h after the last 30-min ICSS training session, baseline sleep-wake EEG activity was recorded for 24 h from ZT 6–6. Beginning at light onset the next day, 18 h after the baseline recording, rats were allowed to self-stimulate for 6 h (ICSS-1; ZT 0–6) in the operant chambers, or were subjected to 6 h GH-SDEP (ZT 0–6) in the recording chambers. The EEG was recorded throughout the 6-h SDEP sessions and for 24 h thereafter (recovery; ZT 6–6). At least 1 w later a new baseline was recorded and the experiment was repeated in a counterbalanced design; rats that previously received ICSS were subjected to GH-SDEP and vice versa. Rats randomly selected for GH-SDEP during the first experiment underwent initial lever press training prior to GH-SDEP, but input/output curve generation and additional training sessions occurred after GH-SDEP. During ICSS-1, rats lever pressed an average of 36.0 (± 6.1 standard error of the mean) times per min, which resulted in 25.8 (± 4.0) stimulations/min.
ICSS-2 Versus NCS (Experiment 2)
A separate cohort of rats (n = 5) were instrumented, trained, and acclimated as previously described. Following a 24-h baseline recording (ZT 6–6), rats were allowed to self-stimulate for 6 h (ICSS-2; ZT 0–6) in the operant chambers. EEG activity was recorded during the 6-h SDEP period and for 24 h thereafter (recovery; ZT 6–6) in the operant chambers. Six days later, baseline was recorded after which rats underwent 6-h NCS (ZT 0–6), which consisted of replaying the individual stimulation patterns from their ICSS-2 session in the absence of the lever. As before, EEG activity was recorded during the 6 h SDEP and for 24 h pre- and post-NCS (baseline and recovery (ZT 6–6), respectively). During ICSS-2, rats lever pressed an average of 29.1 (± 6.8) times per min, which resulted in 22.4 (± 4.2) stimulations/min during both ICSS-2 and NCS.
Histology
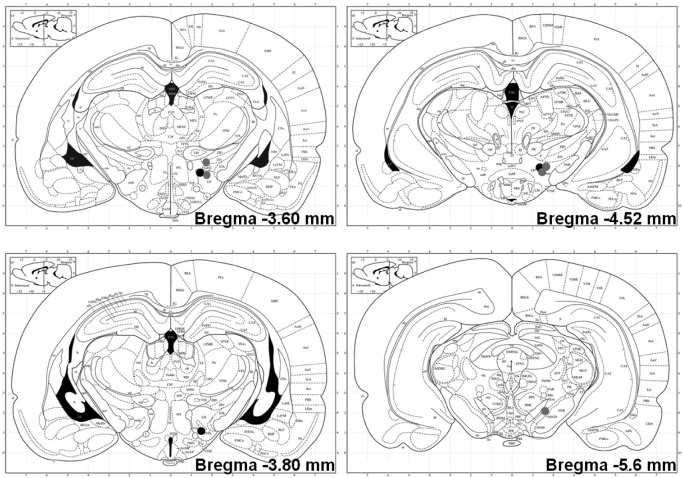
At the end of the experiment, rats were euthanized and their brains were removed, blocked, fixed with 10% formalin, and subsequently submerged in 20% sucrose. Fixed brains were sliced (50 μm), mounted, and stained with cresyl violet. Electrode placement was confirmed using the Rat Brain Atlas by Paxinos & Watson (2004; Figure 1). Precise visualization of the electrode tips in three rats (ICSS-1) could not be determined, but the rats demonstrated behavioral homogeneity with their cohorts and thus their data were included in the analyses.
Figure 1.
Electrode placements for intracranial self-stimulation-1 (ICSS-1; black circles) and ICSS-2/noncontingent stimulation (NCS; gray circles) at A/P −3.60, −3.80, −4.52 and −5.6 (Paxinos & Watson, 2004). Electrode placement for three rats (ICSS-1) could not be determined due to histological error.
Data Analysis
Time in wakefulness during ZT 0–6 was compared between baseline and the various SDEP methods using mixed-effects analyses of variance (ANOVAs) with a fixed effect for condition (baseline versus SDEP). Difference scores (SDEP minus baseline) were used to compare time in wakefulness between methods, using mixed-effects ANOVAs with a fixed effect for method (ICSS-1 versus GH-SDEP, ICSS-2 versus NCS). EEG spectral content during the SDEP was not analyzed due to stimulus artifacts. Raw or normalized data for NREM sleep episode duration, episode frequency, and NREM sleep EEG SWA during the 6-h SDEP periods (ZT 0–6), as well as for time spent in NREM sleep and REM sleep, and NREM sleep EEG SWA during baseline (ZT 6–6), were compared between methods using mixed effects ANOVAs with a fixed-effect for method.
Mixed-effects ANOVAs, using raw, baseline normalized and difference (recovery minus baseline) data, with fixed effects for condition (baseline versus recovery), time (24 h in 6- or 2-h bins) and SDEP method were used to determine the effects of 6-h SDEP on time spent in NREM sleep and REM sleep, NREM sleep and REM sleep episode duration and episode frequency, NREM sleep EEG SWA (1–4 Hz), and REM sleep EEG theta activity (6–9 Hz). NREM sleep and REM sleep EEG power spectra were analyzed using mixed-effects ANOVAs with fixed effects for condition and frequency (1–20 Hz).
Last, data from nine rats that had ICSS as a first condition, four from experiment 1 and five from experiment 2 were used to further examine sleep rebound dynamics, thus, the times in NREM sleep or REM sleep lost during the 6 h of SDEP as compared to the times regained over the 24 h after SDEP. Delta and theta energies were expressed as the time in state differences multiplied by the baseline subtracted absolute NREM sleep EEG delta or REM sleep EEG theta power (μV2). The effect of ICSS SDEP on NREM sleep EEG delta energy and REM sleep EEG theta energy were determined by comparing the energies gained (24-h recovery minus 24-h baseline values) and the energies lost (baseline values ZT 0–6 minus SDEP values). Cumulative values for the SDEP and recovery periods were analyzed using a repeated-measures t-test.
All mixed-effects ANOVAs had a random effect on the intercept and t-tests were conducted when appropriate. Baseline recordings from nine rats had no REM sleep during at least one 2-h or 6-h time bin used for analyses; these bins were excluded from REM sleep episode duration (ICSS-1: 1 bin), REM sleep EEG theta activity (ICSS-1: 5 bins; GH-SDEP: 7 bins; ICSS-2: 3 bins; NCS: 3 bins) and REM sleep spectral power (ICSS-1: 1 bin; GH-SDEP: 3 bins; ICSS-2: 1 bin; NCS: 1 bin) analyses.
RESULTS
ICSS-1 Versus GH-SDEP
SDEP
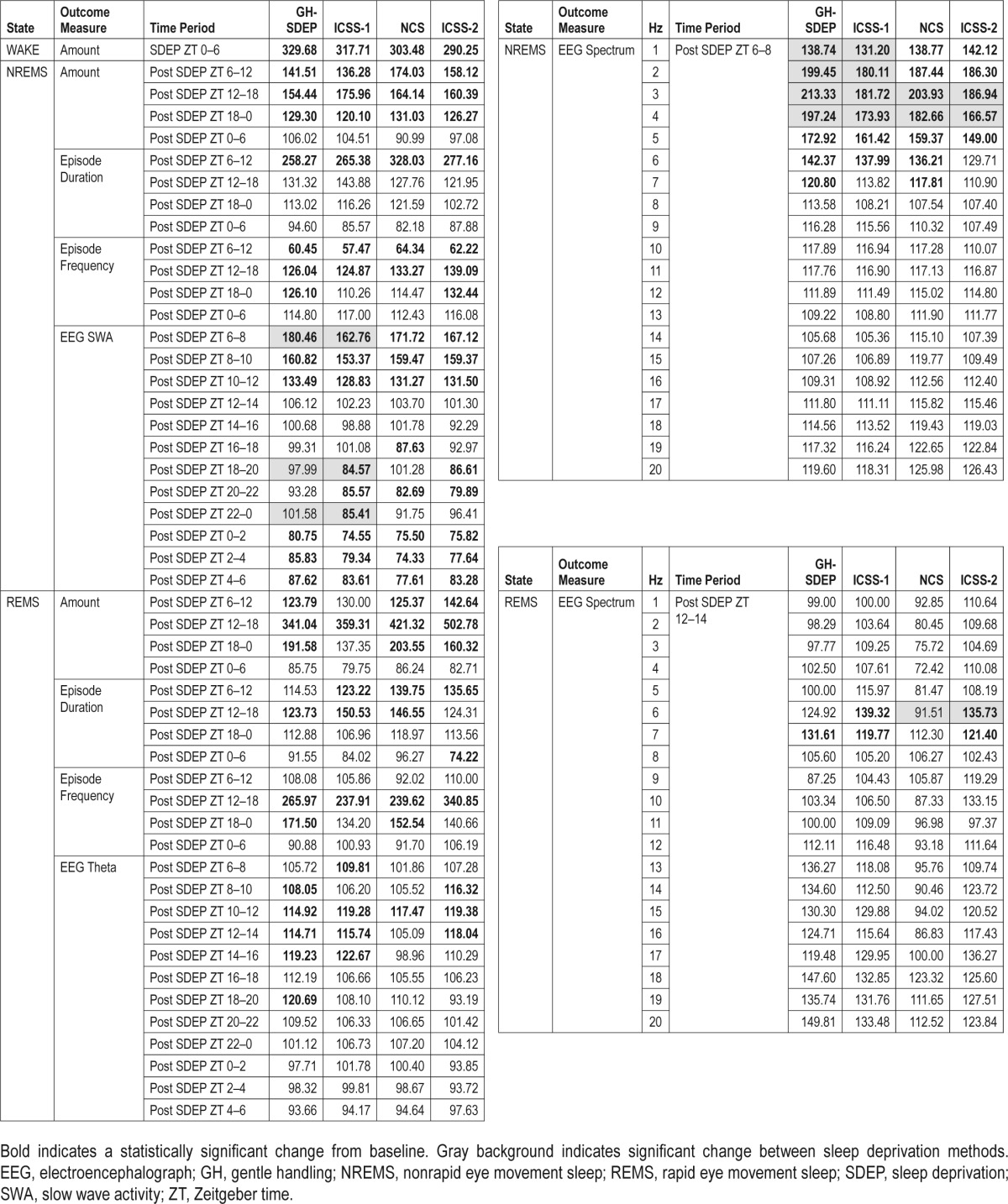
Rats were awake 97.8% of the time between ZT 0–6 of GHSDEP (condition: F1,6 = 684.5, P < 0.01) and at 98.7% of the time during ICSS-1 (condition: F1,6 = 586.9, P < 0.01; Table 1). Comparing ICSS-1 versus GH-SDEP, no statistically signifi-cant differences between methods were detected in time awake, time in NREM sleep, or NREM sleep episode duration or frequency during the SDEP periods. Moreover, no REM sleep occurred during either 6-h SDEP period. Together these data suggest that the effectiveness of ICSS parallels that of GHSDEP. Time in NREM sleep and REM sleep, and NREM sleep EEG SWA did not differ between the separate GH-SDEP and ICSS-1 baseline recordings and exhibited normal rat sleep characteristics when left undisturbed, including NREM sleep EEG SWA peaking during the first 2 h of the light period.27
Table 1.
Percent change from baseline for each sleep deprivation method.
Post-SDEP NREM Sleep
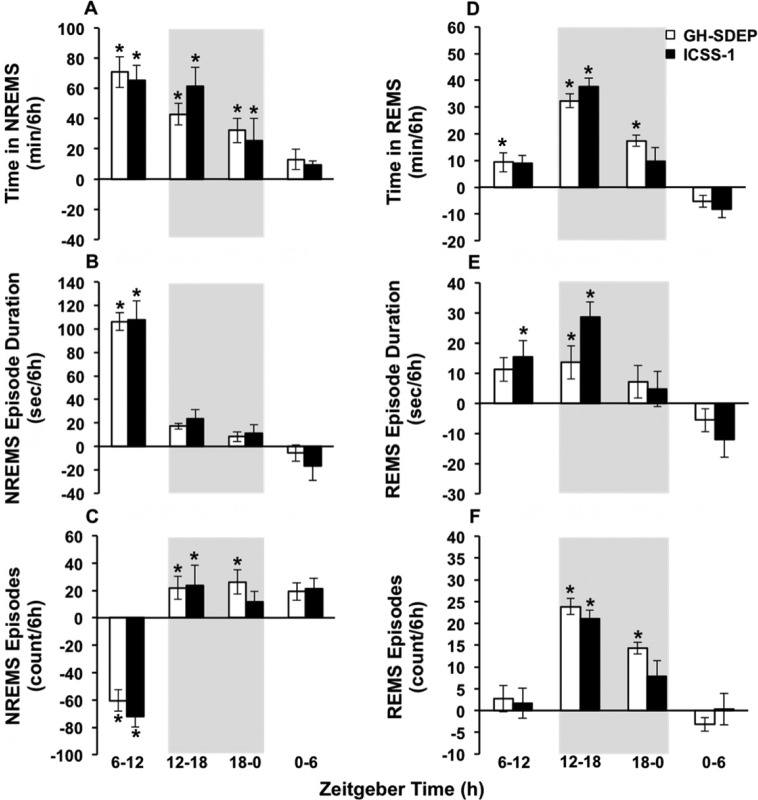
After GH-SDEP, time in NREM sleep (Figure 2A) increased from baseline during ZT 6–12, 12–18 and 18–0 (condition × time: F3,42 = 9.2, P < 0.01). Similarly, after ICSS-1 time in NREM sleep increased from baseline during ZT 6–12, 12–18 and 18–0 (condition × time: F3,42 = 6.1, P < 0.01). After GH-SDEP, NREM sleep episode duration (Figure 2B) increased from baseline during ZT 6–12 and 12–18 (condition × time: F3,42 = 45.4, P < 0.01). After ICSS-1, NREM sleep episode duration increased from baseline during ZT 6–12 (condition × time: F3,42 = 18.3, P < 0.01). After GH-SDEP, NREM sleep episode frequency (Figure 2C) decreased from baseline during ZT 6–12, and increased during ZT 12–18 and 18–0 (condition × time: F3,42 = 16.3, P < 0.01). After ICSS-1, NREM sleep episode frequency decreased from baseline during ZT 6–12. The post-SDEP increased time in NREM sleep were not different between GH-SDEP and ICSS-1. Moreover, the differences in NREM sleep episode duration and NREM sleep episode frequency rebounds between GH-SDEP and ICSS-1 were not significant. These results indicate that the patterns of NREM sleep rebound were similar after both methods of SDEP.
Figure 2.
During recovery intracranial self-stimulation (ICSS) has similar nonrapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS) phenotypes compared to gentle handling-induced sleep deprivation (GH-SDEP). Means and standard errors of (A) time in NREMS, (B) NREMS episode duration, (C) NREMS episode frequency, (D) time in REMS, (E) REMS episode duration and (F) REMS episode frequency after GH-SDEP (white bars) and ICSS-1 (black bars), shown as difference from baseline. Gray background indicates lights off. Baseline versus recovery: *P < 0.05.
Post-SDEP REM Sleep
After GH-SDEP, time in REM sleep (Figure 2D) increased during ZT 6–12, 12–18, and 18–0 (condition × time: F3,42 = 12.9, P < 0.01). After ICSS-1, time in REM sleep increased from baseline during ZT 6–12 (condition × time: F3,42 = 10.5, P < 0.01). After GH-SDEP, REM sleep episode duration (Figure 2E) increased from baseline during ZT 12–18 (condition: F1,42 = 4.5, P < 0.05). After ICSS-1, REM sleep episode duration increased from baseline during ZT 6–12 and 12–18 (condition × time: F 3,40 = 5.9, P < 0.01). After GH-SDEP, REM sleep episode frequency (Figure 2F) increased from baseline during ZT 12–18 and 18–0 (condition × time: F3,42 = 10.7, P < 0.01). After ICSS-1, REM sleep episode frequency increased from baseline during ZT 12–18 (condition × time: F3,42 = 4.2, P < 0.05). The differences in time in REM sleep, REM sleep episode duration, and REM sleep episode frequency between GH-SDEP and ICSS-1 were not significant, indicating that the REM sleep rebound was similar regardless of SDEP technique.
Post-SDEP NREM Sleep SWA
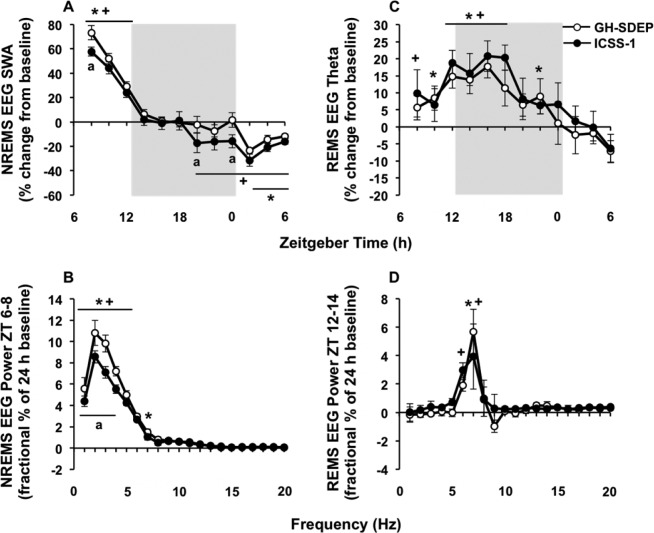
The effects of GH-SDEP on EEG SWA during NREM sleep are biphasic28–30; the greatest increases in EEG SWA occur during the initial 2 h after SDEP, after which it progressively declines with NREM sleep, and a delayed negative rebound occurs approximately 12 h post-SDEP and can last up to 24 h (Figure 3A). We therefore focus our results on these time blocks, ZT 6–8, ZT 18–0, and ZT 0–6 (18 h post-SDEP), analyzed in 2-h bins. After both GH-SDEP and ICSS-1, there were significant condition × time interactions for NREM sleep EEG SWA (Figure 3A; F 11,138 = 40.6, P < 0.01; F11,138 = 29.2, P < 0.01, respectively). After GH-SDEP, NREM sleep EEG SWA increased from baseline during ZT 6–8, did not change during ZT 18–0, and then decreased during ZT 0–6 (18 h post-SDEP); this effect was signifi-cant for each 2-h time bin between ZT 0–6. After ICSS-1, NREM sleep EEG SWA increased from baseline during ZT 6–8, and decreased during ZT 18–0 and ZT 0–6 (18 h post-SDEP); this effect was significant for each 2-h time bin between ZT 18–6. After both GH-SDEP and ICSS-1, NREM sleep spectral power increased from baseline during ZT 6–8 (Figure 3B; condition × frequency: F19,234 = 28.3, P < 0.01; F19,234 = 16.4, P < 0.01, respectively), including in the 1–4 Hz range. The NREM sleep EEG SWA rebound was significantly different between GH-SDEP and ICSS-1. Specifically, the positive NREM sleep EEG SWA rebound during ZT 6–8 was higher after GH-SDEP compared to ICSS-1, and the negative NREM sleep EEG SWA rebound during ZT 18–0 and ZT 0–6 was less negative after GH-SDEP compared to ICSS-1 (method: F1,138 = 17.5, P < 0.01); pairwise comparisons show that this effect was significant for ZT 18–20 and ZT 22–0. NREM sleep EEG SWA during ZT 8–12 did not differ between methods. The increase from baseline in NREM sleep spectral power during ZT 6–8 was higher after GH-SDEP compared to ICSS-1 in the 1–4 Hz range (method × frequency: F 19,234 = 3.3, P < 0.01).
Figure 3.
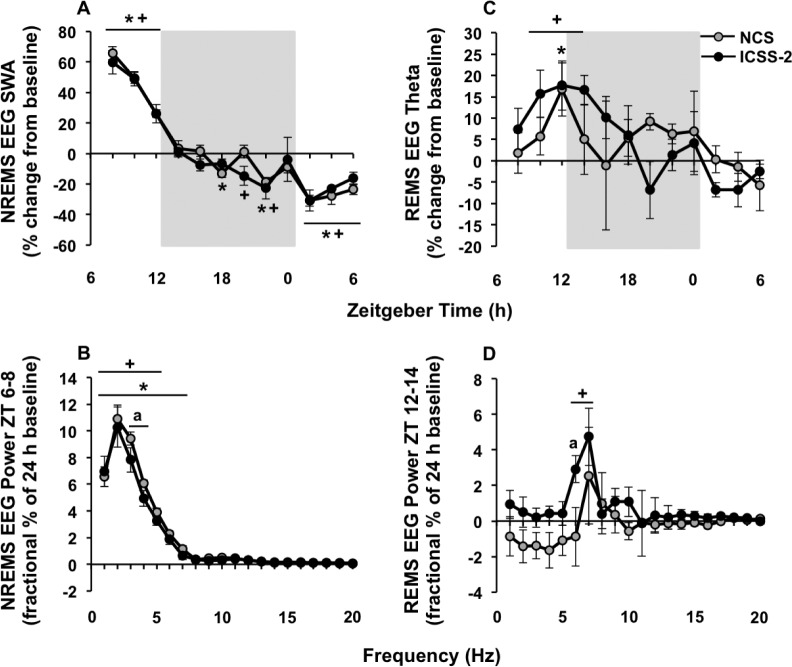
Gentle handling-induced sleep deprivation (GH-SDEP) leads to elevated electroencephalography (EEG) spectral content compared to intracranial self-stimulation (ICSS) during recovery. Means and standard errors of (A) NREMS EEG SWA (1–4 Hz), (B) NREMS EEG spectral power (1–20 Hz) during ZT 6–8, (C) REMS EEG theta activity (6–9 Hz) and (D) REMS EEG spectral power (1–20 Hz) during ZT 12–14 after GH-SDEP (white circles) and ICSS-1 (black circles). Gray background indicates lights off. GH-SDEP, baseline versus recovery: *P < 0.05. ICSS-1, baseline versus recovery: +P < 0.05. ICSS-1 versus GH-SDEP: aP < 0.05. NREMS, nonrapid eye movement sleep; REMS, rapid eye movement sleep; SWA, slow wave activity; ZT, zeitgeber time.
Post-SDEP REM Sleep Theta
The highest REM sleep rebound occurred during ZT 12–18 (Figure 2D), specifically during the first 2 h (ZT 12–14; data not shown). The greatest increase in REM sleep EEG theta activity occurs during the first maximum REM sleep period after SDEP31 and therefore we focus our results regarding REM sleep EEG theta activity (6–9 Hz) and REM sleep spectral power (1–20 Hz) during these time blocks, ZT 12–18 and ZT 12–14, respectively. After both GH-SDEP and ICSS-1, there were significant condition × time interactions for REM sleep EEG theta activity (Figure 3C; F11,124 = 3.5, P < 0.01; F11,128 = 3.0, P < 0.01, respectively); REM sleep EEG theta activity increased from baseline during ZT 12–18, which was significant for each 2-h time bin between ZT 12–18 for both methods. In addition, after both GHSDEP and ICSS-1, REM sleep spectral power increased from baseline during ZT 12–14 for 6 Hz and 6–7 Hz, respectively (Figure 3D; condition × frequency: F19,117 = 1.7, P < 0.05; condition: F1,195 = 7.1, P < 0.01, respectively). The rebounds in REM sleep EEG theta activity or REM sleep spectral power were not significantly different between GH-SDEP and ICSS-1.
ICSS-2 Versus NCS
SDEP
Rats were awake for 99.2% of the time between ZT 0–6 during NCS (condition: F1,4 = 1885.5, P < 0.01) and 98.4% of the time during ICSS-2 (condition: F1,4 = 462.1, P < 0.01). Comparing NCS versus ICSS-2, no statistically significant differences between methods were detected in time awake, time in NREM sleep, and NREM sleep episode duration or frequency during the SDEP period. Moreover, no REM sleep occurred during the 6-h SDEP periods. Together these data suggest coherence of NCS and ICSS in their efficacy of maintaining wakefulness. During the separate 24-h baseline recordings time in NREM sleep, REM sleep, or NREM sleep EEG SWA did not differ between NCS and ICSS-2 groups.
Post-SDEP NREM Sleep
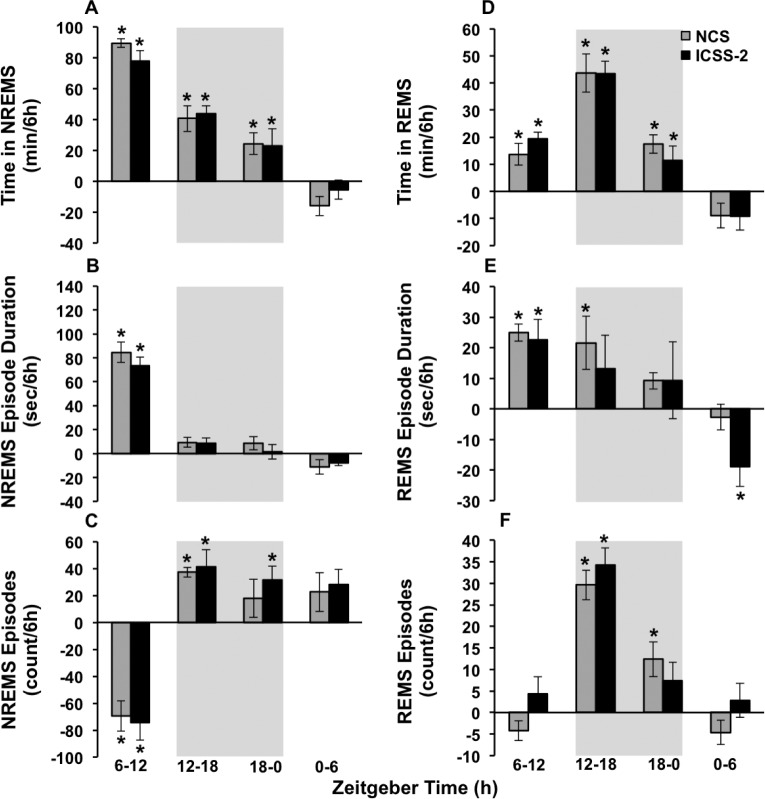
After NCS, time in NREM sleep (Figure 4A) increased from baseline during ZT 6–12, 12–18, and 18–0 (condition × time: F3,28 = 16.8, P < 0.01). Similarly, after ICSS-2, time in NREM sleep increased from baseline during ZT 6–12, 12– 18, and 18–0 (condition × time: F3,28 = 15.0, P < 0.01). After NCS and ICSS-2, NREM sleep episode duration (Figure 4B) increased from baseline ZT 6–12 (condition × time: F 3,28 = 38.4, P < 0.01; condition × time: F3,28 = 49.4, P < 0.01, respectively). After NCS, NREM sleep episode frequency (Figure 4C) decreased from baseline during ZT 6–12, and increased from baseline during ZT 12–18 (condition × time: F3,28 = 10.1, P < 0.01), whereas after ICSS-2, NREM sleep episode frequency decreased from baseline during ZT 6–12, and increased from baseline during ZT 12–18 and 18–0 (condition × time: F3,28 = 13.0, P < 0.01). The post-SDEP changes in time in NREM sleep, NREM sleep episode frequency, and NREM sleep episode duration were not different between NCS and ICSS-2. These results indicate that the NREM sleep rebound is similar between the NCS and ICSS methods.
Figure 4.
ICSS and NCS manifest similar sleep phenotypes during recovery. Means and standard errors of (A) time in NREMS, (B) NREMS episode duration, (C) NREMS episode frequency, (D) time in REMS, (E) REMS episode duration and (F) REMS episode frequency after NCS (gray bars) and ICSS-2 (white bars), shown as difference from baseline. Gray background indicates lights off. Baseline versus recovery: *P < 0.05. ICSS, intracranial self-stimulation; NCS, noncontingent stimulation; NREMS, nonrapid eye movement sleep; REMS, rapid eye movement sleep.
Post-SDEP REM Sleep
Following NCS and ICSS-2, time in REM sleep (Figure 4D) increased from baseline during ZT 6–12, 12–18, and 18–0 (condition × time: F3,28 = 12.3, P < 0.01; condition × time: F3,28 = 16.2, P < 0.01, respectively). After NCS, REM sleep episode duration (Figure 4E) increased from baseline during ZT 6–12 and 12–18 (condition × time: F3,28 = 3.5, P < 0.05), whereas after ICSS-2 increases from baseline were only significant during ZT 6–12 (condition × time: F3,28 = 4.1, P < 0.05). After NCS, REM sleep episode frequency (Figure 4F) increased during ZT 12–18 and 18–0 (condition × time: F3,28 = 9.9, P < 0.01). After ICSS-2, REM sleep episodes frequency increased from baseline during ZT 12–18 (condition × time: F3,28 = 8.1, P < 0.01). The increases in post-SDEP time in REM sleep were not different between NCS and ICSS-2. Likewise, the differences in REM sleep episode duration and REM sleep episode frequency rebounds between NCS and ICSS were not significant, indicating that the REM sleep rebound is similar after NCS and ICSS.
Post-SDEP NREM Sleep SWA
Similar to Experiment 1, we focus our NREM sleep EEG SWA (1–4 Hz) results on ZT 6–8, during which the largest increases occurred (Figure 5A), and on ZT 18–0 and ZT 0–6 (18 h post-SDEP; analyzed in 2-h bins), during which the negative rebound typically manifests. After both NCS and ICSS-2, significant interactions were detected for NREM sleep EEG SWA (condition × time: F3,28 = 53.8, P < 0.01; F 3,28 = 43.4, P < 0.01, respectively). After NCS, NREM sleep EEG SWA increased from baseline during ZT 6–8, and decreased during ZT 18–0 and ZT 0–6 (18 h post-SDEP); this effect was significant for each 2-h time bin between ZT 18–6, except for ZT 18–20 and ZT 22–0. Similarly, after ICSS-2, NREM sleep EEG SWA increased from baseline during ZT 6–8, and decreased during ZT 18–0 and ZT 0–6 (18 h post-SDEP); this effect was significant for each 2-h time bin between ZT 18–6, except for ZT 22–0. After both NCS and ICSS-2, NREM sleep spectral power increased from baseline during ZT 6–8 (Figure 5B; condition × frequency: F19,156 = 79.1, P < 0.01; F19,156 = 29.6, P < 0.01, respectively), including in the 1–4 Hz range. The NREM sleep EEG SWA rebound was not significantly different between NCS and ICSS-2 for either the positive rebound or the negative rebound. However, the increase in NREM sleep spectral power from baseline during ZT 6–8 was higher after NCS compared to ICSS-2 in the 3–4 Hz range (method: F1,156 = 4.1, P < 0.05).
Figure 5.
NCS EEG spectral content is higher than ICSS in NREMS but not in REMS. Means and standard errors of (A) NREMS EEG SWA (1–4 Hz), (B) NREMS EEG spectral power (1–20 Hz) during ZT 6–8, (C) REMS EEG theta activity (6–9 Hz), and (D) REMS EEG spectral power (1–20 Hz) during ZT 12–14 after NCS (gray circles) and ICSS-2 (black circles), shown as difference from baseline. Gray background indicates lights off. NCS baseline versus recovery: *P < 0.05. ICSS-2 baseline versus recovery: +P < 0.05. ICSS-2 versus NCS: aP < 0.05. ICSS, intracranial self-stimulation; NCS, noncontingent stimulation; NREMS, nonrapid eye movement sleep; REMS, rapid eye movement sleep; SWA, slow wave activity; ZT, Zeitgeber time.
Post-SDEP REM Sleep Theta
The highest REM sleep rebound occurred during ZT 12–18 (Figure 4D), especially during the initial 2 h (ZT 12–14; data not shown), similar to Experiment 1. Therefore, we focus our results regarding REM sleep EEG theta activity (6–9 Hz; Figure 5C) and REM sleep spectral power (1–20 Hz; Figure 5D) on these time blocks, ZT 12–18 and ZT 12–14, respectively. After NCS, REM sleep EEG theta activity did not change from baseline during ZT 12–18, but increased after ICSS-2 (condition × time: F11,86 = 3.5, P < 0.01); this effect was significant for ZT 12–14. Similarly, after NCS, REM sleep spectral power did not change from baseline during ZT 12–14, but increased after ICSS-2 in the 6–7 Hz range (condition: F1,117 = 10.7, P < 0.01). The difference in REM sleep EEG theta activity rebound between NCS and ICSS-2 was not significant. However, the difference in the increase in REM sleep spectral power from baseline was significant between methods (method: F1,116 = 11.1, P < 0.01), with ICSS-2 being higher than NCS, specifically at 6 Hz.
NREM Sleep and REM Sleep Energies
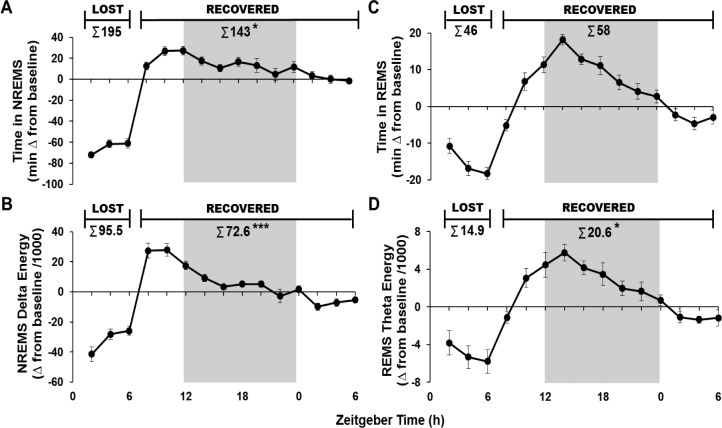
During the 24-h post-SDEP period, the amount of NREM sleep recovered from the nine rats that received ICSS prior to GHSDEP or NCS was 73% (Figure 6A; t-test, P < 0.05), and only 76% of NREM sleep delta energy was recovered (Figure 6B; t-test, P < 0.001). In contrast, about 127% of the time in REM sleep was recovered after SDEP, but this increase was not statistically significant (Figure 6C). In the 24-h post-SDEP period, 138% of REM sleep theta energy was recovered (Figure 6D; t-test, P < 0.05).
Figure 6.
Data from the nine rats that received ICSS as their first condition (n = 4 from Experiment 1 and n = 5 from Experiment 2) Means and standard errors of (A) time in NREMS expressed as minutes change from baseline, (B) NREMS delta energy expressed as a product of absolute NREMS delta power value (μV2) change from baseline and time in NREMS values divided by 1,000. Time in REMS (C) and REMS theta energies (D) were calculated as in (A) and (B) but using REMS and REMS theta (6–9 Hz). Inset values use cumulative values for the 6 h SDEP periods (amount lost) and the 24h recovery period (amount recovered). Gray background indicates lights off. *P < 0.05 ***P < 0.001 for lost versus recovered. ICSS, intracranial self-stimulation; NREMS, nonrapid eye movement sleep; REMS, rapid eye movement sleep; SDEP, sleep deprivation.
DISCUSSION
A primary finding reported herein is that lateral hypothalamic (LH)-ICSS during the first 6 h of the light phase induced wakefulness followed by sleep rebounds. Similarly, in a pilot study,32 sleep rebounds after 6-h LH-ICSS during the last 6 h of the light phase showed increased time in NREM sleep and REM sleep, and NREM sleep EEG SWA compared to baseline. These findings demonstrate that LH-ICSS is effective for inducing voluntary SDEP. Heretofore, it had not been possible to experimentally isolate the controllability aspect of animal SDEP. As such, ICSS-SDEP more closely reflects human SDEP studies, which are predominantly based on voluntary participation. Although some instances of human sleep loss are arguably forced and aversive, many real-world conditions of sleep loss not induced by medical, psychiatric or psychological conditions involve self-selected and reinforced behaviors, e.g., social activities, studying, gaming, work, etc. ICSS-SDEP more fully emulates the latter situations compared to existing animal models of SDEP.
NCS was also capable of inducing wakefulness during the 6-h SDEP period. In fact, every NCS stimulation that occurred during a NREM sleep epoch induced wake in the subsequent epoch. The NCS session was also followed by NREM sleep, REM sleep, and NREM sleep EEG SWA rebounds. Similar to our findings, noncontingent optogenetic activation of mouse LH-hypocretin cells disrupted sleep followed by a subsequent sleep rebound.33 Although that study reported unchanged corticosterone levels with disrupted sleep, in a previous rat study corticosterone concentrations increased compared to baseline controls after short-session ICSS and NCS LH stimulations.34 Regardless, NCS may be used to induce enforced sleep loss and directly examine the effect of controllability on sleep through comparison with ICSS-SDEP.
With minor exceptions, the NREM sleep and REM sleep vigilance states, episode duration, and frequencies rebounds were similar after ICSS-SDEP, GH-SDEP, and NCS-SDEP, suggesting that SDEP methods do not differentially affect time in state. However, after GH-SDEP the increase in NREM sleep EEG SWA was higher compared to ICSS-SDEP. Likewise, after NCS-SDEP, the rebound in the upper NREM sleep EEG SWA range (3–4 Hz) increased compared to ICSS-SDEP. NREM sleep EEG SWA is traditionally regarded as sleep intensity because SWA amplitude increases following SDEP during NREM sleep.35 Local NREM sleep EEG SWA is use-dependent.36 For example, NREM sleep EEG SWA is enhanced in the contralateral somatosensory cortex after palm vibration prior to sleep; conversely, NREM sleep EEG SWA decreases during NREM sleep following arm immobilization.37,38 Finally, NREM sleep EEG SWA is likely regulated independently from NREM sleep duration.39,40
Current animal SDEP methods typically involve uncontrollable and aversive stimuli, and thus it is likely that the SDEP procedures constitute a stressor that may affect sleep loss-induced sleep phenotypes during the rebound phase.1,2 Stressors alone, e.g., immobilization and social conflict, increase NREM sleep EEG SWA for several hours after stressor termination,3,6,7,41–43 and this increase can persist for several days.5 Moreover, some stressors, e.g., social conflict, in combination with GH-SDEP causes a larger increase in post-SDEP NREM sleep EEG SWA than GH-SDEP alone.4 Therefore, it is plausible that the increased NREM sleep EEG SWA rebound following these methods reflects a nonspecific stress effect, in that the increase in the NCS condition is due to the forced nature of the SDEP method, whereas the increase in GH-SDEP reflects the combination of the forced and aversive components of the SDEP method.
The difference in NREM sleep EEG SWA rebounds could reflect a decrease due to nonspecific effects associated with ICSS, such as the significant elevation in dopamine levels due to stimulation of the mesolimbic dopamine system. However, dopamine is a well-conserved brain arousal mechanism44 and sleep loss increases dopamine and dopamine receptors expressions in the brain.45–48 Further, chronic or acute administration of amphetamines, which increases dopamine levels similarly to ICSS, is followed by a sleep rebound, after the initial wake-promoting effects, with49 or without50–52 an increase in NREM sleep EEG SWA. Administration of L-dopa or dopamine D2 receptor agonists induces a similar effect, showing a delayed increase in nonstate-specific EEG delta power.53 Last, the stimulation parameters during ICSS-SDEP and NCS-SDEP were identical, yet NCS-SDEP resulted in a higher subsequent NREM sleep EEG SWA rebound compared to ICSS-SDEP, and in the same direction but with a lesser magnitude than GH-SDEP. Other sleep-related neuromodulators could affect EEG signals during ICSS-SDEP and constitute good targets for follow up studies. For example, orexinergic neurons synapse on ventral tegmental area dopaminergic cells and mediate reward salience, accordingly, application of orexin A or blocking orexin receptors alters ICSS response patterns.54–56 There are intriguing interactions between these receptor systems as melanin-concentrating hormone suppresses LH-orexinergic signaling.57
It is possible that increased physical activity associated with the rigorous lever pressing behavior could result in attenuated NREM sleep EEG SWA specific to ICSS-SDEP. However, GHSDEP also causes increased motor activity relative to baseline, perhaps not to the extent of that observed during ICSS-SDEP. Moreover, physical activity during SDEP, such as that during forced locomotion SDEP methods, increases NREM sleep EEG SWA.58 The pattern of stimulations may also differ between ICSS-SDEP and GH-SDEP. Extended ICSS sessions show a slow decline in lever press behavior, and therefore stimulations as the session progresses, whereas with GH-SDEP the number of experimenter initiated paintbrush encounters increases across the session as sleep propensity builds. Although it is difficult to directly compare these two stimulus types due to their qualitative differences, the overall number of stimuli is an order of magnitude higher in ICSS-SDEP when GH-SDEP stimulations peak and thus do not explain the changes in NREM sleep EEG SWA.
Another probable interpretation of the attenuated NREM sleep EEG SWA may stem from the training procedure for ICSS constituting a chronic stressor due to multiple sessions. A chronic mild stress paradigm, for instance, which involves chronic exposure (2–4 w) to multiple mild, unpredictable stressors, has been associated with a decrease in deep sleep, which is characterized by high EEG SWA.59,60 However, comparing pre-ICSS-SDEP baseline sleep to pre-GH-SDEP baseline sleep did not reveal an effect of the daily ICSS training sessions. In addition, it is unlikely any purported ICSS related stress effects confounded pre-GH-SDEP baseline recordings. Rats were left undisturbed for at least 1 w between ICSS-SDEP and GH-SDEP, allowing them to recover from the possibility of any ICSS-induced stress effects. Moreover, a subset of rats underwent GH-SDEP prior to ICSS-SDEP and received only minimal ICSS training (1–2 daily sessions) several days prior to pre-GH-SDEP baseline recordings. Taken together, an inhibitory effect of ICSS training on NREM sleep EEG SWA does not appear to provide a plausible alternative explanation for the current findings. Nevertheless, the current results should be interpreted with care as ICSS-SDEP involves stimuli not previously studied in relation to NREM sleep EEG SWA.
EEG theta activity in wake correlates with NREM sleep EEG SWA,61 whereas REM sleep EEG theta activity is linked to learning and memory consolidation.62–64 In addition to NREM sleep EEG SWA, the rebound in the lower REM sleep EEG theta range (6–7 Hz) differed between methods; it increased after ICSS-SDEP, but not after NCS-SDEP. Various stressors are associated with a decrease in REM sleep EEG theta activity43 and thus the difference in rebound could reflect a stress effect caused by the lack of control during NCS-SDEP. Alternatively, in contrast to NCS, ICSS is associated with increased body temperature, which may in part be caused, by the increased physical activity.65 An increase in temperature during SDEP is associated with an increase in REM sleep and REM sleep theta power.66 Thus, it is plausible that the difference in REM sleep EEG theta power is caused by the increased physical activity associated with ICSS-SDEP.
Finally, the onset of recovery for NREM sleep outcomes were rapid, peaking during the light period 6 h immediately after ICSS-SDEP ended, compared to REM sleep outcomes which peaked during the first few hours of the dark period. Notably, the NREM sleep amounts and NREM sleep delta energies lost during SDEP are not fully recovered. In contrast, full recovery plus an overshoot occurs for REM sleep amounts and REM sleep theta energies post-SDEP. These effects are consistent with prior reports using other methods of SDEP.28,40,67–69
In conclusion, the current study demonstrated that ICSSSDEP is an effective method for inducing voluntary SDEP. The difference between the SDEP conditions in NREM sleep EEG SWA indicate that the aversive and uncontrollable nature of SDEP methods differentially affects sleep phenotypes, and thus ICSS provides a distinct advantage in the capacity to parse these important psychological factors at an electrophysiological level. The electrophysiological differences between SDEP methods may also elicit unique neuromolecular profiles that could advance our understanding of how the brain reacts to sleep loss.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was funded by NIH grants NS085605 to Dr. Davis, NS025378 to Dr. Krueger, and an equipment grant N66001-09-1-2117 to Jonathan Wisor. Additional support provided by WSU-Spokane. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Drs. David Rector, Jaak Panksepp, and Éva Szentirmai for their contributions to this work, as well as Drs. Jonathan Wisor and Hans Van Dongen. We thank William Clegern for technical help and Theresa Nguyen for assistance in data collection.
REFERENCES
- 1.Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced change in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanford LD, Suchecki D, Meerlo P. Stress, arousal, and sleep. Curr Top Behav Neurosci. 2015;25:379–410. doi: 10.1007/7854_2014_314. [DOI] [PubMed] [Google Scholar]
- 3.Meerlo P, Pragt BJ, Daan S. Social stress induces high intensity sleep in rats. Neurosci Lett. 1997;225:41–4. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- 4.Meerlo P, De Bruin EA, Strijkstra AM, Serge D. A social conflict increases EEG slow-wave activity during subsequent sleep. Physiol Behav. 2001;73:331–5. doi: 10.1016/s0031-9384(01)00451-6. [DOI] [PubMed] [Google Scholar]
- 5.Kinn AM, Grønli J, Fiske E, et al. A double exposure to social defeat induces sub-chronic effects on sleep and open field behavior in rats. Physiol Behav. 2008;95:553–61. doi: 10.1016/j.physbeh.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Kamphuis J, Lancel M, Koolhaas JM, Meerlo P. Deep sleep after social stress: NREM sleep slow-wave activity is enhanced in both winners and losers of a conflict. Brain Behav Immun. 2015;49:149–54. doi: 10.1016/j.bbi.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Chang FC, Opp MR. Role of corticotropin-releasing hormone in stressor-induced alterations of sleep in rat. Am J Physiol Regul Integr Comp Physiol. 2002;283:R400–7. doi: 10.1152/ajpregu.00758.2001. [DOI] [PubMed] [Google Scholar]
- 8.Bodosi B, Obál J, Gardi J, Komlodi J, Fang J, Krueger JM. An ether stressor increases REM sleep in rats: possible role of prolactin. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1590–8. doi: 10.1152/ajpregu.2000.279.5.R1590. [DOI] [PubMed] [Google Scholar]
- 9.Kant GJ, Pastel RH, Bauman RA, et al. Effects of chronic stress on sleep in rats. Physiol Behav. 1995;57:359–65. doi: 10.1016/0031-9384(94)00241-v. [DOI] [PubMed] [Google Scholar]
- 10.Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable foot shock stress in sleep in mice. Sleep. 2010;33:621–30. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 12.Olds J. Hypothalamic substrates of reward. Physiol Rev. 1962;42:554–604. doi: 10.1152/physrev.1962.42.4.554. [DOI] [PubMed] [Google Scholar]
- 13.Routtenberg A, Lindy J. Effects of the availability of rewarding septal and hypothalamic stimulation on bar pressing for food under conditions of deprivation. J Comp Physiol Psychol. 1965;60:158–61. doi: 10.1037/h0022365. [DOI] [PubMed] [Google Scholar]
- 14.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–24. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 15.Valenstein ES, Beer B. Continuous opportunity for reinforcing brain stimulation. J Exp Anal Behav. 1964;7:183–4. doi: 10.1901/jeab.1964.7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terman M, Terman JS. Circadian rhythm of brain self-stimulation behavior. Science. 1970;168:1242–4. doi: 10.1126/science.168.3936.1242. [DOI] [PubMed] [Google Scholar]
- 17.Annau Z, Heffner R, Koob GF. Electrical self-stimulation of single and multiple loci: long term observations. Physiol Behav. 1974;13:281–90. doi: 10.1016/0031-9384(74)90046-8. [DOI] [PubMed] [Google Scholar]
- 18.Bishop MP, Elder ST, Heath RG. Intracranial self-stimulation in man. Science. 1963;140:394–6. doi: 10.1126/science.140.3565.394. [DOI] [PubMed] [Google Scholar]
- 19.Faircloth KP. The importance of subject control in reinforcing brain stimulation. Learn Motiv. 1974;5:16–23. [Google Scholar]
- 20.Ettenberg A, Laferriere A, Milner PM, White N. Response involvement in brain stimulation reward. Physiol Behav. 1981;27:641–7. doi: 10.1016/0031-9384(81)90236-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsang WK, Stutz RM. Subject control as a determinant of the reinforcing properties of intracranial stimulation. Physiol Behav. 1984;32:795–802. doi: 10.1016/0031-9384(84)90197-5. [DOI] [PubMed] [Google Scholar]
- 22.Steiner SS, Ellman SJ. Relation between REM sleep and intracranial self- stimulation. Science. 1972;177:1122–4. doi: 10.1126/science.177.4054.1122. [DOI] [PubMed] [Google Scholar]
- 23.Van Luijtelaar EL, Kaiser J, Coenen AM. Deprivation of paradoxical sleep and intracranial self-stimulation. Sleep. 1982;5:284–9. doi: 10.1093/sleep/5.3.284. [DOI] [PubMed] [Google Scholar]
- 24.Marti-Nicolovius M, Portell-Cortes I, Morgado-Bernal I. Intracranial self- stimulation after paradoxical sleep deprivation induced by the platform method in rats. Physiol Behav. 1984;33:165–7. doi: 10.1016/0031-9384(84)90094-5. [DOI] [PubMed] [Google Scholar]
- 25.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–5. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 26.Routtenberg A. Forebrain pathways of reward in Rattus norvegicus. J Comp Physiol Psychol. 1971;75:269–76. doi: 10.1037/h0030927. [DOI] [PubMed] [Google Scholar]
- 27.Davis CJ, Clinton JM, Taishi P, Bohnet SG, Honn KA, Krueger JM. MicroRNA 132 alters sleep and varies with time in brain. J Appl Physiol. 2011;111:665–72. doi: 10.1152/japplphysiol.00517.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trachsel I, Tobler I, Borbély AA. Sleep regulation in rats: effects of sleep deprivation, light, and circadian phase. Am J Physiol. 1986;255:R27–37. doi: 10.1152/ajpregu.1986.251.6.R1037. [DOI] [PubMed] [Google Scholar]
- 29.Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci Lett. 1991;130:141–4. doi: 10.1016/0304-3940(91)90382-4. [DOI] [PubMed] [Google Scholar]
- 30.Feinberg I, Campbell IC. Total sleep deprivation in the rat transiently abolishes the delta amplitude response to darkness: implications for the mechanism of the “negative delta rebound”. J Neurophysiol. 1993;70:2695–9. doi: 10.1152/jn.1993.70.6.2695. [DOI] [PubMed] [Google Scholar]
- 31.Borbély AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–82. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 32.Clegern W, Szentirmai E, Rector D, Panksepp J, Krueger JM. Voluntary sleep restriction in rats is associated with sleep rebound. Sleep. 2008;31:A122. (Abstract Suppl) [Google Scholar]
- 33.Rolls A, Colas D, Adamantidis A, et al. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci U S A. 2011;108:13305–10. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terry LC, Martin JB. Hypothalamic-pituitary responses to intracranial self-stimulation in the rat. Brain Res. 1978;157:89–104. doi: 10.1016/0006-8993(78)90998-8. [DOI] [PubMed] [Google Scholar]
- 35.Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- 36.Krueger JM, Huang Y, Rector DM, Buysse DJ. Sleep: a synchrony of cell activity-driven small network states. Eur J Neurosci. 2013;38:2199–209. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kattler H, Dijk DJ, Borbély AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 38.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 39.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7:S16–8. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephenson R, Caron AM, Famina S. Behavioral sleep-wake homeostasis and EEG delta power are decoupled by chronic sleep restriction in the rat. Sleep. 2015;38:685–97. doi: 10.5665/sleep.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez-Palacios G, Velazquez-Moctezuma J. Effect of electric foot shocks, immobilization, and corticosterone administration on the sleep-wake pattern in the rat. Physiol Behav. 2000;71:23–8. doi: 10.1016/s0031-9384(00)00285-7. [DOI] [PubMed] [Google Scholar]
- 42.Meerlo P, Turek FW. Effects of social stimuli on sleep in mice: non-rapid-eye- movement (NREM) sleep is promoted by aggressive interaction but not by sexual interaction. Brain Res. 2001;907:84–92. doi: 10.1016/s0006-8993(01)02603-8. [DOI] [PubMed] [Google Scholar]
- 43.Tang X, Yang L, Sanford LD. Interactions between brief restraint, novelty and foot shock stress on subsequent sleep and EEG power in rats. Brain Res. 2007;114:110–8. doi: 10.1016/j.brainres.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nall A, Sehgal A. Monoamines and sleep in Drosophila. Behav Neurosci. 2014;128:264–72. doi: 10.1037/a0036209. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh PK, Hrdina PD, Ling GM. Effects of REMS deprivation on striatal dopamine and acetylcholine in rats. Pharmacol Biochem Behav. 1976;4:401–5. doi: 10.1016/0091-3057(76)90055-1. [DOI] [PubMed] [Google Scholar]
- 46.Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 47.Zant JC, Leenaars CH, Kostin A, Van Someren EJ, Porkka-Heiskanen T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Res. 2011;1399:40–8. doi: 10.1016/j.brainres.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Azogu I, De La Tremblaye PB, Dunbar M, Lebreton M, LeMarec N, Plamondon H. Acute sleep deprivation enhances avoidance learning and spatial memory and induces delayed alterations in neurochemical expression of GR, TH, DRD1, pCREB and Ki67 in rats. Behav Brain Res. 2015;279:177–90. doi: 10.1016/j.bbr.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt MA, Wisor JP. Interleukin 1 receptor contributes to methamphetamine- and sleep deprivation-induced hypersomnolence. Neurosci Lett. 2012;513:209–13. doi: 10.1016/j.neulet.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dugovic C, Meert TF, Ashton D, Clincke GH. Effects of ritanserin and chlordiazepoxide on sleep-wakefulness alterations in rats following chronic cocaine treatment. Psychopharmacology. 1992;108:263–70. doi: 10.1007/BF02245110. [DOI] [PubMed] [Google Scholar]
- 51.Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther. 1997;283:757–69. [PubMed] [Google Scholar]
- 52.Wisor JP, Dement WC, Aimone L, Williams M, Bozyczko-Coyne D. Armodafinil, the R-enantiomer of modafinil_wake-promoting effects and pharmacokinetic profile in the rat. Pharmacol Biochem Behav. 2006;85:492–9. doi: 10.1016/j.pbb.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 53.Dimpfel W. Pharmacological modulation of dopaminergic brain activity and its reflection in spectral frequencies of the rat electropharmacogram. Neuropsychobiology. 2008;58:178–86. doi: 10.1159/000191124. [DOI] [PubMed] [Google Scholar]
- 54.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boutrel B, Kenny PJ, Specio SE, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao Y, Lu M, Marsh DJ, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin-concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–10. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tobler I, Borbély AA. Effects of 20-min forced locomotion on sleep and EEG spectra of the rat. In: Horne JA, editor. Sleep '90. Bochum: Pontenegal Press; 1990. pp. 102–5. [Google Scholar]
- 59.Grønli J, Murison R, Bjorvatn B, Sørensen E, Portas CM, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res. 2004;150:139–47. doi: 10.1016/S0166-4328(03)00252-3. [DOI] [PubMed] [Google Scholar]
- 60.Grønli J, Dagestad G, Milde AM, Murison R, Bramham CR. Post-transcriptional effects and interactions between chronic mild stress and acute sleep deprivation regulation of translation factor and cytoplasmic polyadenylation element- binding protein phosphorylation. Behav Brain Res. 2012;235:251–62. doi: 10.1016/j.bbr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 62.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 63.Hutchison IC, Rathore S. The role of REM sleep theta activity in emotional memory. Front Psychol. 2015;6:1439. doi: 10.3389/fpsyg.2015.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burgess ML, Davis JM, Wilson SP, Borg TK, Burgess WA, Buggy J. Effects of intracranial self-stimulation on selected physiological variables in rats. Am J Physiol. 1993;26:R149–55. doi: 10.1152/ajpregu.1993.264.1.R149. [DOI] [PubMed] [Google Scholar]
- 66.Tobler I, Franken P, Gao B, Jaggi K, Borbély AA. Sleep deprivation in the rat at different ambient temperatures: effect on sleep, EEG spectra and brain temperature. Arch Ital Biol. 1994;132:39–52. [PubMed] [Google Scholar]
- 67.Benington JH, Heller HC. Implications of sleep deprivation experiments for our understanding of sleep homeostasis. Sleep. 1999;22:1033–7. [PubMed] [Google Scholar]
- 68.Rechtschaffen A, Bergmann BM, Gilliand MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep de privation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 69.Schwierin B, Borbély AA, Tobler I. Prolonged effects of 24-h total sleep deprivation on sleep and sleep EEG in the rat. Neurosci Lett. 1999;261:61–4. doi: 10.1016/s0304-3940(98)01006-4. [DOI] [PubMed] [Google Scholar]