Highlights
-
•
The largest renal tumour {3.63 kg} removed in the Western World-the second largest in the World.
-
•
A road map of the steps of removal.
-
•
A pathological review of leiomyosarcomas of the kidney.
Keywords: Renal leiomyosarcoma, Western World’s largest renal tumour
Abstract
Introduction
This paper describes the technique employed for the removal of the largest renal tumour in the Western Hemisphere and the second largest in the World. It is a road map for Surgeons in Training and should be of interest to other Surgeons/Urologists.
This tumour weighed 3.63 kg; the world's largest weighed 5.44 kg.
Presentation of case
A 52 year old male presented with a one year history of progressive weight loss, a gradually enlarging abdomen and no other admissible symptom, including no haematuria. The mass started on his left side of the abdomen.
CT scans showed a large tumour arising from the left kidney.
Discussion
A combined Urological and vascular approach was chosen in view of the CT scans images of huge renal veins and collateral vessels.
The left pleural cavity was elevated by the mass pushing on the left diaphragm and the heart was also displaced cranially as the mass made its own space.
Bowels were displaced as the giant mass reached into his pelvis.
A thoraco abdominal supra12 rib bed approach was adopted. The rib was not resected nor was the pleural cavity opened.
Histological diagnosis was renal leiomyosarcoma.
Conclusion
Large renal tumours or masses are best approached by the Urologist with an experienced vascular/general surgeon as assistant as well as a skilled anesthetist/Intensivist.
Optimisation, critical care and early mobilization of the patient by a dedicated nursing staff are essential to minimize complications and ensure a successful end result.
The success of this operation underscores what is possible in developing countries.
1. Introduction
Renal tumours are known to grow to huge sizes because of their ability to create new vessels to support their growth and their propensity to produce erythropoietin.
In May 2015, The All India Institute of Medical Sciences published the removal of a 5.44 kg renal tumour [1], [2] as the largest renal tumour to be removed in the World, thereby surpassing the then existing record of a 2.72 kg removal in New Delhi some time earlier.
This paper describes the collaborative efforts of an experienced team of Urologist, Vascular surgeon and anesthesiologist, supported by a dedicated operating theatre staff and nurses, in removing a 3.63 kg renal tumour, the largest in the Western Hemisphere. This was done on December 02, 2015.
2. Case presentation
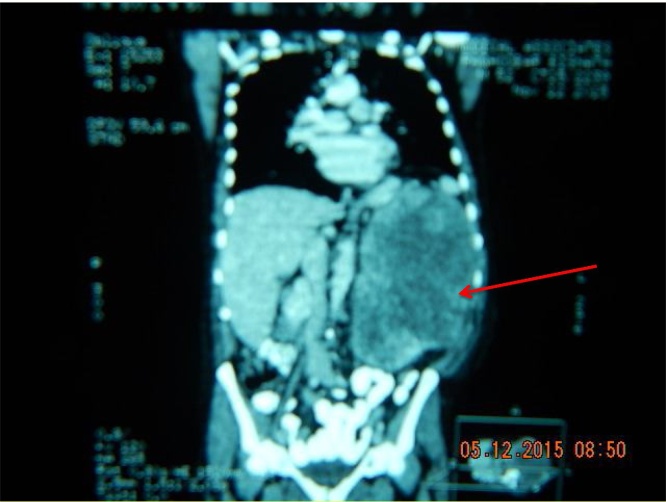
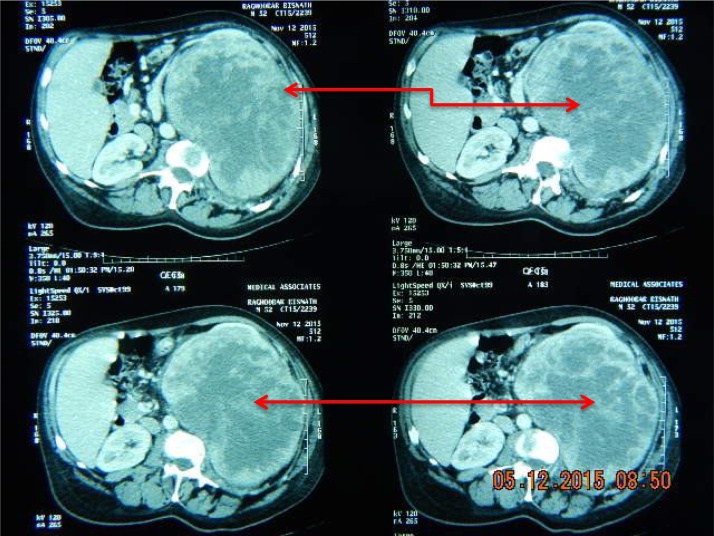
A careful preoperative study and discussion of the CT images, Fig. 1, Fig. 2 is the best preparation, in addition to the clinical examination of the patient. An understanding of the adjacent viscera, especially the vascular structures and close look at the renal vein, vena cava and neo vasculature structures are vital to a successful outcome.
Fig. 1.
The left renal tumour CT image.
Fig. 2.
Red arrows on CT images indicate renal mass.
The patient presented with major vascular challenges due to the very large size of the renal vessels and neovasculature, as the tumour impacted the heart by pushing it in a cephalic direction and compromised the left pleural space by causing the diaphragm to be elevated significantly into the left chest.
The tumour pushed the large bowels in all directions as it sought more space for growth.
Our patient had a mitral valve issue as well as scoliosis. The latter condition narrowed down the working space available to the surgeons.
The patient was placed in the “kidney position ‘ with lumbar elevation and “breaking’ the table to open up the lumbar space as much as possible.
Anticoagulation was not used for fear of uncontrollable bleeding.
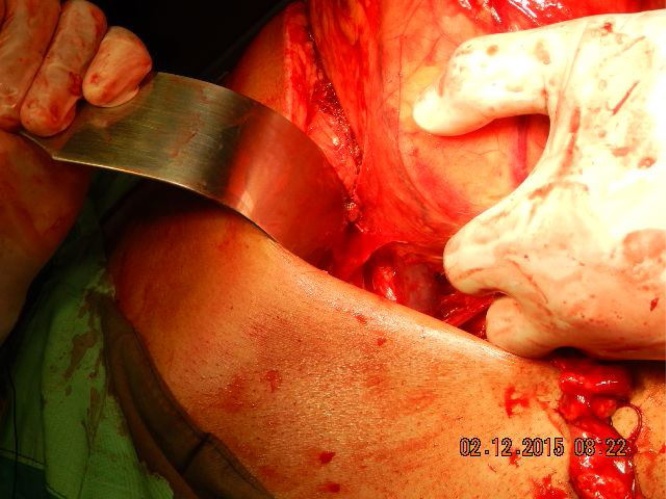
The incision was started in the thorax in the left bed of the 11th rib, just below the 11th and extended towards the abdominal cavity as far caudally as the umbilicus, Fig. 3. No rib resection was needed and the pleural cavity was not opened. An extrapleural approach was maintained throughout the procedure to avoid causing a pneumothorax thereby creating additional respiratory difficulties for the anesthesiologist.
Fig. 3.
Large Thoraco abdominal incision.
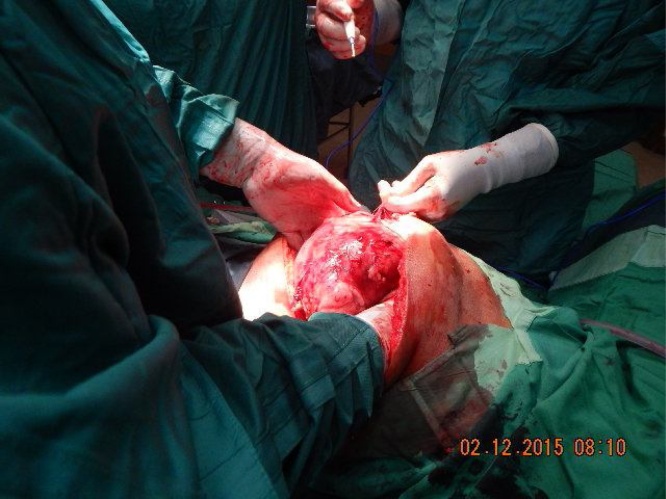
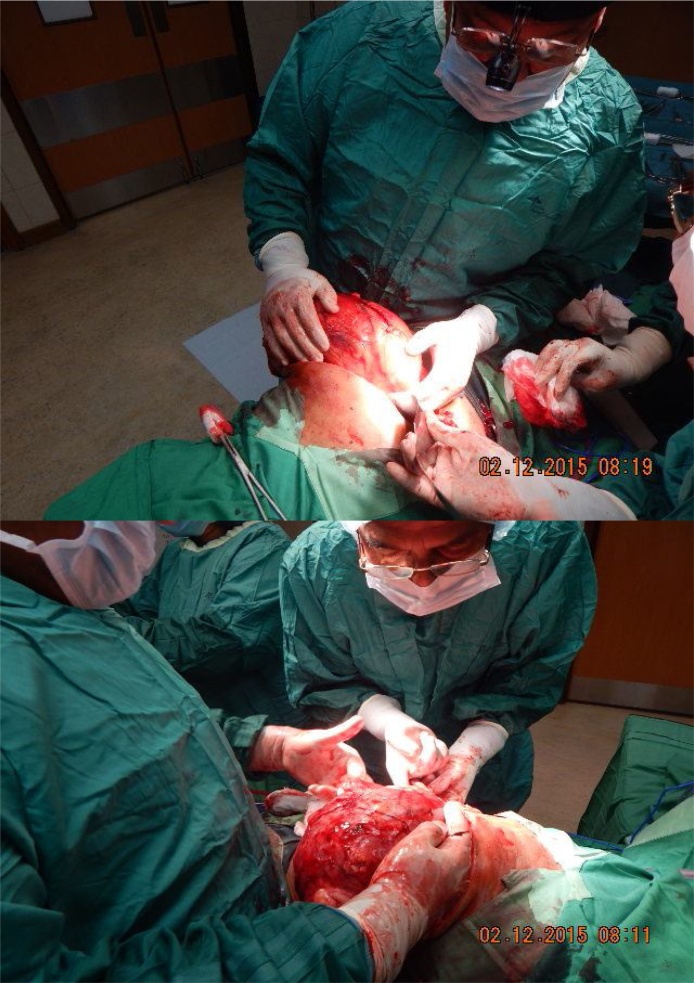
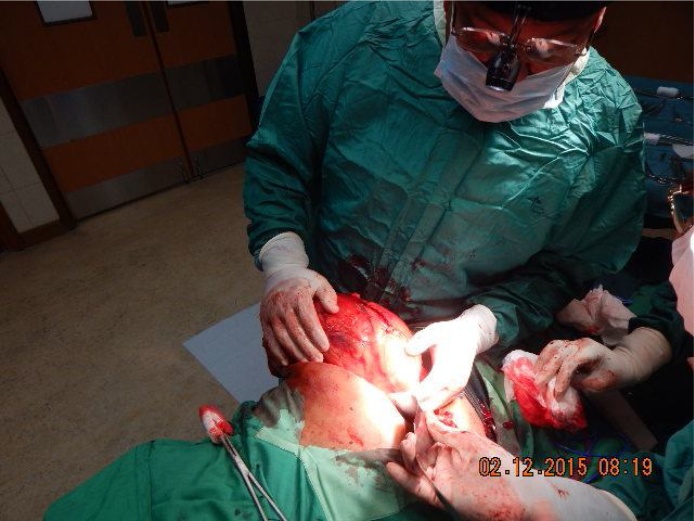
The incision into Gerota’s fascia was initiated on the left side of the tumour, Fig. 4, remaining well into the perirenal fat and Gerota’s fascia, far away as possible from the tumour itself.
Fig. 4.
Lateral wall of mass is being mobilized.
This plane was entered and opened up right along this margin to the adrenal area and carried forward over the renal mass.
The adrenal and other vessels from the upper pole of the mass, Fig. 5, were carefully separated.
Fig. 5.
Attention now to upper pole as its separated from adrenals.
The mass was abutting the pericardial region and a hand was gently persuaded between these structures pulling the renal mass downwards and laterally while sharply dividing all the attachments encountered as well as ligating them carefully.
Attention was next directed at the lower pole and this was mobilized and separated from adjacent bowel and delivered upwards.
At this time attention had to be placed on the pedicle of the mass due to its weight, as its supports were being removed by its dissection.
Any tug on this would avulse the pedicle, causing it to retract behind the large vessels and stemming the torrent of blood would have been life threatening. As such, the weight of the tumour, as it was delivered, was always supported by the hands {and abdomen} of the surgeon and assistant.
The pedicle was then dissected carefully and meticulously.
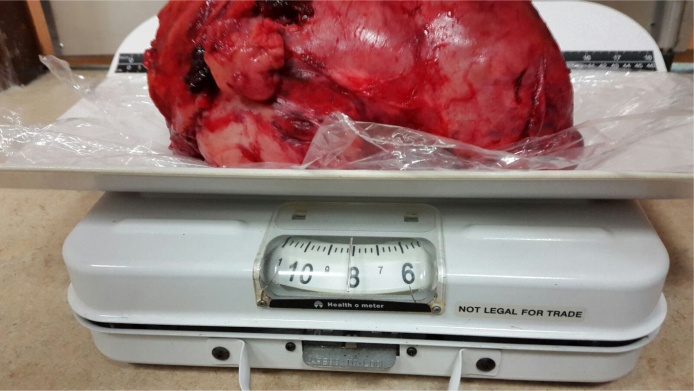
At surgery, the left renal mass was delivered into the wound by a series of traction and pulsion manoeuvres, Fig. 6. The single renal hilar artery and vein were dissected to the aorta and inferior vena cava respectively. They were both very dilated with the vein measuring about 2.5 cm and artery 2 cm. These vessels were skeletonized and transfixed, ligated with 1/0 silk. There was an extensive network of feeding vessels to the mass from surrounding structures. These vessels were all of significant sizes. They were all ligated with 0 vicryl and the tumour delivered and weighed, Fig. 7, Fig. 8.
Fig. 6.
Lateral and apical margins cleared.
Fig. 7.
The 8 pounder is now prepared for final delivery.
Fig. 8.
The specimen weighs in at 8 lbs.
The patient’s blood loss was estimated at 1200 mls.
His admission Haemoglobin level was 7.1 gm/dl.
Three Units of blood were transfused pre operatively and two units of blood were transfused perioperatively.
Immediate post operative haemoglobin was 10.4 gm/dl.
3. Discussion
This paper addresses the surgical and anesthetic challenges encountered in removing very large renal tumours.
In this case, the large size of the tumour posed special obstacles to its complete removal.
The patient had scoliosis, which reduced the working space; he also had mitral insufficiency which could have further complicated his operative course and post operative recovery.
Additionally, his left pleural cavity was reduced as the tumour pushed the diaphragm upwards and compromised the cavity.
It also pushed his heart cranially and to the right.
All his adjacent small and large bowels were pushed away as the tumour expanded into the pelvis. Multiple bowel adhesions were encountered.
All these factors were potential comorbid factors that could have hampered a successful outcome.
In such a small island as Trinidad a surgeon’s career is at risk as bad news travel quickly in an island and he is tried in the Courts of Public opinion and banished rather expeditiously.
Additionally, the island’s population is now well educated in matters medica and access to an abundance of over zealous lawyers willing to sue at no cost to the plaintiff is easy to obtain.
Hence, this type of case is extremely stressful for a Urologist and his team and having this road map to guide another encounter by another team should be very helpful.
4. Pathology of renal mass
4.1. Histopathology
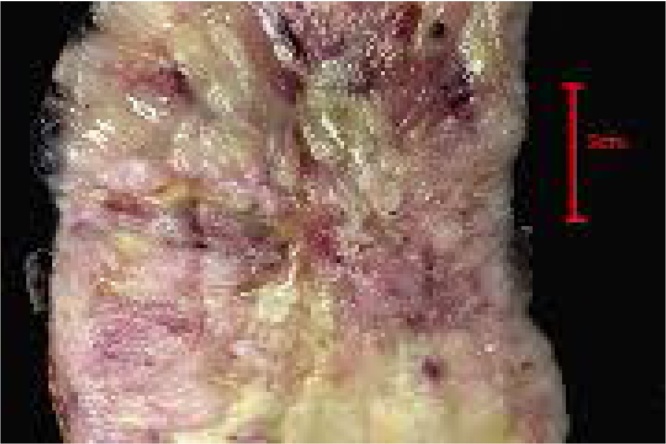
The left kidney was 230 × 129 × 175 mm and weighed 3701 g {8.159 lbs}. It was almost completely replaced by a 214 mm tumour which had a smooth bosselated surface and dilated subcapsular vessels. It had a firm solid grey cut surface with irregular zones of necrosis (Fig. 9). It was well circumscribed and showed no obvious involvement of the perinephric fat. Only a thin rim of residual renal parenchyma could be seen with obliteration of the calyces.
Fig. 9.
Bisected specimen showing near complete replacement by tumour with yellow/white zones of necrosis.
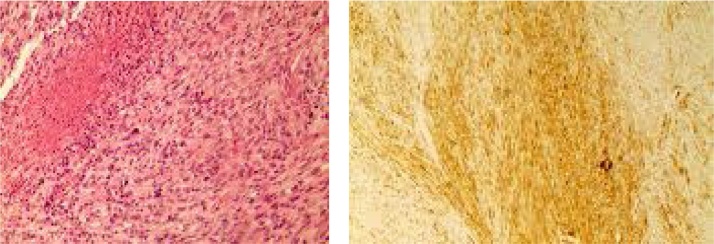
Histologically, this was a solid tumour formed of spindle cells with ample eosinophilic cytoplasm and ovoid blunt-ended nuclei (Fig. 10 left). The cells were arranged in interlacing bundles and fascicles. Focally there was increased cellular atypia characterized by enlarged bizarre nuclei and abnormal mitotic figures. There were zones of coagulative necrosis. The mitotic rate was 24 per high power field.
Fig. 10.
H&E × 200, left, Spindle cell pattern of tumour cells arranged in fascicles. There is coagulative type necrosis seen in the upper left aspect.
The immunohistochemical marker Smooth Muscle Actin (SMA), right, shows variably intense but diffuse positivity.
Immunohistochemically, the tumour showed diffuse expression of smooth muscle actin (Fig. 10 right). It lacked pancytokeratin expression and had no expression of Bcl-2. The tumour was diagnosed as a grade 3 leiomyosarcoma based on the French Federation of Cancer Centres System.
Spindle cell neoplasms of the kidney are not common. The possible diagnoses that were considered included sarcomatoid renal cell carcinoma, synovial sarcoma, leiomyosarcoma, angioleiomyoma, and leiomyoma. The malignant characteristics namely the mitotic rate, atypia and necrosis essentially eliminated the benign diagnoses of leiomyoma and angioleiomyoma. These are both benign mesenchymal tumours with smooth muscle differentiation.
Sarcomatoid renal cell carcinoma (RCC) occurs as a metaplastic transformation of malignant epithelial cells and therefore is not a true sarcoma [7]. It arises from a preexisting clear cell, papillary, chromophobe or collecting duct RCC. There were no preexisting typical RCC elements in this tumour. Cytokeratin expression supports a diagnosis of sarcomatoid RCC although at times these tumours may altogether loose such expression [8]. Our tumour had no cytokeratin expression. Generally these tumours display pronounced cytological atypia and do not show the fascicular arrangement seen in this case.
Primary renal synovial sarcoma is a recently described entity [9]. It may have a spindle cell pattern and is often accompanied by cyst formation. The tumour infiltrates the renal parenchyma and often encircles normal structures. This pattern of invasion was not seen in this lesion. Synovial sarcoma is consistently positive with the Bcl-2, CD99 and vimentin markers [10]. Our patient’s tumour did not display Bcl-2.
Leiomyosarcomas [9], [10] (LMS) of the kidney are rare tumours accounting for only 0.5–1% of all renal malignancies. They do, however, represent the most common renal sarcoma accounting for between 50 and 60% of all cases [11]. Histogenesis remains obscure but they are thought to arise from the renal parenchyma, capsule, main renal vein or smooth muscle of the renal pelvis or rarely from the background of renal angioleiomyoma [12].
Immunohistochemically the tumour cell express the markers smooth muscle actin, Desmin and h Caldesmon. Most are cytokeratin negative [13].
The prognosis of LMS is generally poor; 3 year survival rate was 20% and median survival was 18 months in one large series. The importance of grading the tumour is that most tumours with a low grade had relative good prognosis while those with high grade perform poorly [13], [14].
5. Conclusion
Large renal tumours or masses are best approached by the Urologist with an experienced vascular/general surgeon as assistant as well as a skilled anesthetist/Intensivist.
Optimisation, critical care and early mobilization of the patient by a dedicated nursing staff are essential to minimize complications and ensure a successful end result.
The success of this operation underscores what is possible in developing countries.
Conflicts of interest
Nothing to declare.
Funding
None.
Ethical approval
Patient consent obtained.
Consent
Written consent obtained from patient.
Author contribution
Lall R. Sawh, Urologist, team leader, wrote the Urological aspects of the paper.
Steve Budhooram, vascular surgeon, wrote the vascular aspect of the paper, assistant at surgery.
Peng Ewe, anesthesiologist.
Rudy Rattan, Pathologist. Wrote the Pathological aspects.
Sean L. Sawh collaborated on the study concepts and design of paper.
Sylvia Sawh, reviewed the paper for grammar, language and syntax issues.
Guarantor
Lall R. Sawh.
Acknowledgements
We would like to thank all the staff members of Southern Medical Services Ltd., San Fernando, Trinidad for their support and devotion to duty.
Special mention must be made of Susan Maharaj, head nurse of the operating theatre and her support staff led by Moncie Mathew.
Dr. Sean Sawh is recognised for assisting with compiling this work and for his support and research.
The authors wish to salute the founder of this Medical Center, Dr Rupert Indar Snr, for his visionary leadership as The Center approaches its 50th year of specialist services to the island of Trinidad & Tobago and the rest of the Caribbean.
Mrs. Sylvia Sawh, proof read the article for grammar and syntax corrections and the authors wish to thank her for her help.
Special thanks to the personal assistants, Patricia Ramkissoon and Hemawatee Sahadeo for assisting with the submission.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwj1-_6Guc3KAhULpR4KHb5ZBGcQFggdMAA&url=http%3A%2F%2Ftimesofindia.indiatimes.com%2Findia%2FAIIMS-surgeons-remove-worlds-largest-kidney-tumour-weighing-5-018-kg%2Farticleshow%2F47359053.=cms&usg=AFQjCNHSIU5RxZ0ONQHXlPf_ftBdM43Big&sig2 Q-KeB1HScgQipq-IpGs4Iw gest & third largest renal tumours removed in India.
- 2.‘world's largest kidney tumour' − Daily Mirror www.mirror.co.uk ›…› All-India Institute of Medical Sciences.
- 7.Farrow G.M., Harrison E.G., Jr., Utz D.C. Sarcomas and sarcomatoid and mixed malignant tumors of the kidney in adults I. Cancer. 1968;22(September (3)):545–550. doi: 10.1002/1097-0142(196809)22:3<545::aid-cncr2820220308>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Balercia G., Bhan A.K., Dickersin G.R. Sarcomatoid carcinoma: an ultrastructural study with light microscopic and immunohistochemical correlation of 10 cases from various anatomic sites. Ultrastruct. Pathol. 1995;19:249–263. doi: 10.3109/01913129509064228. [DOI] [PubMed] [Google Scholar]
- 9.Argani P., Faria P.A., Epstein J.I. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am. J. Surg. Pathol. 2000;24(August (8)):1087–1096. doi: 10.1097/00000478-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kim D.H., Sohn J.H., Lee M.C. Primary synovial sarcoma of the kidney. Am. J. Surg. Pathol. 2000;24(August (8)):1097–1104. doi: 10.1097/00000478-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Deyrup A.T., Montgomery E., Fisher C. Leiomyosarcoma of the kidney: a clinicopathologic study. Am. J. Surg. Pathol. 2004;28(February (2)):178–182. doi: 10.1097/00000478-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Ng W.D., Chan K.W., Chan Y.T. Primary leiomyosarcoma of renal capsule. J. Urol. 1985;(May (5)):834–835. doi: 10.1016/s0022-5347(17)49245-7. [DOI] [PubMed] [Google Scholar]
- 13.Sharma D., Pradhan S., Aryya N.C. Leiomyosarcoma of kidney: a case report with long term result after radiotherapy and chemotherapy. Int. Urol. Nephrol. 2007;39(2):397–400. doi: 10.1007/s11255-006-9022-8. [DOI] [PubMed] [Google Scholar]
- 14.Grignon D.J., Ayala A.G., Ro J.Y. Primary sarcomas of the kidney. A clinicopathologic and DNA flow cytometric study of 17 cases. Cancer. 1990;65(April (7)):1611–1618. doi: 10.1002/1097-0142(19900401)65:7<1611::aid-cncr2820650727>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]