Highlights
-
•
Management of cervical leiomyosarcoma in pregnancy requires a multidisciplinary approach.
-
•
Ovarian preservation is preferred in young patients with early stage cervical leiomyosarcoma.
-
•
Routine lymphadenectomy in patients with early stage cervical leiomyosarcoma is not useful.
1. Introduction
Genital sarcomas in pregnancy are extremely rare. A review by Matsuo et al. covering the period 1955 to 2007 revealed a total of 40 cases of female genital sarcomas diagnosed during pregnancy; 37.5% uterine, 27.5% retroperitoneal, 22.5% vulvar sarcoma, and 12.5% vaginal (Matsuo et al., 2009). Mean age at diagnosis was 27.8 years and the majority of cases were diagnosed in the third trimester. The 5 year survival for all patients was quite poor at 22.2%. Leiomyosarcoma of the uterine cervix associated with pregnancy has not been previously reported. In this report we describe the case of a gravid patient with a leiomyosarcoma confined to the cervix.
1.1. Case
An 18 year-old female Gravida 3 Para 1011 presented to an outside clinic with several months of vaginal bleeding and abdominal pain. She was diagnosed with an intrauterine pregnancy with an estimated gestational age (EGA) of 27 weeks based on dating by ultrasound. She was also noted to have an exophytic mass protruding from the endocervical canal. Subsequent examination under anesthesia revealed a 4.2 × 3.9 cm, pedunculated mass, arising from the endocervical canal. The mass was resected and pathology was consistent with a leiomyosarcoma.
The patient was referred to our institution at 31 weeks EGA for consultation with maternal fetal medicine (MFM) and gynecologic oncology. Physical examination was consistent with a normally developed young woman, normotensive, with a BMI of 26. Ultrasound evaluation showed an appropriately grown female fetus with a normal anatomic survey. Pelvic examination showed normal external female genitalia and vaginal tube. The cervix was 50% effaced and the external cervical os was 1 cm dilated. There was a remnant of a stalk at the 9:00 position in the proximal endocervical canal.
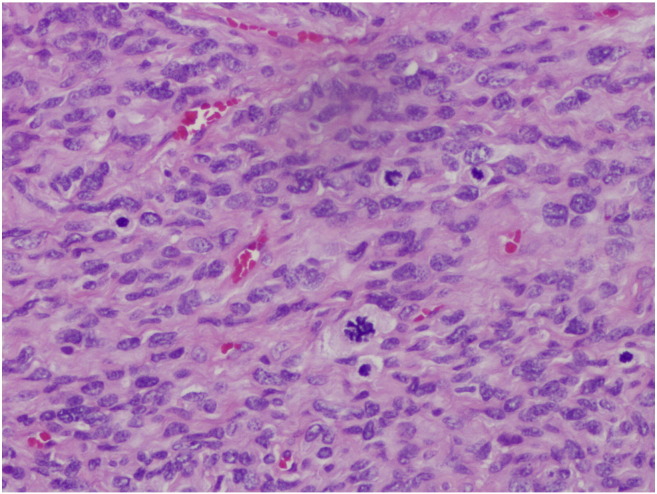
Pathology review at our institution revealed a 4.2 cm smooth polypoid fleshy mass on a narrow stalk. Microscopic examination revealed a cellular spindle cell neoplasm composed of highly atypical and focal bizarre giant cells with a high mitotic rate (11 mitoses/10 high power fields). Marked cytologic atypia was diffuse, but tumor cell necrosis was not prominent (Fig. 1, Fig. 2). Subsequent immunostaining showed the tumor to be focally positive for smooth muscle actin and muscle specific actin. Desmin, caldesmon, AE1/AE3, S100 and HMB45 were negative; p53 stained a few scattered nuclei; p16 showed strong diffuse block staining; Ki67 showed a high proliferation rate. A diagnosis of high grade leiomyosarcoma was made which was confirmed by expert consultation. The non-ulcerated surface was covered by benign endocervical epithelium confirming a submucosal configuration (Fig. 3). The tumor appeared to involve the entire polyp. While there was a small amount of stroma at the surgical margin, a definitive assessment of the margin status was not possible.
Fig. 1.
The tumor is composed of atypical spindle cells (H&E, 20 ×).
Fig. 2.
The tumor contains numerous mitoses (H&E, 400 ×).
Fig. 3.
The pedunculated tumor is lined by endocervical epithelium which is apposed to the underlying endocervical canal (H%E, 400x).
Computerized tomography of the chest, abdomen, and pelvis demonstrated a gravid uterus with no evidence of metastatic disease. After counseling by MFM and based on the pathology showing a high grade leiomyosarcoma the decision was made for delivery of the fetus at 33 to 34 weeks. She completed corticosteroids at 31 2/7 weeks. She was admitted with preterm labor and received a rescue dose of corticosteroids at 33 1/7 weeks. Assessment of fetal lung maturity was not performed. The patient desired definitive management of the high grade leiomyosarcoma and underwent primary low transverse cesarean section followed by exploratory laparotomy, total abdominal hysterectomy, and bilateral salpingectomy at 33 2/7 weeks. She delivered a viable female infant with APGARS 6 and 8 and weight of 2180 g. Intra-operative findings included normal ovaries and fallopian tubes and no evidence of extra-uterine disease. Final pathology showed no residual leiomyosarcoma. The patient had an uncomplicated postoperative course and has no evidence of recurrent disease by clinical exam and CT scan of the chest, abdomen, and pelvis at 13 months from her diagnosis. The infant was admitted to the neonatal intensive care unit post-delivery. She was treated for 3 days with phototherapy for hyperbilirubinemia. She required supplemental oxygen therapy for 9 days for transient tachypnea of the new born. She was discharge to home on day of life 19 with no feeding or other issues. At 1 year of life her pediatric examination showed normal growth and she had met her developmental milestones.
2. Discussion
We performed a literature search in PubMed and Medline databases without language restriction from 1960 to 2016. All articles were initially screened for title and abstract and full texts of eligible articles were subsequently selected. The search terms were “sarcoma, cervix, pregnancy, and leiomyosarcoma”. Four case reports were discovered. Table 1 shows a summary of reports of cervical sarcomas in pregnancy. The 3 early case reports did not characterize the type of sarcomas using modern diagnostic criteria and nomenclature (Diaz-Bazan and Masferrer, 1960, Adinolfi and Persico, 1961, Onarir et al., 1967). The modern diagnosis of leiomyosarcoma is based on the work of Bell et al. (1994) and includes a combination of features including diffuse moderate to severe cellular atypia, a mitotic count of ≥ 10 MFs/10 HPFs, and coagulative tumor cell necrosis (Bell et al., 1994). Generally tumors with any 2 of these 3 features are diagnosed as clinically malignant leiomyosarcoma. The clinical circumstances of the case report by Schiavone et al. was quite similar to the current case, although the sarcoma in their case was called “high-grade” rather than leiomyosarcoma based on inconclusive immunohistochemical staining (Schiavone et al., 2011).
Table 1.
Literature review of cervical sarcoma in pregnancy.
| Source | Age, y | Gravity, parity | EGA | Mode of diagnosis | Gross findings | Microscopic diagnosis | Treatment | Pathology | Follow up |
|---|---|---|---|---|---|---|---|---|---|
| Diaz-Bazan and Masferrer (1960) | 24 | G3P2 | 7 | Dilation and evacuation. Excision of cervical lesion | Cervical lesion (NOS) | Polypoid form, fusiform type, sarcoma | Radical hysterectomy, BSO, LND | No residual sarcoma | NED at 5 years |
| Adinolfi and Persico (1961) | 29 | G7P5025 | pp | Cervix biopsies | 5 cm cervical mass | Small blue cell, alveolar type, sarcoma | Hysterectomy and radiation therapy | Residual sarcoma | ND |
| Onarir et al. (1967) | 30 | ND | 9 | Cervix biopsies | Cervical lesion (NOS) | Sarcoma (NOS) | Hysterectomy with fetus in-utero | Residual sarcoma | ND |
| Schiavone et al. (2011) | 27 | G2P1 | 25 | Removal of endocervical polyp | Small endocervical polyp | High grade sarcoma (NOS) | Cesarean hysterectomy, healthy fetus |
No residual sarcoma | ND |
| Whitcombe et al. | 18 | G2P1 | 27 | Excision of endocervical mass | 4 cm mass endocervical mass | Leiomyosarcoma | Cesarean hysterectomy, healthy fetus | No residual sarcoma | NED at 1 year |
Note: y — year; PP — postpartum; EGA — estimated gestational age; NOS — not otherwise specified; BSO — bilateral salpingo-oophorectomy; LND — pelvic and para-aortic lymph node dissection; NED — no evidence of disease; ND — no data.
The diagnosis of leiomyosarcoma diagnosed during pregnancy requires expert pathological evaluation. The diagnosis of sarcoma in pregnancy can be difficult due to pregnancy induced histologic changes seen in benign leiomyomata including hemorrhage, necrosis, and degenerative changes. Muezzinoglu et al. in a review of leiomyomas in pregnancy described several cases of smooth muscle tumors with extensive hyaline and infarct necrosis (Muezzinoglu and Corakci, 1996). Coagulative cell necrosis was not observed. Cellular atypia when present was mild and focal and usually associated with hyaline type necrosis. Mitotic activity was low, less than 5/10 HPF. The diagnosis of leiomyosarcomas that meet the “three-feature” Bell criteria are considered high grade tumors (Bell et al., 1994). In the current case the tumor met the criteria proposed by Bell and was considered a high grade leiomyosarcoma.
Fertility sparing management options should be considered and discussed with patients desirous of future childbearing. In certain selected cases, surgical procedures such as myomectomy, polypectomy, and conization, during or after pregnancy, and dilation and curettage after delivery, could be performed. If no residual disease is found, surveillance rather than hysterectomy could be considered. Good outcomes and subsequent pregnancy have been reported with conservative surgery for patents with uterine smooth muscle tumor of uncertain malignant potential and low grade uterine leiomyosarcoma (Campbell et al., 2015, Salman et al., 2007). In the current case expert pathologic examination confirmed a high grade cervical leiomyosarcoma. Extrapolating from the literature on uterine leiomyosarcoma, high grade has been shown to a significant risk factor for recurrence and death and hysterectomy is considered the treatment of choice (Giuntoli et al., 2003).
In the current case CT scan was used as the imaging modality and the chest, abdomen, and pelvis were evaluated. The dose of radiation delivered to the fetus was 20 to 50 mGy which is below the dose threshold for malformations, growth retardation, mental retardation, and death; however, the risk of carcinogenesis increases approximately by a factor of 2, although it remains low in absolute term, less than one in 250 (Tremblay et al., 2012). Ultrasound and magnetic resonance imaging are not associated with risk and are the imaging modalities of choice in a pregnant patient.
The decision to deliver at 33 2/7 weeks EGA was based on several factors. The timing of delivery is based on the balance between the risk of cancer progression and the risk of delivery of a premature fetus. Leiomyosarcomas are aggressive tumors and patients whose disease appears to be initially confined to the uterus may experience a clinically aggressive disease course (Giuntoli et al., 2003). There is quite limited experience with leiomyosarcomas in pregnancy. Based on the uncertain pathologic margin status and the appearance of a residual cervical stalk on speculum exam there was a concern for residual disease. The patient was extensively counseled concerning natural history of high grade leiomyosarcomas and the risks of premature delivery at 33 2/7 weeks.
Prevention of iatrogenic prematurity is an important part of the treatment strategy for managing pregnant patients with cancer. Van Calsteren et al. recently published their experience examining 215 pregnancies complicated by cancer (Van Calsteren et al., 2010). The authors found that iatrogenic prematurity complicated 54.2% of cases. ACOG defines late preterm birth as the delivery of an infant between 34 and 36 weeks and 6 days of gestation. During the birth hospitalization, the late preterm infants compared with term infants are more likely to have hypothermia, hypoglycemia, respiratory distress, apnea, hyperbilirubinemia, and feeding difficulties (Leone et al., 2012). There is conflicting data concerning long-term neurodevelopmental morbidity including cognition, achievement, social skills, and behavioral/emotional problems (Gurka et al., 2010, Harris et al., 2013). Our institution is a tertiary care, referral medical center, with a level 4 NICU, with maternal fetal medicine and gynecologic oncology services. The intact survival at 33 to 34 weeks at our institution very much depends on the condition of the fetus, i.e. presence or absence of congenital abnormalities, IUGR, etc., and the indication for delivery, i.e. premature rupture of membranes, intra-amniotic infection, etc. In the current case we had a healthy, appropriately grown, fetus and the indication for delivery was based on maternal indications. At our institution the intact survival in this scenario is the same as term infants. At 1 year of life the infant shows normal growth and neurocognitive development. It is important that pregnant cancer patients are treated in a multidisciplinary setting with access to maternal fetal medicine specialists, neonatologists, and oncologists. It is imperative that these patients are treated in a hospital with access to neonatal intensive care units as iatrogenic preterm delivery in not uncommon. Long term care of the premature infant should include regular neurocognitive and developmental assessments.
A simple hysterectomy rather than radical hysterectomy was performed in the current case. The tumor was clinically confined to the cervix with no parametrial or vaginal extension. Leiomyosarcomas typically metastasize via a hematogenous route. There have been no reported cases of cervical leiomyosarcoma with occult parametrial involvement and lymphatic metastasis is a rare event (Bansal et al., 2010). In contrast, carcinoma of the cervix typically invades the cervical stroma and lymphatic vascular spaces with subsequent metastasis to the parametrial and pelvic lymph nodes (Girardi et al., 1989). The radical hysterectomy is used primarily to treat early stage carcinoma of the cervix. Surgical management includes removal of the primary tumor with hysterectomy and assessment for metastatic disease. Exploration of the abdomen and pelvis is performed and suspicious lesions biopsied. The ovaries may be preserved in selected patients with early-stage uterine leiomyosarcoma who wish to retain hormonal function. No difference in disease-specific survival and recurrence rates were found between patients with and without oophorectomy (Kapp et al., 2008, Leitao et al., 2003). In the current case based on the patient's young age and extrapolating from the uterine data, the ovaries were preserved. Routine lymphadenectomy at primary surgery is not recommended for uterine leiomyosarcoma. Lymphadenectomy failed to have any prognostic significance, as the 5-year disease-specific survival was similar between those who had and had not undergone lymphadenectomy (Kapp et al., 2008, Leitao et al., 2003). Bansal et al. analyzing SEER data from 1988 to 2005 identified 67 non-gravid patients with cervical leiomyosarcoma (Bansal et al., 2010). None of the patients had positive lymph nodes. In the current case there was no evidence of lymphadenopathy on CT scan or at surgical exploration and a lymphadenectomy was not performed. Our patient did not receive postoperative chemotherapy. Adjuvant chemotherapy for patients with stage I uterine leiomyosarcoma is controversial (Hensley et al., 2013).
The surveillance recommendations for high grade cervical leiomyosarcoma were extrapolated from the NCCN guidelines version 2.2016 for high grade uterine sarcoma (Guidelines, 2015). The recommendations include history and physical examination every 3 months for 2 years, then every 6 to 12 months, CT imaging of the chest, abdomen, and pelvis every 3 to 6 months for 2 to 3 years, then every 6 months for the next two years, then annually, and education regarding the symptoms of potential recurrence, lifestyle, obesity, exercise, and nutrition, and smoking cessation counseling. Patients should be educated regarding sexual health, vaginal dilator use, and vaginal lubricants and moisturizers.
In summary, we report a case of leiomyosarcoma of the cervix diagnosed in pregnancy with a good outcome for both mother and baby. We advocate expert pathology review in the clinical setting of suspected sarcoma in pregnancy. The decision of timing of delivery is made after thorough patient counseling by MFM, neonatology, and oncology concerning the risks of prematurity and the risk of cancer progression. Due to the rarity of cervical leiomyosarcomas management recommendations are based on data extrapolated from experience with uterine leiomyosarcoma; including hysterectomy, ovarian preservation, and omission of lymphadenectomy.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
The authors have no conflict of interest to disclose.
References
- Adinolfi G., Persico M. Primary sarcoma of the cervix in pregnancy. Arch. Ostet. Ginecol. 1961;66:174–186. [PubMed] [Google Scholar]
- Bansal S., Lewin S.N., Burke W.M., Deutsch I. Sarcoma of the cervix: natural history and outcomes. Gynecol. Oncol. 2010;118:134–138. doi: 10.1016/j.ygyno.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Bell S.W., Kempson R.L., Hendrickson M.R. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am. J. Surg. Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- Campbell J.E., Knudtson J.F., Valente P.T., Robinson R.D., Kost E.R. Successful pregnancy following myomectomy for uterine smooth muscle tumor of uncertain malignant potential: A case report and review of the literature. Gynecol. Oncol. Rep. 2015 Aug 7;15:1–3. doi: 10.1016/j.gore.2015.07.005. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Bazan N., Masferrer R. Cervical sarcoma associated with pregnancy. Review of the literature and report of a case. Arch. Col. Med. El Salv. 1960;13:157–172. [PubMed] [Google Scholar]
- Girardi F., Lichteneggar W., Tamussino K., Haas J. The importance of parametrial lymph nodes in the treatment of cervical cancer. Gynecol. Oncol. 1989;34:206–211. doi: 10.1016/0090-8258(89)90143-1. [DOI] [PubMed] [Google Scholar]
- Giuntoli R.L., II, Metzinger D.S., DiMarco C.S., Cha S.S. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol. Oncol. 2003;89:460–469. doi: 10.1016/s0090-8258(03)00137-9. [DOI] [PubMed] [Google Scholar]
- NCCN Guidelines . 2015. Uterine sarcoma. Version 2.2016, 11/12/2016, National Comprehensive Cancer Network, Inc. [DOI] [PubMed] [Google Scholar]
- Gurka M.J., LoCasale-Crouch J., Blackman J.A. Long-term cognition, achievement, socioeconomic, and behavioral development of healthy late-preterm infants. Arch. Pediatr. Adolesc. Med. 2010;164:525. doi: 10.1001/archpediatrics.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M.N., Voigt R.G., Barbaresi W.J. ADHD and learning disabilities in former late preterm infants: a population-based birth cohort. Pediatrics. 2013;132:647. doi: 10.1542/peds.2012-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley M.L., Wathen J.K., Maki R.G., Araujo D.M. Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma. Cancer. 2013;119:1555–1561. doi: 10.1002/cncr.27942. [DOI] [PubMed] [Google Scholar]
- Kapp D.S., Shin J.Y., Chan J.K. Prognostic factors and survival in 1396 patients with uterine leiomyosarcoma: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112:820–830. doi: 10.1002/cncr.23245. [DOI] [PubMed] [Google Scholar]
- Leitao M.M., Sonoda Y., Brennan M.F., Barakat R.B. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol. Oncol. 2003;91:209–212. doi: 10.1016/s0090-8258(03)00478-5. [DOI] [PubMed] [Google Scholar]
- Leone A., Ersfeld P., Adams M. Neonatal morbidity in singleton late preterm infants compared with full-term infants. Acta Paediatr. 2012;101 doi: 10.1111/j.1651-2227.2011.02459.x. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Eno M.L., Im D.D., Rosenshein N.B. Pregnancy and genital sarcoma: a systemic review of the literature. Am. J. Perinatol. 2009;26(7):507–518. doi: 10.1055/s-0029-1215428. [DOI] [PubMed] [Google Scholar]
- Muezzinoglu B., Corakci A. Pathological characteristics and clinical outcome of uterine leiomyomas associated with pregnancy. Pathol. Res. Pract. 1996;207(11):691–694. doi: 10.1016/j.prp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Onarir R., Hilagu C., Kazancigil A. Sarcoma of the uterine cervix observed during pregnancy. Tip Fak. Mecm. 1967;30(1):113–117. [PubMed] [Google Scholar]
- Salman M.C., Guler O.T., Kucukali T., Karaman N., Ayhan A. Fertility-saving surgery for low-grade uterine leiomyosarcoma with subsequent pregnancy. Int. J. Gynaecol. Obstet. 2007;98(2):160–161. doi: 10.1016/j.ijgo.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Schiavone M.B., Smok D., Wright T.C., Wright J.D. High-grade uterine sarcoma during pregnancy. Gynecol. Oncol. 2011;123:424–425. doi: 10.1016/j.ygyno.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Therasse E., Thomassin-Naggara I., Trop I. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics. 2012;32:897–911. doi: 10.1148/rg.323115120. [DOI] [PubMed] [Google Scholar]
- Van Calsteren K., Heyns L., De Smet F., Van Eycken L., Gziri M.M., Van Gemert W., Halaska M., Vergote I., Ottevanger N., Amant F. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and neonatal outcomes. J. Clin. Oncol. 2010 Feb 1;28(4):683–689. doi: 10.1200/JCO.2009.23.2801. [DOI] [PubMed] [Google Scholar]