Abstract
Introduction
With the increasing use of intravitreal administration of corticosteroids in macular edema, steroid-induced intraocular pressure (IOP) rise is becoming an emergent issue. However, for patients in whom intravitreal steroids are indicated, there are no specific recommendations for IOP monitoring and management after intravitreal administration of corticosteroids.
Method
An expert panel of European ophthalmologists reviewed evidence on corticosteroid-induced IOP elevation. The objective of the panel was to propose an algorithm based on available literature and their own experience for the monitoring and management of corticosteroid-induced IOP elevation, with a focus on diabetic patients.
Results
Data from trials including diabetic patients with a rise of IOP after intravitreal steroid administration indicate that IOP-lowering medical treatment is sufficient for a large majority of patients; only a small percentage underwent laser trabeculoplasty or filtering filtration surgery. A 2-step algorithm is proposed that is based on the basal value of IOP and evidence for glaucoma. The first step is a risk stratification before treatment. Patients normotensive at baseline (IOP ≤ 21 mmHg), do not require additional baseline diagnostic tests. However, patients with baseline ocular hypertension (OHT) (IOP > 21 mmHg) should undergo baseline imaging and visual field testing. The second step describes monitoring and treatment after steroid administration. During follow-up, patients developing OHT should have baseline and periodical imaging and visual field testing; IOP-lowering treatment is proposed only if IOP is >25 mmHg or if diagnostic tests suggest developing glaucoma.
Conclusion
The management and follow-up of OHT following intravitreal corticosteroid injection is similar to that of primary OHT. If OHT develops, IOP is controlled in a large proportion of patients with standard IOP treatments. The present algorithm was developed to assist ophthalmologists with guiding principles in the management of corticosteroid-induced IOP elevation.
Funding
Alimera Sciences Limited.
Keywords: Diabetic macular edema, Intravitreal implants, Intravitreal steroid injection, Ocular hypertension, Steroid-induced glaucoma, Steroid-induced ocular hypertension
Introduction
Diabetic macular edema (DME) is a common complication of diabetes involving the microvasculature of retina. DME is the consequence of a breakdown in the blood-retina barrier which leads to retinal thickening resulting from the accumulation of fluid and exudates [1]. DME is the primary reason of vision loss in diabetic retinopathy, which is a leading cause of blindness in countries with a developed economy [2, 3].
The goal of therapy in DME is to preserve retinal function by reducing vascular leakage causing edema (expressed as retinal thickening). Laser photocoagulation and intravitreal administration of anti-vascular endothelial growth factor (anti-VEGF), as first line therapy, or corticosteroids, as second line therapy, have demonstrated efficacy [1]. In contrast with anti-VEGFs, which inhibit only the effect of VEGF, corticosteroids have multiple mechanisms of action on various pathways of the inflammatory cascade thus, reducing the effects of VEGF, inflammatory cytokines and other mechanisms involved in the pathogenesis of DME [1].
With the increasing use of corticosteroids, steroid-induced elevated intraocular pressure (IOP) or ocular hypertension (OHT) are becoming an emergent problem with the reports of incidence ranging from 11% to 79% [4]. The risk of IOP elevation depends on the dose and chemical structure of the steroid, duration of therapy and route of administration [5]. The variable risk according to the route of administration has motivated some authors to evaluate a provocation test before an intravitreal injection. Risk factors for a steroid-induced rise in IOP have been identified: primary open-angle glaucoma (POAG), family history of glaucoma, diabetes, myopia, rheumatoid arthritis, old age or age less than 6 years [5, 6]. However, there are no specific recommendations regarding patient selection and monitoring following intravitreal use of corticosteroids in the setting of diabetic retinopathy. The guidelines of the European Glaucoma Society (EGS) provide recommendations for the management of glaucoma due to corticosteroid treatment, but the common situation of diabetic patients with macular edema being treated with intravitreal corticosteroids is not specifically addressed [5].
The objective of this article was to review current evidence on steroid-induced IOP elevation and OHT and to provide guidance for the monitoring and management of IOP following treatments with corticosteroids in DME.
Methods
The authors of this article are an expert panel of European ophthalmologists who met in Paris in March 2015. The panel’s intention was first to review evidence on corticosteroid-induced IOP elevation in DME patients. Since data are sparse or incomplete regarding the specific use of corticosteroids in DME, the panel also considered the relationship between OHT and onset of glaucoma, mechanisms of corticosteroid-induced IOP elevation and current guidelines for the management of corticosteroid-induced IOP elevation.
A monitoring and management algorithm has been proposed that was designed to be acceptable for use across Europe and that can be adapted in local countries. The proposed algorithm is evidence based as far as possible. When necessary, the recommendations were based on panel’s experience.
This article is intended to be a practical tool for ophthalmologists in the management of corticosteroid-induced IOP elevation. This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Intravitreal Corticosteroids Used in DME
The direct injection of corticosteroid into the vitreous allows the administration of a large bolus of drug. The therapeutic level is rapidly achieved and the drug is administered in the vicinity of the target tissue. Moreover, systemic absorption and systemic side effects are minimized. The most common corticosteroids used for intravitreal administration are triamcinolone acetonide, fluocinolone acetonide and dexamethasone.
Triamcinolone acetonide is minimally water soluble and is administered as a suspension. Most frequently the 4 mg dose is used and its duration of action is approximately 3 months [7]. Compared with triamcinolone, dexamethasone is more potent with a shorter duration of action [8]. The intravitreal administration of dexamethasone has been studied at 0.4 and 0.8 mg. Due to its short duration of action, a single injection is not sufficient to achieve a sustained therapeutic effect in DME which is a chronic disease [9].
Repeated intravitreal administration of corticosteroids for maintaining therapeutic effects increases the risk of adverse events, such as endophthalmitis or vitreous haemorrhage [7, 10]. Sustained-release implants have, therefore, been developed to reduce injection frequency and reduce the risk of such complications [11]. These implants are either biodegradable or non-biodegradable. With non-biodegradable implants, the release of the corticosteroid is more precise with a longer therapeutic effect [4].
Retisert® (Bausch and Lomb, Inc., Rochester, NY, USA) is a non-biodegradable implant that is inserted via the pars plana. It is sutured to the sclera and fluocinolone acetonide is released for 30 months at a controlled rate [12]. This drug has no license in Europe and will not be discussed in this review.
ILUVIEN® (Alimera Sciences, Inc, Alpharetta, GA, USA) is a non-biodegradable implant inserted into the vitreous cavity via the pars plana through a 25-gauge needle. Fluocinolone acetonide is released at a rate of 0.2 µg per day for up to 36 months [13].
Ozurdex® (Allergan, Inc, Irvine, CA, USA) is a biodegradable sustained-release device that is inserted into the vitreous cavity using a stepped incision through a 23-gauge needle. One dexamethasone implant contains 700 μg of dexamethasone which is released for up to 6 months [14].
Pharmacokinetics of Intravitreal Corticosteroids
Steroids administered in the vitreous are eliminated via two main pathways. The flow of aqueous humor through the anterior chamber angle is the first pathway and the second is posteriorly through the blood-retinal barrier [11, 15]. The concentration of the intravitreal corticosteroids, and consequently its duration of action, depends on the clearance rate from the vitreous [16, 17].
Direct measurements of corticosteroid concentrations in vitreous are difficult and experimental animal models have been developed: triamcinolone acetonide in rabbit [18, 19], dexamethasone implants (Ozurdex) in monkey [20], fluocinolone acetonide (ILUVIEN) in rabbit [21]. Nevertheless, small series have been performed in human for triamcinolone acetonide [7], fluocinolone acetonide (ILUVIEN) [22, 23]. No study has been published for dexamethasone implants (Ozurdex) in the human eye.
From these pharmacokinetic studies, the duration of the therapeutic window has been evaluated to be ~1–3 months for triamcinolone acetonide 4 mg, ~2–4 months for Ozurdex (700 µg of dexamethasone) and up to 36 months for ILUVIEN (0.2 µg/day of fluocinolone acetonide) [24].
Evidence Base for Corticosteroid-Induced Ocular Hypertension
Topical, intravitreal and high-dose and long-term systemic corticosteroid therapy can induce subacute or chronic elevation of IOP and particularly in patients with POAG [25, 26]. Thus, IOP elevation has been observed in 40% of normal subjects after 4–6 weeks of treatment with topical 0.1% dexamethasone and 100% of patients with POAG [4].
It has been suggested that POAG and steroid-induced glaucoma share common pathogenic mechanisms; OHT induced by corticosteroids is similar to POAG since, in both cases, the outflow facility is decreased [27]. The ultrastructural basis of corticosteroid-induced obstruction within the trabecular meshwork remains poorly understood. Some studies suggested that extracellular matrix alterations contribute to decreased outflow facility within the trabecular meshwork or the juxtacanalicular connective tissue [28]. The upregulation of the gene of myocilin has been evidenced in cultured trabecular meshwork cells [29]. Moreover, mutations of this gene have been found to be associated with POAG [30].
Whichever corticosteroid drug is administered into the vitreous, the incidence of OHT increases with the dose: 32.1% and 45.9% for triamcinolone 4 and 25 mg [4], 37.1% and 45% for ILUVIEN 0.2 and 0.5 µg/day [31], 27% and 32% for Ozurdex 0.35 and 0.7 mg [32], respectively. There is a correlation between the steroid concentration in aqueous humor and the increased risk of OHT in DME patients after intravitreal administration of fluocinolone acetonide [23]. The authors concluded that, in susceptible patients, prolonged aqueous levels of fluocinolone acetonide >1 ng/ml moderately increases the risk of IOP rise and levels >6 ng/mL are associated with a markedly increased risk. Compared to the control arm, significant anatomical signs of development of glaucoma were not detected in the ILUVIEN studies [33].
The time course of OHT after intravitreal administration of corticosteroids varies according to the molecule and dose. Moreover, comparisons between studies are limited by the various definitions used for the steroid induced rise of IOP. In the systematic review of Kiddee et al. the onset of OHT defined as IOP ≥21 or ≥10 mmHg from baseline was 2–4 weeks in randomized controlled trials and 1–8 weeks in non-randomized studies after administration of triamcinolone 4 mg [4]. However, in some studies onset could be observed as early as 1 week or on the contrary 20–24 weeks after administration. With the ILUVIEN 0.2 µg/day implants, the onset of OHT began within 2–4 weeks with a maximum at 24–48 weeks and a return to basal values 9–12 months after implantation [22, 34, 35]. The time course of OHT has not been reported with the dexamethasone implant. Nevertheless, the peak IOP occurred 60 days after implantation and returned to basal values within 6 months [36–39].
Although diabetes has been reported as a risk factor of development of POAG after intravitreal triamcinolone administration in a small retrospective study (p = 0.050) [40], diabetes was not considered a predictor of development of POAG in both independent Ocular Hypertension Treatment Study (OHTS) (ClinicalTrials.gov identifier, NCT00000125) and European Glaucoma Prevention Study [6, 7]. Elevated IOP >21 mmHg after administration of 20 or 25 mg of triamcinolone was statistically independent of diabetes (p = 0.74 and p = 0.37, respectively) [41, 42]. Another study with triamcinolone 4 or 8 mg did not find a relationship between an IOP rise and diabetes [hazard ratio, 0.91; 95% confidence interval (CI), 0.47–1.61, p = 0.760] [43]. There is an increased risk of glaucoma in patients with diabetes regardless of IOP level [44], possibly explained by vascular mechanisms, as diabetes causes microvascular damage and may affect vascular autoregulation of the retina and optic nerve.
Some authors hypothesized that side effects related to corticosteroids may be explained in part by the differences in drug lipophilicity and partitioning into trabecular meshwork and lens [45]. However, these results have been recently rejected in the scientific literature [46].
Evidence Base for Elevated IOP After Corticosteroids in DME
The effects on IOP of intravitreal administration of corticosteroids during large trials for the treatment of DME are summarized in Table 1. The results of IOP elevation are, however, difficult to compare since IOP was not a primary endpoint and only reported as an adverse event. Moreover, definitions of IOP were not standardized with different cut-offs for IOP elevation or OHT. Overall, the safety results regarding elevated IOP in patients with DME are similar to those reported in the systematic review of Kiddee et al. which included patients regardless of diabetes status [4]. Nevertheless, a large proportion of patients with DME received IOP-lowering medical treatments and were largely well controlled (Table 1). Some patients underwent laser trabeculoplasty or incisional glaucoma surgery, but the proportion was low, except for one study of triamcinolone in which 9% underwent glaucoma surgery [47].
Table 1.
Intravitreal corticosteroid in randomized controlled trials for the treatment of DME
| Study | Corticosteroid | Number of eyes | Study design | Follow-up | Conclusion on IOP for corticosteroid groups (labeled arm if any) | |
|---|---|---|---|---|---|---|
| IOP events | IOP management | |||||
| Gillies [47] | Triamcinolone acetonide | 69 | Triamcinolone (4 mg) vs laser + placebo | 5 years | ΔIOP ≥+5 mmHg: 79% |
IOP-lowering medication: 56% Glaucoma surgery: 9% |
| DRCR.net [64] | Triamcinolone acetonide | 840 | Triamcinolone (1 or 4 mg) vs laser | 3 years | IOP ≥21 mmHg: 15% and 10% |
IOP-lowering medications: 2% and 12% Laser trabeculoplasty (n = 1) and glaucoma surgery (n = 3) with 4 mg |
| DRCR.net [65] | Triamcinolone acetonide | 854 | Bevacizumab with deferred or prompt laser vs triamcinolone (4 mg) with deferred or prompt laser | 1 year | Up to 14 weeks and from 14 to 56 weeks for events and management: ΔIOP ≥+10 mmHg: 17% and 10% IOP-lowering medication: 2% and 9% IOP ≥30 mmHg: 4% and 4% Glaucoma surgery: 0% and 1% | |
| Callanan [66] | Dexamethasone (Ozurdex) | 253 | Dexamethasone implant 6 M fixed dosing + laser at M1 vs sham + laser at M1 | 1 year |
ΔIOP ≥+10 mmHg: 15.2% IOP ≥25 mmHg: 16.8% IOP ≥35 mmHg: 4.0% |
IOP-lowering medication: 15.9% Glaucoma surgery: 0% |
| Ramu [67] | Dexamethasone (Ozurdex) | 100 | Dexamethasone implant 5 M fixed vs prn dosing | 1 year | IOP >30 mmHg: 20% and 34% |
IOP lowering medication: 28 pts Glaucoma surgery: 0% |
| Heng [68] | Dexamethasone (Ozurdex) | 80 | Dexamethasone implant prn + laser vs laser alone | 1 year | Not reported |
IOP lowering medication: 20% Glaucoma surgery: 0% |
| Boyer [32] | Dexamethasone (Ozurdex) | 1048 | Dexamethasone implant 6 M fixed dosing (350 or 700 µg) vs sham | 3 years |
ΔIOP ≥+10 mmHg: 27.7% IOP ≥25 mmHg: 32.0% IOP ≥35 mmHg: 6.6% |
IOP-lowering therapy: 41.5% Laser trabeculoplasty: 0% Glaucoma surgery: 0.9% |
| Gillies [69] | Dexamethasone (Ozurdex) | 88 | Dexamethasone implant prn (700 µg) vs bevacizumab | 1 year |
ΔIOP ≥+5 mmHg: 46% ΔIOP ≥+10 mmHg: 20% |
Laser trabeculoplasty: 1 patient |
| Campochiaro [31] | Fluocinolone acetonide (ILUVIEN) | 953 | ILUVIEN insert (0.2 or 0.5 μg/day) vs sham/laser | 3 years | Increased IOP: 37.1% |
IOP-lowering therapy: 38.4% Laser trabeculoplasty: 1.3% Glaucoma surgery: 4.8% |
DME diabetic macular edema, IOP intraocular pressure, prn pro re nata
Outcomes comparable to those seen in controlled trials were obtained in recent real-world studies [48–51]. The study of Lam et al. assessed the intravitreal dexamethasone implant (Ozurdex) in 120 study eyes (including 34 eyes with diagnosis of DME) in patients with ≥3 months of follow-up after the initial dexamethasone implant [48]. An IOP rise ≥+10 mmHg from baseline, ≥25 and ≥35 mmHg were reported for 20.6%, 26.5% and 2.9% of patients with DME. Glaucoma surgery was performed for 1.7% of all patients (no patient with DME had surgery). IOP elevation in DME patients or patients with retinal vein occlusion were comparable and IOP-lowering medical treatment was administered to 29.4% of patients with DME and 16.7% of patients with retinal vein occlusion.
The recent 1-year retrospective study of Mazzarella et al. evaluated the effect of intravitreal injection of dexamethasone (Ozurdex) on IOP in 92 patients with macular edema (including 36.5% with diabetic retinopathy) either without OHT (group 1; n = 65) or associated with glaucoma or OHT (group 2; n = 27) [49]. After injection, OHT (>21 mmHg) was observed in 21.5% of eyes of group 1 and 59.3% in group 2. In group 2, all eyes required IOP-reducing treatment vs. 12.3% in group 1. In general the rise of IOP was transitory and only one patient required filtration surgery. These results underscore the need to monitor patients during corticosteroid treatment, especially patients with a previous history of OHT or glaucoma.
The 1-year prospective study of Massin et al. evaluated the fluocinolone acetonide intravitreal implant (ILUVIEN) in 16 patients (17 eyes) with DME insufficiently responsive to laser and anti-VEGF therapy. Patients with a history of an IOP rise after intravitreal corticosteroids were excluded. Elevation of IOP was reported in three patients who were well controlled by IOP-lowering eyedrops [50].
Overall, these studies indicate that diabetic patients with intravitreal steroids have an incidence of IOP increase and OHT comparable with those of non-diabetic patients. When necessary, IOP-lowering medical treatment or laser trabeculoplasty is sufficient for a large majority of patients and glaucoma surgery is needed for only a small proportion of patients.
Evidence Base for Onset of Glaucoma Related to Ocular Hypertension
The OHTS was a multicenter, randomized, prospective clinical trial designed to assess the relationship between primary OHT and onset of glaucoma. For this purpose, 1636 patients aged from 40 to 80 years with high IOP were randomized between IOP-lowering treatment and no treatment [52]. The goal was to achieve IOP <24 mmHg and an IOP decrease of at least 20% from baseline. The primary outcome was the development of open-angle glaucoma (visual field defects or optic disc deterioration). The IOP was reduced by 22.5% in the treated group and 4.4% in the no treatment group [53]. The main results indicated that around 90% of patients with OHT (baseline IOP between 24 and 32 mmHg) who are not treated had no glaucoma after 5 years. IOP reduction by at least 20% was found to decrease the risk of conversion from OHT to glaucoma by 50%. This large randomized controlled study confirmed that IOP reduction decreases the incidence of glaucoma in patients with OHT [5]. The risk of developing glaucoma as a result of a corticosteroid-induced IOP rise may not be the same as that for the development of primary open-angle glaucoma in patients with OHT. Hence, new studies are still needed to identify the risk of developing glaucoma after treatment with a steroid.
Is A ‘Steroid Provocative Test’ Useful?
Few studies have directly addressed this point i.e. initiating a short-acting steroid treatment to assess a ‘steroid response’ on IOP before initiating a sustained steroid release treatment. However, previous studies on the effect of topical corticosteroids on IOP are available. Thus, pioneering studies in the 1960s showed that topical corticosteroids caused elevated IOP in a large proportion of patients [27, 54, 55]. These early studies showed that increased IOP is observed in approximately one-third of people after administration of topical corticosteroids for several days (more than 90% in patients with chronic glaucoma). Among ‘responders’, the majority were low responders (IOP rise <6 mmHg) and one-third were intermediate responders (IOP rise from 6 to 15 mmHg) and 4–6% were high responders (IOP rise >15 mmHg) [27, 54–56]. This IOP elevation was usually transient, with a return to normal within 2 weeks after discontinuation, provided the treatment duration was short. Persistent IOP elevations refractory to IOP-lowering treatment were observed in 1–5% of cases [56].
More recently, Breusegem et al. investigated the diagnostic value for a steroid response of a topical dexamethasone provocative test before intravitreal triamcinolone acetonide injection [57]. The dexamethasone test had a sensitivity of 25% (CI 95%, 0.07–0.52), a specificity of 100% (95% CI, 0.83–1.00), a positive predictive value of 100% (95% CI, 0.40–1.00) and a negative predictive value of 62% (95% CI, 0.44–0.79). It was thus concluded that a positive response to topical dexamethasone is a strong predictor of the response to intravitreal triamcinolone acetonide; however, a negative response does not guarantee there will be no response to intravitreal triamcinolone acetonide.
In the panel’s opinion, the benefit of a corticosteroid provocation test is equivocal. Since the great majority of steroid-induced OHT can be managed with medical or laser therapy, a positive steroid challenge does not necessarily imply that a patient would not benefit from intravitreal steroids; the clinician and patient should consider the benefit-risk balance before deciding on the best course of action.
Recommendations
There is no evidence regarding the choice of IOP-lowering treatment or the targeted IOP for steroid-induced OHT in DME patients. The guidelines of the EGS recommend: (1) discontinuation of corticosteroid therapy or switch to weaker steroid; (2) administration of topical or systemic IOP-lowering medication; (3) laser trabeculoplasty and (4) glaucoma surgery in intractable cases [5]. These recommendations are, however, not specific for intravitreal administration, nor for patients with DME. A systematic review by Kiddee addressed the management of IOP but did not discuss the specifics of the diabetic population studied [4].
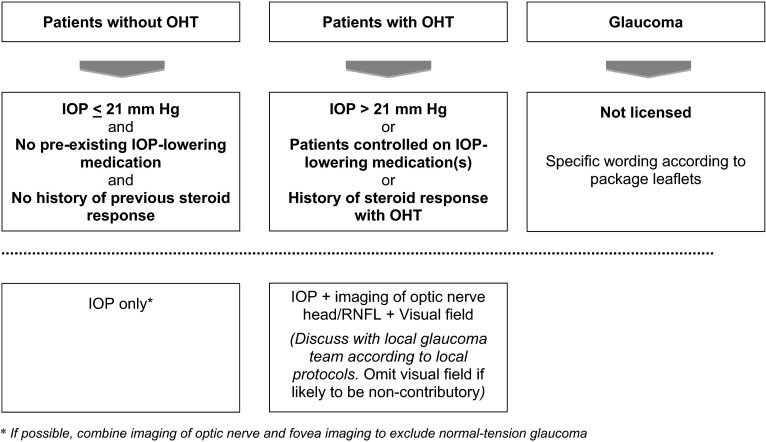
We propose two consecutive algorithms for DME patients treated with intravitreal corticosteroids—one for baseline risk stratification (Fig. 1) and prior to administering a corticosteroid and the second is for the monitoring and management of IOP after a corticosteroid has been administered (Fig. 2). In the risk stratification algorithm, patients with pre-existing or advanced glaucoma are not eligible for treatment according to respective package leaflets of fluocinolone acetonide (ILUVIEN) and dexamethasone (Ozurdex) implants [58, 59] (Fig. 1). Some authors have suggested that pre-existing glaucoma should be a relative contraindication for steroid use because intravitreal steroids may be the only effective treatment for some patients [6, 56]. The use of intravitreal steroids in such patients would be ‘off-label’ as the products are not licensed for use in these patients. For monitoring of patients with IOP ≤21 mmHg, without a history of IOP-lowering medication or IOP increase after corticosteroid treatment, measurement of IOP alone is sufficient, without the need for supplementary examinations. For patients with OHT (IOP > 21 mmHg), or patients already receiving IOP-lowering medications or with a history of OHT after corticosteroid treatment, baseline imaging (such as optical coherence tomography of optic nerve head and/or retinal nerve fibre layer) and visual field testing should be performed for future reference.
Fig. 1.
Algorithm for the management of IOP elevation by retinal specialists: pre-injection considerations. IOP intraocular pressure, OHT ocular hypertension, RNFL retinal nerve fibre layer
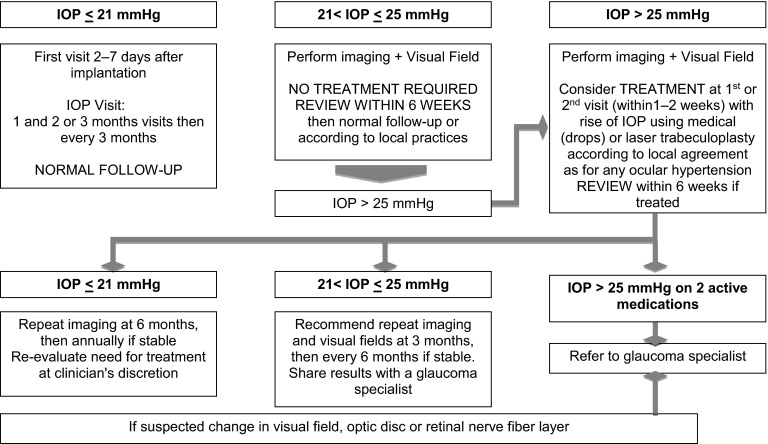
Fig. 2.
Algorithm for the management of IOP elevation by retinal specialists: Post-injection IOP management. IOP intraocular pressure
The second algorithm concerns monitoring and management (Fig. 2). For patients with IOP ≤21 mmHg, the standard follow-up protocol applies. If a patient develops 21 < IOP ≤ 25 mmHg, the next visit should be within 6 weeks; if the IOP remains in this window, then the standard follow-up protocol applies. If not previously done, the patient should have baseline imaging and visual field testing. For patients developing an IOP >25 mmHg, treatment should be considered with IOP-lowering medication or laser trabeculoplasty according to local practices. If IOP remains ≥25 mmHg on two active medications, then the patient should be referred to a glaucoma specialist. Overall, the management of IOP rise in DME patients who received intravitreal corticosteroid is not different to other patients with primary OHT since there is no evidence for an additional risk for diabetic patients. The panel agreed there is no need for specific management of IOP elevation induced by steroids compared with other causes of IOP rise. Moreover, transitory OHT per se should not be considered a problem as long as the risk of conversion to glaucoma is reduced. Anecdotal experience in treating steroid-induced OHT suggests that laser trabeculoplasty is an effective treatment.
In a meta-analysis of randomized controlled trials, the most efficient IOP-lowering drugs were prostaglandins followed by non-selective beta-blockers, alpha-adrenergic agonists, selective beta-blockers and topical carbonic anhydrase inhibitors [60]. However, the panel considered that there were neither particular preferences nor exclusions of specific classes of medical treatment in DME patients with corticosteroid-induced OHT; treatment should start with monotherapy, as set out in the EGS guidelines [5]. The use of prostaglandin analogues is not contraindicated but treating clinicians need to be aware of the uncommon occurrence of macular edema from prostaglandin analogue use [61].
Contraindications particular to each class of topical anti-glaucoma drugs should be considered on a case-by-case basis [5].
Conclusion
In diabetic patients having intravitreal steroids, clinical studies indicated that the rates of elevated IOP and OHT were comparable with those of non-diabetic patients (for example patients with uveitis). The panel agreed that there is no need for specific management protocol for IOP elevation induced by steroids compared with other causes of raised IOP. When necessary, IOP-lowering medical treatment or laser trabeculoplasty is sufficient for a large majority of patients and glaucoma surgery is needed for only a small proportion of patients.
These principles have been implemented in an algorithm that is intended to be simple and practical to provide guiding principles to ophthalmologists in the management of corticosteroid-induced IOP elevation.
Acknowledgments
The meeting was supported by Alimera Sciences Limited. Sponsorship and article processing charges for this study were funded by Alimera Sciences Limited. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Francisco Goni declares that he doesn’t have any financial/personal interest or belief that could affect his objectivity, or inappropriately influence his actions on the submitted work. Ingeborg Stalmans declares consultant honoraria from Alcon, Alimera, Allergan, Amakem, AqueSys, EyeTechCare, Isarna, Santen and Théa. Simon Taylor declares that he has received research funding from UK National Institute of Health Research, UK BBSRC, GlaxoSmithKline, Novartis and declares speaker honoraria, advisory boards, travel grants with Alimera, Novartis, Bayer, GlaxoSmithKline, Santen, Allergan. Philippe Denis declares consultant honoraria from Alimera and Allergan. Jean-Philippe Nordmann declares speaker honoraria, advisory boards, travel grants with Allergan, Alcon, Pfizer and Alimera. Michael Diestelhorst declares that he has received a speaker honorarium from Drug Company. Antonio Figueiredo declares that he has received speaker honorarium from Alimera Sciences, Allergan, Pfizer and Thea. Ted Garway Heath declares that he receives research funding/support from the NHS’s National Institute for Health Research, Pfizer Inc and Heidelberg Engineering and declares Speaker honoraria or advisory boards with Alcon, Aerie, Alimera, Allergan, Pfizer, Santen.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/45C4F0604DE73D4B.
References
- 1.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375–1394. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2010;117:63–70. doi: 10.1016/j.ophtha.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 4.Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol. 2013;58:291–310. doi: 10.1016/j.survophthal.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 5.European Glaucoma Society . Terminology and guidelines for glaucoma. 4. Savona: PubliComm; 2014. [Google Scholar]
- 6.Razeghinejad MR, Katz LJ. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012;47:66–80. doi: 10.1159/000328630. [DOI] [PubMed] [Google Scholar]
- 7.Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–686. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 8.Yeung CK, Chan KP, Chan CK, Pang CP, Lam DS. Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: comparison with dexamethasone and hydrocortisone. Jpn J Ophthalmol. 2004;48:236–242. doi: 10.1007/s10384-003-0053-8. [DOI] [PubMed] [Google Scholar]
- 9.Chan CK, Mohamed S, Lee VY, Lai TY, Shanmugam MP, Lam DS. Intravitreal dexamethasone for diabetic macular edema: a pilot study. Ophthalmic Surg Lasers Imaging. 2010;41:26–30. doi: 10.3928/15428877-20091230-05. [DOI] [PubMed] [Google Scholar]
- 10.Graham RO, Peyman GA. Intravitreal injection of dexamethasone. Treatment of experimentally induced endophthalmitis. Arch Ophthalmol. 1974;92:149–154. doi: 10.1001/archopht.1974.01010010155016. [DOI] [PubMed] [Google Scholar]
- 11.Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffe GJ, Yang CH, Guo H, Denny JP, Lima C, Ashton P. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000;41:3569–3575. [PubMed] [Google Scholar]
- 13.Kane FE, Burdan J, Cutino A, Green KE. ILUVIEN: a new sustained delivery technology for posterior eye disease. Expert Opin Drug Deliv. 2008;5:1039–1046. doi: 10.1517/17425247.5.9.1039. [DOI] [PubMed] [Google Scholar]
- 14.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13:135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Choonara YE, Pillay V, Danckwerts MP, Carmichael TR, du Toit LC. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J Pharm Sci. 2010;99:2219–2239. doi: 10.1002/jps.21987. [DOI] [PubMed] [Google Scholar]
- 17.Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011;16:270–277. doi: 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Kamppeter BA, Cej A, Jonas JB. Intraocular concentration of triamcinolone acetonide after intravitreal injection in the rabbit eye. Ophthalmology. 2008;115:1372–1375. doi: 10.1016/j.ophtha.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Ye YF, Gao YF, Xie HT, Wang HJ. Pharmacokinetics and retinal toxicity of various doses of intravitreal triamcinolone acetonide in rabbits. Mol Vis. 2014;20:629–636. [PMC free article] [PubMed] [Google Scholar]
- 20.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 21.Kane FE, Green KE. Ocular pharmacokinetics of fluocinolone acetonide following ILUVIEN implantation in the vitreous humor of rabbits. J Ocul Pharmacol Ther. 2015;31:11–16. doi: 10.1089/jop.2014.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campochiaro PA, Hafiz G, Shah SM, Bloom S, Brown DM, Busquets M, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117(1393–9):e3. doi: 10.1016/j.ophtha.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Campochiaro PA, Nguyen QD, Hafiz G, Bloom S, Brown DM, Busquets M, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120:583–587. doi: 10.1016/j.ophtha.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Bailey C, Loewenstein A, Massin A. Intravitreal corticosteroids in diabetic edema: pharmacokinetic considerations. Retina. 2015;35(12):2440–2449. doi: 10.1097/IAE.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol. 1965;4:198–205. [PubMed] [Google Scholar]
- 26.Becker B, Hahn KA. Topical corticosteroids and heredity in primary open-angle glaucoma. Am J Ophthalmol. 1964;57:543–551. doi: 10.1016/0002-9394(64)92500-0. [DOI] [PubMed] [Google Scholar]
- 27.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- 28.Overby DR, Bertrand J, Tektas OY, Boussommier-Calleja A, Schicht M, Ethier CR, et al. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci. 2014;55:4922–4933. doi: 10.1167/iovs.14-14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211(3):126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- 30.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 31.Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–2132. doi: 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Boyer DS, Yoon YH, Belfort R, Jr, Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Public Assessment Report (PAR) for ILUVIEN® 190 micrograms intravitreal implant in applicator (PL 41472/0001; UK/H/3011/001/E01).
- 34.Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–635. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Ramchandran RS, Fekrat S, Stinnett SS, Jaffe GJ. Fluocinolone acetonide sustained drug delivery device for chronic central retinal vein occlusion: 12-month results. Am J Ophthalmol. 2008;146:285–291. doi: 10.1016/j.ajo.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915–923. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 37.Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 38.Haller JA, Dugel P, Weinberg DV, Chou C, Whitcup SM. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina. 2009;29:46–51. doi: 10.1097/IAE.0b013e318188c814. [DOI] [PubMed] [Google Scholar]
- 39.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(1134–46):e3. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Ansari EA, Ali N. Intraocular pressure following intravitreal injection of triamcinolone acetonide. Open Ophthalmol J. 2008;2:119–122. doi: 10.2174/1874364100802010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112:593–598. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 42.Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003;87:24–27. doi: 10.1136/bjo.87.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inatani M, Iwao K, Kawaji T, Hirano Y, Ogura Y, Hirooka K, et al. Intraocular pressure elevation after injection of triamcinolone acetonide: a multicenter retrospective case–control study. Am J Ophthalmol. 2008;145:676–681. doi: 10.1016/j.ajo.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122:72–78. doi: 10.1016/j.ophtha.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 45.Thakur A, Kadam R, Kompella UB. Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol. 2011;129:914–920. doi: 10.1001/archophthalmol.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bressler NM, Bauchner H. Expression of concern: Thakur A, Kadam R, Kompella UB. Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol. 2011;129(7):914–20. JAMA Ophthalmol. 2015;133:375. [DOI] [PMC free article] [PubMed]
- 47.Gillies MC, Simpson JM, Gaston C, Hunt G, Ali H, Zhu M, et al. Five-year results of a randomized trial with open-label extension of triamcinolone acetonide for refractory diabetic macular edema. Ophthalmology. 2009;116:2182–2187. doi: 10.1016/j.ophtha.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 48.Lam WC, Albiani DA, Yoganathan P, Chen JC, Maberley DA, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:255–268. doi: 10.2147/OPTH.S80500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzarella S, Mateo C, Freixes S, Bures-Jelstrup A, Rios J, Navarro R, et al. Effect of intravitreal injection of dexamethasone 0.7 mg (Ozurdex(R)) on intraocular pressure in patients with macular edema. Ophthalmic Res. 2015;54:143–149. doi: 10.1159/000438759. [DOI] [PubMed] [Google Scholar]
- 50.Erginay A, Dupas B, Garrec J, Tadayoni R, Massin P. Real-life experience following the usage of 0.2 mg/day fluocinolone acetonide implant in diabetic macular edema patients. In: ARVO annual meeting, May 3–7, 2015, Denver, Colorado.
- 51.Manna A, Dhillon N, Patra S, Vemala R, Tsaloumas M. Multicentre case series evaluating intravitreal fluocinolone implant in the treatment of patients with chronic diabetic macular edema (DME) insufficiently responsive to current treatment options. In: 15th Euretina Congress, 17–20 September 2015, Nice, France.
- 52.Gordon MO, Kass MA. The ocular hypertension treatment study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 53.Miglior S, Pfeiffer N, Torri V, Zeyen T, Cunha-Vaz J, Adamsons I. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 54.Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963;70:500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- 55.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. Ii. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- 56.Dot C, El Chehab H, Russo A, Agard E. Ocular hypertension after intravitreal steroid injections: clinical update as of 2015. J Fr Ophtalmol. 2015;38:656–664. doi: 10.1016/j.jfo.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Breusegem C, Vandewalle E, Van Calster J, Stalmans I, Zeyen T. Predictive value of a topical dexamethasone provocative test before intravitreal triamcinolone acetonide injection. Invest Ophthalmol Vis Sci. 2009;50:573–576. doi: 10.1167/iovs.08-2625. [DOI] [PubMed] [Google Scholar]
- 58.European Medicines Agency. Ozurdex 700 micrograms intravitreal implant in applicator. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001140/WC500095499.pdf.
- 59.European Medicines Agency. ILUVIEN 190 micrograms intravitreal implant in applicator. Summary of product characteristics. http://www.medicines.org.uk/emc/medicine/27636.
- 60.van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112:1177–1185. doi: 10.1016/j.ophtha.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 61.Digiuni M, Fogagnolo P, Rossetti L. A review of the use of latanoprost for glaucoma since its launch. Expert Opin Pharmacother. 2012;13:723–745. doi: 10.1517/14656566.2012.662219. [DOI] [PubMed] [Google Scholar]
- 62.Bandello F, Preziosa C, Querques G, Lattanzio R. Update of intravitreal steroids for the treatment of diabetic macular edema. Ophthalmic Res. 2014;52:89–96. doi: 10.1159/000362764. [DOI] [PubMed] [Google Scholar]
- 63.Stewart MW. Corticosteroid use for diabetic macular edema: old fad or new trend? Curr Diab Rep. 2012;12:364–375. doi: 10.1007/s11892-012-0281-8. [DOI] [PubMed] [Google Scholar]
- 64.Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–251. doi: 10.1001/archophthalmol.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Googe J, Brucker AJ, Bressler NM, Qin H, Aiello LP, Antoszyk A, et al. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina. 2011;31:1009–1027. doi: 10.1097/IAE.0b013e318217d739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120:1843–1851. doi: 10.1016/j.ophtha.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 67.Ramu J, Yang Y, Menon G, Bailey C, Narendran N, Bunce C, et al. A randomized clinical trial comparing fixed vs pro-re-nata dosing of Ozurdex in refractory diabetic macular oedema (OZDRY study) Eye (Lond). 2015;29:1603–1612. doi: 10.1038/eye.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heng LZ, Sivaprasad S, Crosby-Nwaobi R, Saihan Z, Karampelas M, Bunce C, et al. A prospective randomised controlled clinical trial comparing a combination of repeated intravitreal Ozurdex and macular laser therapy versus macular laser only in centre-involving diabetic macular oedema (OZLASE study). Br J Ophthalmol. 2015. [DOI] [PubMed]
- 69.Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121:2473–2481. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]