Abstract
Scrotal leiomyosarcoma is a rare tumor arising from the dartos layer of the scrotum presenting as firm, rubbery, non-tender, irregular mass. To date about 37 cases of leiomyosarcoma of scrotum have so far been reported. Treatment involves wide surgical excision with tumor free margins. We report a case of scrotal leiomyosarcoma in a 48-year-old man which was treated by a wide surgical excision and follow up of 14 months showed no recurrence of tumor.
Keywords: Scrotum, Leiomyosarcoma, Dartos
Introduction
Leiomyosarcomas of the scrotum, not involving the testis, epididymis or spermatic cord, belong to the group of subcutaneous superficial leiomyosarcomas. Over 95% of all paratesticular leiomyosarcomas are located in the spermatic cord or epididymis, and their location in the scrotal skin or subcutaneous position in the scrotum is exceptionally rare. Johnson H Jr. in 19871 reported the first known case of leiomyosarcoma of the scrotum and to date about 37 cases of leiomyosarcoma of scrotum have been reported in literature.2 The prognosis in general after a wide surgical excision has been reported to be good because lymphatic metastases is very rare; however a local recurrence rate of 40% has been reported.3 We report a case of leiomyosarcoma scrotum in a 48-year old patient presenting as a painless mass lesion.
Case report
A 40-year-old man presented with a firm, non-tender lump of 4 cm size in the posterior surface of scrotum in the subcutaneous location (Fig. 1). The lump was mobile but attached to the primary scar on the scrotal skin and not adherent to the underlying structures. This lesion first appeared 2-years previously and a partial excision of lump was done elsewhere, the histopathology of the same was reported to be inconclusive and it gradually increased in size in the past 6 months. Both testes epididymis and the spermatic cords were clinically normal, and there was no inguinal lymphadenopathy. There was no history of previous local irradiation or long-term anabolic steroid abuse in the patient.
Figure 1.
Clinical picture showing mass in the posterior surface of scrotum.
Hematological examination and routine biochemical investigations were within normal range. Ultrasound (USG) of the scrotum revealed a well-circumscribed hypoechoic lesion 4 cm × 3 cm in the subcutaneous plane separate from underlying testes and epididymis.
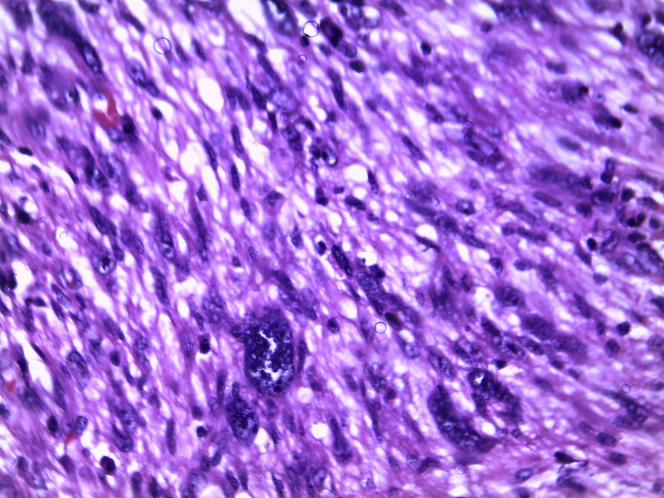
Wide excision of lump was done with 1 cm margin around. The histology report showed spindle cells of tumor were arranged in fascicles containing eosinophilic cytoplasm prominent nucleoli and presence of atypical mitosis suggestive of well differentiated leiomyosarcoma (Fig. 2). Tumor Margins and base were free from tumor extention. Immunohistochemistry showed the presence of vimentin, desmin confirming the smooth muscle origin of tumor. A regular follow up for a period of 14 months did not show evidence of local recurrence or distant metastasis.
Figure 2.
Histology showed spindle cells of tumor arranged in fascicles with eosinophilic cytoplasm.
Discussion
Soft tissue sarcomas account for 1% of all malignancies. Leiomyosarcomas constitute 10–20% of soft tissue sarcomas. Subcutaneous leiomyosarcomas account for 1–2% of all superficial soft tissue malignancies.4 Some of the more frequent malignant sarcomatous tumors in scroum are-
-
•
Rhabdomyosarcoma (RMS)
-
•
Leiomyosarcoma
-
•
Liposarcoma
-
•
Fibrosarcoma
-
•
Malignant fibrous histiocytoma (MFH)
-
•
Desmoplastic round cell
Leiomyosarcoma
Leiomyosarcomas are malignant mesenchymal neoplasms arising from smooth muscle. Leiomyosarcoma of the scrotum is a very rare tumor presenting between the fourth and 8 decades of life as a painless, slow-growing skin lesion of size 2–9 cm. Their location in the scrotal skin or subcutaneous position in the scrotum is exceptionally rare to date about 37 cases of leiomyosarcoma of scrotum have been reported in literature.2 The etiology of leiomyosarcomas remains unclear, though some authors suggest local irradiation during childhood as a potential cause.
The diagnosis of leiomyosarcoma is based upon histological examination of biopsy specimens. On histology they show spindle cells with cigar shaped nuclei arranged in interweaving fascicles. On immunohistochemistry leiomyosarcomas are positive for actin, desmin and CD 34.5 The mode of spread of leiomyosarcoma is primarily hematogenous to lung, liver and bone. The prognosis of leiomyosarcoma depends upon the size, depth and grade of the tumor and presence of distant metastases at diagnosis.
Rhabdomyosarcoma
The paratesticular region is the commonest site for the development of RMS accounting for 20%. Although most of these tumors occur in children and adolescents and 80% of RMS occur before the age of 21-years. Clinically RMS present as an intrascrotal mass, usually large. Histologically commonest subtypes seen are embryonal, alveolar, pleomorphic and mixed. Embryonal RMS is the commonest variety in the paratesticular region.6, 7
Liposarcoma
Liposarcomas account for ≈ 20% of sarcomas seen in the scrotum. From clinicopathological and cytogenetic features, three distinct categories of liposarcoma are recognized; well differentiated/de-differentiated, myxoid/round cell and pleomorphic. Most paratesticular liposarcomas are well differentiated. They typically present in older adults as a large fatty appearing mass.8, 9
Malignant fibrous histiocytoma
MFH is thought to account for a significant proportion of tumors in the adult paratesticular region. There are four histological subtypes, i.e. myxoid, storiform-pleomorphic, inflammatory and giant cell Most of the paratesticular MFH are of the storiform-pleomorphic type. These tumors have a high local recurrence rate, reportedly up to 51% after apparently complete surgical excision.10
Desmoplastic round cell tumor
Desmoplastic round cell tumors (DRCTs) are highly aggressive sarcomas. They are rare in the paratesticular region and occur in the young adult, with an age distribution of 17–43-years. Cytogenetically, DRCTs have a unique translocation, t(11; 22) (p13; p12), that results in fusion of the ESW gene on chromosome 22 to the WT-1 gene on chromosome 11.11
Management of paratesticular sarcomas
Sarcomas, like most malignant tumors, are classified according to their grade and stage. The TNM system grades tumors according to their degree of differentiation into four grades (G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; and G4, undifferentiated).
Treatment of paratesticular sarcomas
The optimum local and systemic treatment for these tumors remains controversial, but there is a general consensus that all scrotal sarcomas in adults should be managed with complete surgical resection with safe margin. However, an oncologically accepted safe margin of >10 mm, which gives a 84% local recurrence-free interval at 5-years for soft tissue sarcomas has been reported by McKee et al.12
Soft tissue sarcomas in most sites of the body have a tendency for local recurrence after inadequate resection, and dissemination of the disease is common for high-grade tumors. The reported local relapse rate in the scrotum is 25–44%.3
Radiation therapy is also of doubtful value in treatment of leiomyosarcoma except for palliation. Adjuvant locoregional radiation and/or surgery apparently reduces the risk of local recurrence.13
The role of retroperitoneal lymph node dissection (RPLND) in paratesticular sarcomas remains controversial is not advocated; unless a high degree of suspicion of for lymph node metastasis is present. RPLND has been recommended by some authors especially for patients with RMS, high-grade MFH and fibrosarcoma, as these patients are at high risk of retroperitoneal lymph node failure. RPLND is not recommended in leiomyosarcoma.14
The role of adjuvant chemotherapy in adult paratesticular sarcomas is not fully established. In children with paratesticular RMS adjuvant chemotherapy has had a dramatic effect on the overall survival.15 Thus, use of adjuvant chemotherapy for adult high-grade sarcoma is controversial. Chemotherapy with gemcitabine, paclitaxel, vincristine, doxorubicin, actinomycin-D has been used with limited success.
Recurrence most commonly tends to be local but distant metastases in the bones and lungs have been reported.1 Nonetheless, long-term follow up of patients with cutaneous leiomyosarcoma is mandatory to detect local recurrence and distant metastases that can occur years after the initial excision.
Conflict of interest
No conflict of interest.
References
- 1.Johnson H., Jr. Leiomyosarcoma of scrotum. Urology. 1987;29(4):436–438. doi: 10.1016/0090-4295(87)90520-6. [DOI] [PubMed] [Google Scholar]
- 2.Venyo A.K., Baiden-amissah K., Paiva-correia A.J. Subcutaneous scrotal leiomyosarcoma presenting as pedunculated multi-locular cystic growth in the scrotum mimicking a sebaceous cyst: A case report and review of the literature. WebmedCentral. 2011;2(10):WMC002388. UROLOGY. [Google Scholar]
- 3.Flotte T.J., Bell D.A., Sidhu G.S., Plair C.M. Leiomyosarcoma of the dartos muscle. J Cutan Pathol. 1981;8(1):69–74. doi: 10.1111/j.1600-0560.1981.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaushal V., Singh H., Gill M. Recurrent leiomyosarcoma of the scrotum. JK Sci. 2009;11:97–98. [Google Scholar]
- 5.Fisher C., Goldblum J.I., Montgomerry E. Leiomyosarcoma of the paratesticular region: a clinicopathologic study. Am J Surg Pathol. 2001;25(9):1143–1149. doi: 10.1097/00000478-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton C.R., Pinkerton R., Horwich A. The management of paratesticular rhabdomyosarcoma. Clin Radiol. 1989;40:314–317. doi: 10.1016/s0009-9260(89)80222-3. [DOI] [PubMed] [Google Scholar]
- 7.Stewart L.H., Lioe T.F., Johnston S.R. Thirty-year review of intrascrotal rhabdomyosarcoma. Br J Urol. 1991;68:418–420. doi: 10.1111/j.1464-410x.1991.tb15364.x. [DOI] [PubMed] [Google Scholar]
- 8.Mentzel T., Fletcher C.D. Lipomatous tumours of soft tissues: an update. Virchows Arch. 1995;427:353–363. doi: 10.1007/BF00199383. [DOI] [PubMed] [Google Scholar]
- 9.Dei Tos A.P., Dal Cin P. The role of cytogenetics in classification of soft tissue tumours. Virchows Arch. 1997;431:83–94. doi: 10.1007/s004280050073. [DOI] [PubMed] [Google Scholar]
- 10.Kearney M.M., Soule E.H., Ivins J.C. Malignant fibrous histiocytoma. A retrospective study of 167 cases. Cancer. 1980;45:167–178. doi: 10.1002/1097-0142(19800101)45:1<167::aid-cncr2820450127>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Sawyer J.R., Tryka A.F., Lewis J.M. A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round–cell tumor. Am J Surg Pathol. 1992;16:411–416. doi: 10.1097/00000478-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 12.McKee M.D., Liu Dong Feng, Brooks J. The prognostic significance of margin width for extremity and trunk. J Surg Oncol. 2004;85:68–76. doi: 10.1002/jso.20009. [DOI] [PubMed] [Google Scholar]
- 13.Russo P., Brady M.S., Conlon K. Adult urological sarcoma. J Urol. 1992;147:1032–1036. doi: 10.1016/s0022-5347(17)37456-6. [DOI] [PubMed] [Google Scholar]
- 14.Catton C.N., Cummings V., Fornasier B. Adult paratesticular sarcomas. A review of 21 cases. J Urol. 1991;146:342–345. doi: 10.1016/s0022-5347(17)37787-x. [DOI] [PubMed] [Google Scholar]
- 15.De Vries J.D. Paratesticular rhabdomyosarcoma. World J Urol. 1997;13:219–225. doi: 10.1007/BF00182966. [DOI] [PubMed] [Google Scholar]