Abstract
Background
Schizotypal personality disorder (SPD) is a schizophrenia-spectrum disorder characterized by odd or bizarre behavior, strange speech, magical thinking, unusual perceptual experiences, and social anhedonia. Schizophrenia proper has been associated with anomalies in dopaminergic neurotransmission and deficits in neurophysiological markers of self-monitoring, such as low amplitude in cognitive event-related brain potentials (ERPs) like the error-related negativity (ERN), and the error positivity (Pe). These components occur after performance errors, rely on adequate fronto-striatal function, and are sensitive to dopaminergic modulation. Here we postulated that analogous to observations in schizophrenia, SPD individuals would show deficits in self-monitoring, as measured by the ERN and the Pe. We also assessed the capacity of dopaminergic antagonists to reverse these postulated deficits.
Methods
We recorded the electroencephalogram (EEG) from 9 SPD individuals and 12 healthy controls in two separate experimental sessions while they performed the Eriksen Flanker Task, a classical task recruiting behavioral monitoring. Participants received a placebo or 1 mg risperidone according to a double-blind randomized design.
Results
After placebo, SPD individuals showed slower reaction times to hits, longer correction times following errors and reduced ERN and Pe amplitudes. While risperidone impaired performance and decreased ERN and Pe in the control group, it led to behavioral improvements and ERN amplitude increases in the SPD individuals.
Conclusions
These results indicate that SPD individuals show deficits in self-monitoring analogous to those in schizophrenia. These deficits can be evidenced by neurophysiological measures, suggest a dopaminergic imbalance, and can be reverted by dopaminergic antagonists.
Keywords: Schizotypal personality disorder, Behavioral monitoring, Neurophysiology, Error-related negativity, Dopaminergic antagonism
Highlights
-
•
We assessed self-monitoring in schizotypal personality disorder (SPD) using ERPs.
-
•
SPD patients showed reduced amplitude of the error-related negativity (ERN).
-
•
Dopamine antagonism improved behavior and enhanced the ERN in patients only.
-
•
An inhibited ERN is a potential endophenotype of schizophrenia-spectrum disorders.
-
•
Results show impaired self-monitoring in SPD associated with dopamine disbalance.
1. Introduction
Schizotypal personality disorder (SPD) is characterized by odd or bizarre behavior, strange speech, magical thinking, unusual perceptual experiences, and social anhedonia according to the DSM-5 (American Psychiatric Association, 2013). From a nosological perspective, a categorical approach has conceptualized SPD as an isolated personality disorder that can be diagnosed when certain diagnostic criteria are met (DSM-5). Alternatively, a dimensional approach views SPD as an attenuated form of schizophrenia and considers schizotypy a personality trait that can range in a continuum from low values in health to very high values in full-blown schizophrenia (Raine, 2006).
Individuals with high trait schizotypy show cognitive deficits that are analogous to those found in schizophrenia. High scores in schizotypy correlate with poor attention and memory, deficits in verbal learning, spatial working memory, cognitive flexibility, executive function and abstraction (Raine et al., 1995, Siever et al., 2002, Rosell et al., 2014). This pattern of alterations suggests that schizotypal personality and schizophrenia share a common neuropathological basis involving impairment of higher cognition (Siever et al., 2002). An unexplored aspect of cognitive function in SPD is the potential presence of deficits of self- or behavioral monitoring, a facet of executive functioning that is crucial to differentiate between internally and externally generated actions and thoughts. These deficits are central to schizophrenia (Frith and Done, 1989, Frith, 1995, Stephan et al., 2009) and may explain auditory hallucinations and delusions of control as misattributions of inner speech and thoughts (Stephan et al., 2009).
The behavioral monitoring system has been studied extensively using the error-related negativity or ERN. The ERN is an event-related brain potential observed following errors in behavioral tasks (Falkenstein et al., 1991, Gehring et al., 1993). Its generators have been located in the frontal lobe including the anterior cingulate cortex (ACC) (Luu and Tucker, 2001), and it is considered a correlate of the error detection process by which the behavioral monitoring system compares emitted responses with an internal representation of alternative options (Holroyd and Coles, 2002). The ERN is followed by the error positivity or Pe (Falkenstein et al., 1991). Some authors have specifically associated the Pe with the awareness of error commission (Nieuwenhuis et al., 2001). Studies in psychiatric populations have shown reduced ERN and Pe amplitudes in patients with schizophrenia (Bates et al., 2002, Simmonite et al., 2012, Foti et al., 2012, Houthoofd et al., 2013) indicating deficits in the neural system involved in the generation of this component. These reductions have been proposed as an endophenotypic marker for schizophrenia spectrum disorders (Manoach and Agam, 2013).
Neurochemically, the ERN has been proposed to be driven by phasic decreases in the firing of mesencephalic dopaminergic neurons coding reinforcement-learning signals (Holroyd and Coles, 2002). ERN amplitude is sensitive to dopaminergic antagonists. In healthy volunteers, typical and atypical antipsychotics reduce its amplitude (Zirnheld et al., 2004, de Bruijn et al., 2006), suggesting that D2 receptor blockade disrupts the reinforcement-learning signals and impairs behavioral monitoring. Low ERN amplitude values are observed in schizophrenia, and these abnormal measures normalize following the administration of atypical antipsychotics such as risperidone (Bates et al., 2004). A potential explanation for these opposite effects is that antipsychotics reduce abnormally high dopaminergic tone in schizophrenia. Neuroimaging studies using 18F-dopa have found excessive dopamine synthesis potential in this population (Howes et al., 2013). Dopamine levels and cognitive performance follow an inverted U shaped function. Below or above an optimal dopamine level, cognitive function rapidly deteriorates (Cools and D'Esposito, 2011).
As a working hypothesis, we conceived that if SPD and schizophrenia share a common neurochemical basis, DA tone could also be abnormally high in SPD distorting reinforcement-learning signals and impairing behavioral monitoring. We also postulated, that deficits associated with excessive dopaminergic tone could potentially be reversed by antipsychotic drugs with DA blocking effects.
In the present study, we assessed the behavioral monitoring system in individuals diagnosed with SPD and a control group. We measured the amplitude of the ERN and Pe after a placebo and 1 mg risperidone. We postulated that individuals with SPD would show deficits in behavioral monitoring, as measured by the ERN and the Pe, and that these deficits would be reverted by risperidone. As described in the methods section and in the Supplementary information file, recruitment proved very difficult and the final study sample was modest. The results of the present investigation should consequently regarded as preliminary.
2. Methods
2.1. Ethics
The study was conducted in accordance with the Declarations of Helsinki and its updates concerning experimentation on humans, and was approved by the hospital's ethics committee and the Spanish Ministry of Health. All participants gave their written informed consent prior to participation.
2.2. Participants
Detailed information on the recruitment procedure is provided in the Supplementary information file. The final study sample involved 9 participants (7 women) diagnosed with schizotypal personality disorder and 12 healthy controls (5 women). Individuals with SPD were diagnosed using the Spanish version of the Structured Clinical Interview for the DSM-IV Axis II Personality Disorders (SCID-II) (First et al., 1999), were not seeking treatment, and had never been hospitalized or exposed to antipsychotic medications. Scores on two questionnaires measuring schizotypal personality traits, the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE) and the Schizotypal Personality Questionnaire (SPQ), were also obtained (see Supplementary information). Scores on the different subscales and factors of the O-LIFE and SPQ questionnaires are shown in Table 1.
Table 1.
Scores on the different subscales and factors of the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE) and the Schizotypal Personality Questionnaire (SPQ) for the patient sample and the controls. Data expressed as mean (standard deviation).
| Instrument | Between groups comparison |
||||
|---|---|---|---|---|---|
| Controls n = 12 |
Patients n = 9 |
t-value df(19) |
p value | Cohen's d | |
| O-LIFE | |||||
| Total score | 22.33 (12.05) | 61.67 (9.41) | − 8.10 | < 0.001 | 3.64 |
| Unusual experiences | 6.08 (4.89) | 22.33 (3.74) | − 8.30 | < 0.001 | 3.74 |
| Cognitive disorganization | 5.83 (4.69) | 17.89 (3.79) | − 6.31 | < 0.001 | 2.83 |
| Introvertive anhedonia | 3.67 (2.43) | 10.67 (4.72) | − 4.44 | < 0.001 | 1.86 |
| SPQ | |||||
| Total score | 14.00 (7.79) | 44.67 (6.75) | − 9.44 | < 0.001 | 4.21 |
| Ideas of reference | 1.92 (1.73) | 4.22 (1.56) | − 3.146 | 0.005 | 1.40 |
| Odd beliefs/magical thinking | 1.42 (1.88) | 4.67 (1.66) | − 4.12 | 0.001 | 1.83 |
| Unusual perceptual experiences | 0.92 (1.17) | 5.78 (2.05) | − 6.90 | < 0.001 | 2.05 |
| Paranoid ideation | 1.50 (1.45) | 3.67 (1.87) | − 2.99 | 0.007 | 1.87 |
| Social anxiety | 1.75 (1.87) | 5.33 (2.65) | − 3.65 | 0.002 | 2.65 |
| No close friends | 1.92 (1.31) | 3.78 (1.72) | − 2.82 | 0.011 | 1.22 |
| Constricted affect | 1.17 (1.12) | 3.67 (1.50) | − 4.39 | < 0.001 | 1.89 |
| Eccentric/odd behavior | 0.58 (0.99) | 4.89 (2.62) | − 5.25 | < 0.001 | 2.62 |
| Odd speech | 1.33 (0.89) | 5.00 (1.50) | − 7.02 | < 0.001 | 2.98 |
| Cognitive-perceptual factor | 5.75 (4.88) | 18.33 (3.78) | − 6.41 | < 0.001 | 2.88 |
| Interpersonal factor | 6.33 (3.85) | 16.44 (4.36) | − 5.63 | < 0.001 | 2.46 |
| Disorganized factor | 1.92 (1.31) | 9.89 (3.56) | − 7.20 | < 0.001 | 2.97 |
All participants gave their written informed consent to participate prior to the experimental sessions.
2.3. Drugs
Participants received oral doses of 1 mg of risperidone or a placebo (lactose capsule). This relatively low dose of risperidone was chosen for two reasons: 1) it was within the dose range previously shown to reduce symptoms in SPD patients in a study that had used an upward titration from 0.25 to 2 mg/day (Koenigsberg et al., 2003); and 2) it was low enough to prevent observations in healthy volunteers from being confounded by excessive somnolence.
2.4. Experimental design
The study was carried out according to a double-blind randomized cross-over design. SPD and controls participated in two different experimental sessions in which they were tested after receiving the placebo or the 1 mg oral dose of risperidone. Experimental days were one week apart. Upon arrival in the laboratory, electrodes were applied to the scalp and medication was given. During each recording session, volunteers remained in a quiet room and were asked to stay alert throughout the experiment. The behavioral task and electroencephalography recording (EEG) were conducted 2 h after drug administration when the peak risperidone plasma levels were expected.
2.5. Behavioral task and EEG recording
The electroencephalogram (EEG) was recorded continuously from the scalp while participants performed the Eriksen Flanker Task (Eriksen and Eriksen, 1974), a classical task recruiting behavioral monitoring. A series of behavioral parameters and event-related brain potentials (ERPs) were derived from the task. A detailed description of the task, recording procedure, signal processing, and component quantification is provided in the Supplementary information file.
2.6. Statistical analyses
Sociodemographic (age and sex), behavioral and ERP data are presented in summaries as means and standard deviation (SD). Sample matching variables were analyzed using independent samples, Student's t-tests and the χ2 test (sex distribution).
The statistical analysis of the behavioral and ERP data was designed to evidence: 1) trait group differences between SPD individuals and controls; and 2) state differences associated with risperidone administration.
To test for trait differences between groups, behavioral and ERP data in the placebo condition were compared between groups using independent samples t-tests. In the case of the ERP data, the statistical comparisons were conducted at the three midline electrodes (Fz, Cz, Pz).
To test for differences in the pharmacological intervention, behavioral and ERP data were analyzed using two-way ANOVAs with the within-subjects Treatment factor (placebo vs. risperidone) and the between-subjects factor Group (controls vs. SPD individuals). In the specific case of the ERP data, to simplify the interpretation of the two-way ANOVA and to avoid triple interactions, separate analyses were conducted at each of three midline electrodes (Fz, Cz, Pz). The contrast of interest was the interaction Treatment by Group. A significant interaction indicated the differential modulation of a behavioral or ERP variable by the risperidone treatment.
To test for compatibility effects on the P2 and P3 components of the stimulus-locked averages and their modulation by risperidone, a three-way ANOVA was conducted with factor Compatibility (compatible vs. incompatible stimuli), Group (controls vs. SPD individuals) and Treatment (placebo vs. risperidone). Again, to simplify interpretation of results, separate analyses were conducted at each of three midline electrodes (Fz, Cz, Pz). Two were the contrasts of interest in this analysis: First, the Compatibility by Group interaction. It indicated a differential processing of conflict-eliciting stimuli (the incompatible stimuli) between groups. Second, the Treatment by Compatibility by Group interaction. It indicated a differential impact of risperidone on one subgroup and stimulus type.
Statistical analyses were conducted using the SPSS software. Additionally, effect size (Cohen's d and Cohen's f) was calculated using the G*Power software (Faul et al., 2007). Using G*Power we estimated that with 9 SPD patients and 12 controls we had 80% power to detect an effect of d = 1.14 in the independent samples t-tests, and an effect of f = 0.32 in the within-between interaction of the ANOVA. For all ANOVA results, p values after Greenhouse-Geisser correction are given. Differences were considered statistically significant for p < 0.05.
3. Results
3.1. Behavior
Summary behavioral data from the Eriksen Flanker task and the results of the statistical analyses are shown in Table 2. The analysis of behavior in the absence of pharmacological treatment showed trait differences between the two participant groups. Under placebo, performance was worse in the SPD individuals than in the control group. Reaction times to correctly-responded stimuli were significantly slower for SPD individuals than for controls, without this leading to greater accuracy (no statistical differences in the % of correct and erroneous responses). The time taken to correct choice errors was also greater in the SPD than in the control group. The mean percentage of erroneous responses that were subsequently corrected was also lower for SPD individuals, but the statistical comparison only showed a trend. No other significant differences were found.
Table 2.
Behavior in the Eriksen Flanker Task, expressed as mean (standard deviation). RT = reaction time. Post-error slowing: RT increase in correct trials that follow an error trial relative to correct trials that follow another correct trial. RT incompatibility: reaction time increase in correctly-responded incompatible (HHSHH and SSHSS) relative to correctly-responded compatible trials (HHHHH and SSSSS). Error incompatibility: increase in the number of committed errors in incompatible relative to compatible trials. d: Cohen's d; f: Cohen's f.
| Placebo |
Between groups comparison |
Risperidone |
Treatment × group interaction |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | t-value | p value | d | Controls | Patients | F(1,19) | p value | f | |
| Total responses | 1126 (96) | 989 (262) | 1.48 | 0.171 | 0.69 | 1068 (114) | 1015 (197) | 1.42 | 0.248 | 0.27 |
| Omitted responses | 47 (50) | 191 (247) | − 1.73 | 0.120 | 0.81 | 93 (77) | 163 (187) | 1.78 | 0.197 | 0.31 |
| % correct responses | 78.8 (12.3) | 79.2 (9.7) | − 0.07 | 0.944 | 0.03 | 78.3 (10.7) | 81.2 (5.1) | 0.59 | 0.452 | 0.18 |
| % errors | 21.2 (12.3) | 20.8 (9.7) | 0.07 | 0.944 | 0.04 | 21.7 (10.7) | 18.8 (5.1) | 0.59 | 0.452 | 0.18 |
| % corrected errors | 88.8 (10.3) | 69.1 (29.7) | 1.91 | 0.087 | 0.89 | 86.2 (10.9) | 66.4 (29.0) | 0.00 | 0.991 | 0.01 |
| RT correct responses | 339 (74) | 419 (73) | − 2.48 | 0.023⁎ | 1.09 | 360 (73) | 435 (85) | 0.11 | 0.745 | 0.07 |
| RT corrected errors | 185 (52) | 320 (98) | − 3.77 | 0.003⁎ | 1.72 | 213 (71) | 313 (88) | 6.91 | 0.017⁎ | 0.60 |
| Post-error slowing | 17 (33) | 27 (54) | − 0.53 | 0.660 | 0.22 | 13 (28) | 22 (56) | 0.01 | 0.933 | 0.02 |
| RT incompatibility | 19 (11) | 26 (13) | − 1.47 | 0.159 | 0.58 | 23 (22) | 27 (11) | 0.21 | 0.649 | 0.11 |
| Error incompatibility | 9.0 (5.4) | 7.6 (5.3) | 0.61 | 0.545 | 0.26 | 10.4 (7.8) | 10.2 (3.4) | 0.29 | 0.599 | 0.12 |
p < 0.05.
The analysis of the impact of the pharmacological intervention is shown in the two-way ANOVA with Group and Treatment as factors. This analysis showed a significant Treatment by Group interaction for the time taken to correct erroneously responded stimuli. Risperidone administration modulated this variable differentially between groups. While correction time increased in the control group under risperidone, the drug decreased it in the SPD group.
3.2. Response-locked ERPs
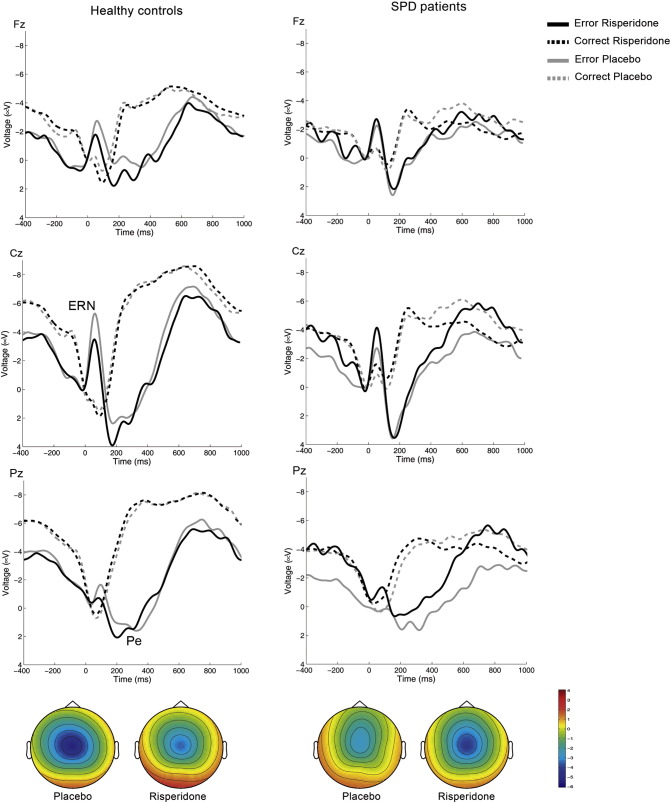
Response-locked averages associated with correct and erroneous responses for SPD individuals and controls are shown in Fig. 1. Mean amplitude values are shown in Table 3. In the averages associated with erroneous responses a negative-going deflection, the ERN, was observed in the ERP. The ERN can be seen to rise immediately following the choice errors, peaking within the next 100 ms. This component was followed by a positive deflection, the Pe.
Fig. 1.
Response-locked ERPs associated with the Eriksen Flanker task. Grand-mean averages at Fz, Cz and Pz for erroneously- and correctly-responded stimuli. The left panels show averages for the control group and the right panels for the SPD group. The negative-going deflection following a choice error is the ERN, and the subsequent positivity, the Pe. Note the reduced amplitude of the ERN and Pe waves following placebo (black solid lines) in the SPD group as compared to the healthy controls group. After risperidone (grey lines), the ERN is reduced in the control group and increased in the SPD group. The grand-averages have been band-pass filtered (2–8 Hz) for display purposes. The topographical map shows the peak activity of the ERN expressed as the difference wave between error - correct responses. Relative scaling was used. Minimum and maximum values: − 6/+4 μV.
Table 3.
Peak amplitude values for the ERP components obtained from the response-locked averages, i.e., the ERN and the Pe. Peak amplitudes are expressed in microvolts and latencies in milliseconds. d: Cohen's d; f: Cohen's f.
| Electrode | Placebo |
Between groups comparison |
Risperidone |
Treatment × group interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | t-value | p value | d | Controls | Patients | F(1,19) | p value | f | |
| Response-locked ERP amplitude values after incorrect responses (ERN) | ||||||||||
| Fz | − 4.78 (2.38) | − 2.93 (2.50) | − 1.73 | 0.100 | 0.76 | − 3.56 (2.21) | − 4.04 (2.38) | 6.49 | 0.020⁎ | 0.59 |
| Cz | − 6.75 (3.08) | − 3.38 (2.42) | − 2.71 | 0.014⁎ | 1.22 | − 4.77 (2.90) | − 5.26 (3.48) | 11.62 | 0.003⁎ | 0.78 |
| Pz | − 2.51 (2.19) | − 1.20 (1.50) | − 1.54 | 0.141 | 0.70 | − 1.98 (1.15) | − 2.08 (1.75) | 2.61 | 0.122 | 0.37 |
| Response-locked ERP amplitude values after incorrect responses (Pe) | ||||||||||
| Fz | 4.80 (2.96) | 5.33 (3.83) | − 0.36 | 0.726 | 0.15 | 5.49 (2.44) | 5.27 (2.75) | 0.33 | 0.571 | 0.13 |
| Cz | 9.10 (4.58) | 7.03 (4.73) | 1.01 | 0.324 | 0.44 | 9.47 (4.08) | 8.40 (4.38) | 0.46 | 0.505 | 0.16 |
| Pz | 4.71 (2.59) | 2.64 (1.61) | 2.11 | 0.049⁎ | 0.96 | 5.47 (2.89) | 4.14 (3.21) | 0.36 | 0.553 | 0.14 |
p < 0.05.
To test for trait differences between groups in the amplitude of the ERN and Pe we performed between-subjects comparisons of the peak values of these components at the three midline electrodes (Fz, Cz, Pz) following placebo. Mean peak values indicated larger (i.e., more negative) ERN amplitudes in the controls than in the SPD group. The statistical analysis showed that these differences were statistically significant at Cz (see Table 3). Mean peak Pe values were also larger for the controls at Cz and Pz. The statistical comparison was significant at Pz.
To test for differential drug effects we conducted the two-way ANOVA with Treatment and Group as factors at each of the three midline electrodes. As shown in Table 3, we found significant Treatment by Group interactions for the ERN at Fz and Cz. While risperidone decreased ERN amplitude in the controls, it led to increases in the SPD group. No main effects of Treatment or Group were observed at any of the three leads in the ANOVA. Regarding the Pe, no main effects or interactions were observed in the two-way ANOVA for this component.
Unexpectedly, the visual inspection of the response-locked correct responses showed a rudimentary negativity immediately following the emission of the correct response. This wave, which is known as the correct-related negativity or CRN, was seen in the controls and the SPD individuals at Fz and more markedly in the SPD group at Cz. To look for trait differences and treatment effects, we analyzed the CRN measuring the peak positive value in the 0–100 ms time window in the individual averages of the correct responses and using the same statistical approach employed for the ERN and the Pe.
Mean (SD) CRN values were more negative in the SPD than in the control group, but no statistically significant differences were found: Fz: SPD − 0.19 (2.59) μV, controls 0.22 (4.17), t(19) = 0.26, n.s.; Cz SPD − 0.61 (2.84) μV, controls 0.93 (5.21), t(19) = 0.79, n.s.; Pz SPD − 0.49 (2.15) μV, controls 0.53 (3.93), t(19) = 0.70, n.s. The two-way ANOVA with Treatment and Group as factors showed a significant interaction at Cz [F(1,19) = 4.82, p = 0.041], reflecting a CRN enhancing effect of risperidone in the SPD group only.
3.3. Stimulus-locked ERPs
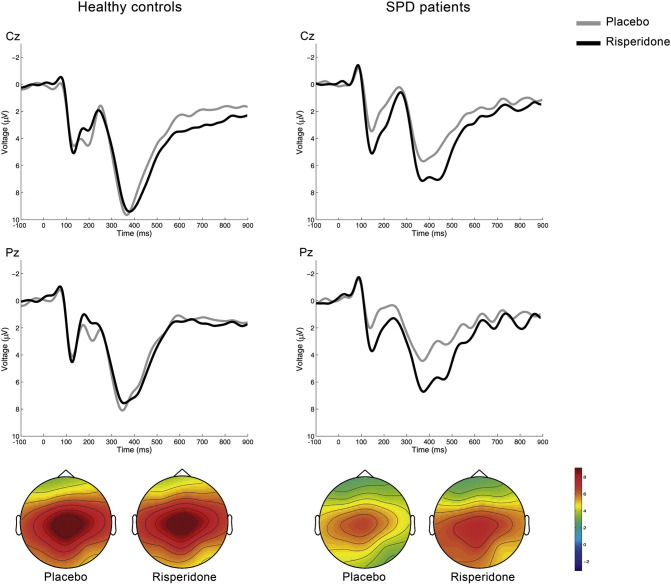
Stimulus-locked averages after correct responses for compatible and incompatible trials combined are shown for each participant group in Fig. 2, and mean amplitude values in Table 4. The most prominent features observed in the averages are two centro-parietal positive-going deflections, the P2 peaking between 100 and 200 ms and the P300 peaking between 200 and 300 ms post-stimulus.
Fig. 2.
Stimulus-locked ERPs associated with the Eriksen flanker task. Grand-mean averages at Cz and Pz for correctly-responded stimuli. Two positive-going deflections can be seen in the averages. The P200 peaks around 200 ms, and the P300 between 300–500 ms after stimulus presentation. The grand-averages have been band-pass filtered (2–8 Hz) for display purposes. The topographical map shows the peak activity of the P300. Relative scaling was used. Minimum and maximum values: − 1/+9 μV.
Table 4.
Amplitude and latency values for the ERP components obtained from the stimulus-locked averages, i.e., the P2 and the P300. Peak amplitudes are expressed in microvolts and latencies in milliseconds. d: Cohen's d; f: Cohen's f.
| Electrode | Placebo |
Between groups comparison |
Risperidone |
Treatment × group interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | t-value | p value | d | Controls | Patients | F(1,19) | p value | f | |
| Stimulus locked ERP amplitude after correct responses (P2) | ||||||||||
| Fz_amplitude | 4.9 (3.2) | 3.5 (2.7) | 1.01 | 0.325 | 0.47 | 5.5 (3.7) | 5.1 (2.5) | 0.74 | 0.401 | 0.20 |
| Cz_amplitude | 5.7 (3.5) | 3.7 (3.1) | 1.37 | 0.188 | 0.60 | 6.0 (3.6) | 5.7 (3.08) | 4.39 | 0.050⁎ | 0.48 |
| Pz_amplitude | 5.2 (2.7) | 2.6 (2.3) | 2.36 | 0.029⁎ | 1.04 | 5.3 (2.6) | 5.1 (2.5) | 22.2 | < 0.001⁎ | 1.08 |
| Stimulus locked ERP amplitude after correct responses (P300) | ||||||||||
| Fz_latency | 377 (31) | 417 (56) | − 1.95 | 0.075 | 0.88 | 393 (39) | 428 (63) | 0.13 | 0.720 | 0.08 |
| Cz_latency | 371 (24) | 416 (54) | − 2.37 | 0.038⁎ | 1.08 | 383 (31) | 432 (50.1) | 0.04 | 0.837 | 0.04 |
| Pz_latency | 362 (30) | 419 (56) | − 2.76 | 0.018⁎ | 1.27 | 370 (38) | 432 (53) | 0.12 | 0.733 | 0.08 |
| Fz_amplitude | 6.4 (5.3) | 5.3 (2.7) | 0.68 | 0.504 | 0.26 | 7.0 (4.3) | 6.5 (3.3) | 0.11 | 0.746 | 0.08 |
| Cz_amplitude | 10.8 (5.9) | 7.4 (4.2) | 1.45 | 0.164 | 0.66 | 10.7 (5.1) | 9.7 (4.9) | 1.91 | 0.183 | 0.32 |
| Pz_amplitude | 9.3 (4.7) | 6.1 (3.3) | 1.73 | 0.101 | 0.79 | 9.1 (5.1) | 8.7 (3.5) | 2.97 | 0.101 | 0.40 |
p ≤ 0.05.
The between-subjects comparison of the P2 peak amplitudes showed significantly smaller values in the SPD group after placebo. These smaller trait values were normalized by risperidone. The two-way ANOVA found a significant Treatment by Group interaction at Pz and a marginally significant one at Cz. While the drug did not induce any changes in P2 amplitude in the controls, it increased P2 amplitude in the patient group.
The between-subjects comparison of the P300 variables showed significant differences in latency at Cz and Pz, with values being larger in the patient group. Although the ERP averages showed a smaller P300 amplitude also in the SPD group, this trait difference was not significant. The visual inspection of the waves showed that following risperidone, amplitude and latency were not modified in the control group. On the other hand, the P300 appeared larger and peaking later in the SPD group. However, the two-way ANOVA did not find any significant main effect of Treatment or a Treatment by Group interaction at any of the three electrodes.
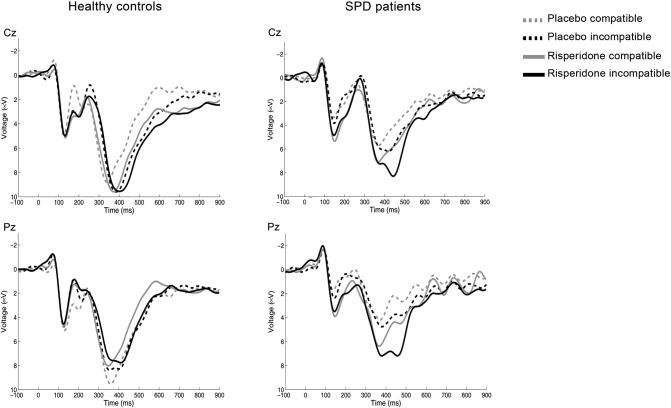
Fig. 3 shows the averages of the correctly-responded stimulus-locked averages separately for compatible and incompatible stimuli. As shown in the figure, a compatibility effect was observable in the P300 for the SPD group only. This compatibility effect consisted in larger peak amplitude values after incongruent (SSHSS and HHSHH) than congruent (SSSSS and HHHHH) stimuli. Also, the visual inspection of the traces suggested that risperidone did not modify the amplitude values in the control group, but increased them in the SPD individuals, especially in the incompatible condition. Mean peak amplitude values for each group, condition and treatment are shown in Table 5.
Fig. 3.
Stimulus-locked averages at Cz and Pz, shown separately for compatible and incompatible correctly-responded stimuli. Note the smaller P200 in the patient group after placebo, and the increase in amplitude following risperidone. Note also the compatibility effect on the P300 after placebo, which is observed in the patient group only. Risperidone showed a trend to selectively enhance P300 amplitude in the SPD group only and specifically for the incompatible trials (Treatment × Compatibility × Group interaction: Fz: F(1,19) = 3.73, p = 0.069; Cz: F(1,19) = 4.29, p = 0.052). The grand-averages have been band-pass filtered (2–8 Hz) for display purposes.
Table 5.
Amplitude values for the P300 shown separately for compatible and incompatible trials. Peak amplitudes are expressed in microvolts.
Comp: compatible; Incomp: incompatible; Compat: compatibility; Treat: treatment; degrees of freedom (1,19); d: Cohen's d; f: Cohen's f; p: p value.
| Controls |
SPD patients |
Three-way ANOVA |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo |
Risperidone |
Placebo |
Risperidone |
Compat × group |
Treat × Compat × group |
|||||||||
| Electrode | Comp | Incomp | Comp | Incomp | Comp | Incomp | Comp | Incomp | F | p | d | F | p | f |
| Fz | 6.3 (3.6) | 6.8 (4.6) | 7.5 (4.7) | 6.9 (4.2) | 5.0 (2.6) | 5.5 (2.4) | 6.0 (2.4) | 7.4 (3.8) | 2.92 | 0.104 | 0.39 | 3.73 | 0.069 | 0.44 |
| Cz | 10.6 (5.5) | 11.1 (6.0) | 11.2 (6.0) | 10.9 (5.0) | 6.9 (4.1) | 7.7 (4.2) | 8.8 (3.1) | 11.4 (5.8) | 5.03 | 0.037⁎ | 0.51 | 4.29 | 0.052 | 0.47 |
| Pz | 9.4 (4.7) | 10.2 (4.8) | 9.6 (5.6) | 9.3 (4.8) | 5.8 (3.4) | 6.7 (3.3) | 8.3 (2.5) | 10.1 (4.6) | 5.40 | 0.031⁎ | 0.53 | 2.76 | 0.113 | 0.38 |
p < 0.05.
To analyze the compatibility effect, a three-way ANOVA was conducted with Treatment (placebo vs. risperidone) and Compatibility (compatible vs. incompatible) as within-subjects factors and Group (controls vs. SPD) as between-subjects factor at each of the three midline electrodes. The results showed a significant interaction Compatibility by Group at Cz and Pz, corroborating the selectivity of the compatibility effect for the SPD group. Marginally significant triple interactions Treatment by Compatibility by Group were observed at Fz and Cz. These interactions supported as well a selective effect of risperidone on the incongruent trials in the SPD patient group.
3.4. Comparative analysis of effect size and power
The main finding of the present study is the reduced amplitude of the ERN in SPD patients and its enhancement by the antipsychotic drug risperidone. Given the small patient sample recruited, we wished to compare the size of these effects with those of analogous studies published in the literature. To do this we used Cohen's d and Cohen's f. As indicated in the methods section, we had calculated that with our participant sample (9 patients and 12 controls) we had 80% power to detect an effect of d = 1.14 in the independent samples t-tests (pre-treatment between-groups comparisons), and an effect of f = 0.32 in the within-between interaction of the ANOVA, i.e., in the Treatment by Group interaction. Size of the effect in studies by other research groups were calculated post-hoc using the means and standard deviations published in four different papers. These studies assessed ERN amplitude in individuals with high trait schizotypy (Kim et al., 2015) and in schizophrenia patients (Bates et al., 2004; Morris et al., 2011; Simmonite et al., 2012).
Summary data are presented in Table 6. As shown therein, the effect size of the pre-drug comparison of ERN amplitude between controls and SPD patients in our study was within the range of previously published values (0.46–2.37).
Table 6.
Comparison table showing effect sizes of ERN differences between healthy controls, SPD patients, high schizotypal trait individuals, and schizophrenia patients across studies. d: Cohen's d; HC: healthy controls; SPD: schizotypal personality disorder patients; HTS: high schizotypal trait individuals; SCHZ: schizophrenia patients. The “Controls”, “Patients” and “Amplitude in Patients” columns show mean (SD) amplitude values for each subgroup. For comparison purposes, in the present study, effect size (Cohen's d) in the between-groups comparison was 0.76 at Fz and 1.22 at Cz.
| Study | Sample | Electrode | Between groups comparison |
Treatment effect⁎ |
|||
|---|---|---|---|---|---|---|---|
| Controls | Patients | d | Amplitude in patients | d | |||
|
Kim et al. (2015) n1 = 20 n2 = 17 |
HC vs. HTS | Fz | − 3.76 (3.41) | − 2.48 (1.91) | 0.46 | – | – |
| Cz | − 5.92 (2.5) | − 3.25 (2.41) | 1.09 | – | – | ||
|
Simmonite et al. (2012) n1 = 35 n2 = 29 |
HC vs. SCHZ | Fz | − 6.67 (4.89) | − 1.25 (4.34) | 1.17 | – | – |
| Cz | − 4.7 (4.59) | − 0.29 (3.93) | 1.03 | – | – | ||
|
Morris et al. (2011) n1 = 21 n2 = 15 |
HC vs. SCHZ | FCz | − 11.46 (10.26) | − 4.66 (4.96) | 0.84 | – | – |
|
Bates et al. (2004) n1 = 9 n2 = 9 |
HC vs. SCHZ | Fz | − 9.86 (2.69) | − 4.34 (3.54) | 1.76 | − 7.77 (3.72) | 0.94 |
| Cz | − 12.5 (3.35) | − 4.27 (3.6) | 2.37 | − 8.46 (3.90) | 1.11 | ||
Treatment effect calculated only for patients, using paired samples Student's t-test.
4. Discussion
Here we showed that compared to controls, SPD individuals show abnormally small amplitudes of the ERN and the Pe, two neurophysiological correlates of behavioral monitoring. These neurophysiological abnormalities were paralleled by worse performance in the Eriksen Flanker task. Individuals with SPD were slower in their response to the targets and their corrective actions after choice errors. Importantly, ERN amplitude deficits and some aspects of behavior were reversed in the SPD group with the administration of risperidone. While risperidone reduced ERN amplitude and worsened performance in the healthy controls, it improved correction time and increased ERN amplitude in the SPD group.
The analysis of the stimulus-locked averages also showed alterations in stimulus perception and categorization in the SPD group. The amplitude of the P2, an exogenous component associated with stimulus identification (Crowley and Colrain, 2004), was reduced in the SPD sample. SPD individuals also showed larger P300 latencies in the placebo condition, suggesting a slowed stimulus categorization in this group. Interestingly, the conflict-inducing incompatible stimuli produced an increase in P300 amplitude in the patient group only. While previous studies have usually found increased P300 latencies and blunted amplitudes in schizophrenia patients (Allen et al., 2009), to our knowledge this compatibility effect has not been described previously. This finding can be interpreted as the patients having experienced an exaggerated increase in processing demands when target letter and flankers diverged (Rusnáková et al., 2011). Risperidone was able to normalize the amplitude values of the P2 in the patient group, both in compatible and incompatible trials. It also selectively increased P300 amplitude in the incompatible trials, potentially reflecting a beneficial effect of the drug in networks processing conflict. Importantly, no drug effects were observed on the P2 or P300 amplitudes in the healthy volunteers that could suggest a general unspecific reduction in general vigilance induced by the risperidone dose administered.
The findings discussed above suggest that individuals with SPD show deficits in behavioral monitoring, but also in stimulus processing. These results extend previous findings that showed neurophysiological anomalies in SPD. A prior study had identified alterations in the mismatch negativity in this population (Hong et al., 2012), suggesting impairment in automatic detection of deviant characteristics of stimuli. In another study, the authors found decreased phase-locking following auditory stimulus presentation (Shin et al., 2010). In line with the self-monitoring deficits found in our study, several research groups have found that SPD patients show reduced N400 amplitudes, indicating poor semantic monitoring in this population (Debruille et al., 2007, Wang et al., 2013).
As hypothesized, ERN and Pe amplitude were reduced in agreement with previous findings in patients with schizophrenia (Mathalon et al., 2002, Morris et al., 2011, Simmonite et al., 2012, Foti et al., 2012). Interestingly, the size of the effect of ERN amplitude differences between participants found in our study at the Fz electrode (d = 0.76) is higher than that reported by Kim and coworkers in their sample of healthy individuals scoring high on schizotypy (Fz: 0.46), but lower than the values reported for schizophrenia patients in several studies (Fz: 1.17, Simmonite et al., 2012; FCz: 0.84, Morris et al., 2011; Fz: 1.76, Bates et al., 2004). These differences suggest an increasing impairment of self-monitoring from health to high trait schizotypy/SPD and to full-blown schizophrenia.
In the only other study that we know of assessing the effects of antipsychotics on the ERN of patients, Bates and colleagues showed low ERN amplitude after commission errors in schizophrenia patients performing a go-no go task. Despite the small sample recruited by these authors (9 patients and 9 controls), they also showed that pharmacological treatment was able to increase ERN amplitude, reversing the initially observed deficits after six weeks of treatment (Bates et al., 2004). These results suggest that an abnormally small ERN is a deficit present in both disorders. The idea of a trait alteration in the neural networks subserving this neurophysiological marker in schizophrenia spectrum disorders is further supported by the fact that our SPD sample was free of the usual confounds present in studies on schizophrenia. Such confounds include disease progression, the acute effects of antipsychotic medication and neural adaptations to pharmacological treatment.
Our results add to previous studies in schizophrenia showing deficits of self-monitoring, defined as the ability to control self-initiated behavior and cognitive processes. Deficits in the correct attribution of actions and thought processes to internal or external sources may be at the core of schizophrenia (Frith and Done, 1989, Frith, 1995, Stephan et al., 2009) in the absence of attentional deficits (Turken et al., 2003). Reduced functional connectivity between areas mediating an action (speech generation) and those mediating certain aspects of that action (speech perception) has also been described (Ford et al., 2002). Defective self-monitoring would explain the attribution of inner speech to external sources in auditory hallucinations, and phenomena such as thought insertion or control (Stephan et al., 2009).
It is noteworthy that risperidone administration led to opposite effects on the ERN in the SPD and control populations. This was observed both at the neurophysiological and behavioral levels. Significant drug × group interactions were seen for ERN amplitude and for the time taken to correct a choice error. While risperidone reduced ERN amplitude and increased correction time in the healthy controls, it led to the reverse effects in the SPD patients. Prior studies involving antipsychotic drug administration to healthy subjects have found an inhibitory effect of both the typical and atypical drugs on this ERP, such as haloperidol (Zirnheld et al., 2004, de Bruijn et al., 2006) and olanzapine (de Bruijn et al., 2006). In line with the paradoxical effect observed in our SPD sample, Bates and colleagues found that whereas the ERN was reduced in schizophrenia patients in the early stages of an acute episode, it's amplitude increased following pharmacological treatment with atypical antipsychotics (Bates et al., 2004). Although no previous studies exist on the effects of antipsychotics on the ERN in SPD, Koenigsberg and colleagues have shown that a low dose regime of risperidone is effective at reducing positive and negative symptoms in this population (Koenigsberg et al., 2003).
Our findings suggest a normalizing effect of risperidone on neurophysiological and behavioral deficits in the disease. The ERN has been associated with a neural network involving the ACC (Dehaene et al., 1994, Ridderinkhof et al., 2004, Doñamayor et al., 2012), the substantia nigra/VTA (Dehaene et al., 1994), the dorsolateral prefrontal cortex (Gehring and Knight, 2000) and the basal ganglia (Falkenstein et al., 2001). Holroyd and Coles have proposed a model in which mesencephalic dopamine pathways code a reinforcement-learning signal that is conveyed to the ACC. According to this model, when an error is committed, decreases in phasic dopamine release disinhibit ACC neurons that generate the ERN (Holroyd and Coles, 2002). While some reports have suggested a role of other neuromodulatory systems (Riba et al., 2005), the available pharmacological evidence suggests a heavy influence of the dopaminergic system. Within this framework, the present findings of a reduced ERN and its enhancement by risperidone point at a dysregulation of dopaminergic neurotransmission in the meso-cortico-striatal network. In support of this possibility, a recent neuroimaging study has found that improvement in psychotic symptoms after risperidone is associated with increased functional connectivity between the striatum and the anterior cingulate and dorsolateral prefrontal cortex (Sarpal et al., 2015).
Dopaminergic dysregulation in SPD patients is also supported by post-mortem and neuroimaging studies showing elevated dopaminergic activity in schizophrenia spectrum disorders. Patients with schizophrenia show elevated midbrain dopamine synthesis capacity and increased 18F-DOPA uptake in the substantia nigra and the striatum (Howes et al., 2013), findings that extend to individuals showing prodromal symptoms (Howes et al., 2009). 18F-DOPA uptake in the striatum is increased in subjects at ultra-high risk for psychosis (Egerton et al., 2013). High schizotypal personality traits have also been associated with abnormalities in dopaminergic neurotransmission. Individuals scoring high in the SPQ questionnaire show exacerbated dopamine release in the striatum following amphetamine challenge (Woodward et al., 2011). Dopamine release levels in diagnosed SPD patients are higher than in controls, and analogous to those in remitted schizophrenia patients (Abi-Dargham et al., 2004).
Additional evidence of modifications in dopaminergic neurotransmission include decreased D2 receptor binding in the ACC (Suhara et al., 2002) and the substantia nigra/ventral tegmental area (Kessler et al., 2009). Schizotypy as a trait has been associated with alterations in D2 receptor availability in the striatum (Mohr and Ettinger, 2014). Also, analogously to observations in schizophrenia, increased dopamine release following amphetamine challenge has been shown in SPD patients using SPECT (Abi-Dargham et al., 2004). In healthy volunteers, dopamine release after amphetamine challenge correlates positively with schizotypal traits (Woodward et al., 2011).
The differential modulation by risperidone found here may be explained by differences in dopaminergic tone between controls and SPD individuals and the inverted-U-function between dopamine levels and performance proposed by Cools and D'Esposito (Cools and D'Esposito, 2011). In the controls, D2 receptor blockade leads to lower-than-normal dopamine-D2 receptor interactions and thus to sub-optimal dopaminergic tone in the meso-cortico-striatal network that gives rise to the ERN and regulates behavioral monitoring. In the patient group, an abnormally enhanced dopaminergic tone may have been tuned-down by risperidone improving cognitive function and increasing the ERN. Importantly, this beneficial effect was observed with a low dose of risperidone that must have led only to partial D2 receptor occupancy (Kapur et al., 1999). Analogous baseline-dependent effects on performance have been observed in healthy volunteers administered the dopaminergic agonist bromocriptine. The study found that individuals with low dopamine synthesis capacity had their performance improved by the drug in a reversal learning task, while subjects with high dopamine synthesis capacity were impaired by the drug (Cools et al., 2009).
The relevance of our present findings goes beyond the study of SPD. This disorder is a useful target for the definition of endophenotypes in schizophrenia spectrum disorders. Research into SPD allows the assessment of biological and cognitive markers without the confounding factors of a major disorder, such as neural changes associated with the natural course of the disease and neural adaptations secondary to chronic pharmacological treatment (Raine et al., 1995). Our findings also support the use of the ERN as a correlate of behavioral monitoring in these populations. Recently, Manoach and Agam proposed the use of neurophysiological correlates of errors as endophenotypes in psychiatric disorders and as surrogate markers of response to treatment (Manoach and Agam, 2013). A blunted ERN is also present in other psychotic disorders where self-monitoring and insight are compromised (Foti et al., 2012). Simmonite and colleagues have shown reduced ERNs in symptom-free schizophrenia patients' siblings (Simmonite et al., 2012). Their sample showed ERNs of intermediate amplitude, i.e. lower than those of healthy controls, but higher than those of patients. Importantly, while in our study the SPD individuals shared symptoms analogous to the schizophrenia prodrome, the unaffected siblings in the study by Simmonite and coworkers did not show any prodromal symptoms of schizophrenia. Nevertheless, their ERN was reduced compared to healthy controls. Contrary to the present observations, the ERN is increased in obsessive-compulsive disorder patients and their unaffected first-degree relatives (Riesel et al., 2011, Ullsperger et al., 2014)
The main limitation of the present study is the relatively small sample recruited. Although many individuals showed initial interest they were discouraged by what they perceived as the pathologization of their perceptual and cognitive experiences, and also by the use of an antipsychotic in the study. Consequently, these results must be considered preliminary.
To sum up, individuals with SPD showed deficits in self-monitoring analogous to those in schizophrenia, highlighting a common endophenotype in schizophrenia spectrum disorders. These deficits suggest a dopaminergic imbalance also in SPD, can be evidenced by neurophysiological measures, and can be reverted by antipsychotic medication. Future studies should attempt the assessment of neurophysiological measures of behavioral monitoring in larger SPD patient samples.
Funding
This study was funded by “Fundació La Marató de TV3”, grant number: 0113310. The funding source had no role in study design, data collection and analysis, interpretation of results and preparation of the manuscript.
Contributors
The study was conceived by Víctor Pérez, Iluminada Corripio, Jordi Riba and the late Prof. Manel J. Barbanoj. Subject recruitment and data collection was performed by Mireia Rabella and Eva Grasa. Rosa Maria Antonijoan provided logistical support. Data was analyzed by Mireia Rabella, Sergio Romero, Miquel Àngel Mañanas, Thomas Münte and Jordi Riba. All authors participated in data interpretation and article preparation, and approved the final manuscript version.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgements
We would like to thank patients and healthy volunteers for their participation. This study is dedicated to Prof. Manel J. Barbanoj in memoriam.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.05.019.
Appendix A. Supplementary data
Supplementary material.
References
- Abi-Dargham A., Kegeles L.S., Zea-Ponce Y. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol. Psychiatry. 2004;55:1001–1006. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Allen A.J., Griss M.E., Folley B.S. Endophenotypes in schizophrenia: a selective review. Schizophr. Res. 2009;109:24–37. doi: 10.1016/j.schres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bates A.T., Kiehl K.A., Laurens K.R., Liddle P.F. Error-related negativity and correct response negativity in schizophrenia. Clin. Neurophysiol. 2002;113:1454–1463. doi: 10.1016/s1388-2457(02)00154-2. [DOI] [PubMed] [Google Scholar]
- Bates A.T., Liddle P.F., Kiehl K.A., Ngan E.T.C. State dependent changes in error monitoring in schizophrenia. J. Psychiatr. Res. 2004;38:347–356. doi: 10.1016/j.jpsychires.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Cools R., D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R., Frank M.J., Gibbs S.E. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J. Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley K.E., Colrain I.M. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- de Bruijn E.R.A., Sabbe B.G.C., Hulstijn W. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Debruille J.B., Kumar N., Saheb D. Delusions and processing of discrepant information: an event-related brain potential study. Schizophr. Res. 2007;89:261–277. doi: 10.1016/j.schres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Posner M.I., Tucker D.M. Localization of a neural system for error detection and compensation. Psychol. Sci. 1994;5:303–305. [Google Scholar]
- Doñamayor N., Heilbronner U., Münte T.F. Coupling electrophysiological and hemodynamic responses to errors. Hum. Brain Mapp. 2012;33:1621–1633. doi: 10.1002/hbm.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A., Chaddock C.A., Winton-Brown T.T. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol. Psychiatry. 2013;74:106–112. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of target letters in a non-search task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hielscher H., Dziobek I. Action monitoring, error detection, and the basal ganglia: an ERP study. Neuroreport. 2001;12:157–161. doi: 10.1097/00001756-200101220-00039. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Smith B.S. 1999. Guia del usuario para la entrevista clínica estructurada para los trastornos de la personalidad del eje II del DSM-IV: SCID-II. (Structured clinical interview for DSM-IV axis II personality disorders (SCID-II)) (Masson, Barcelona, Spain) [Google Scholar]
- Ford J.M., Mathalon D.H., Whitfield S. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol. Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Foti D., Kotov R., Bromet E., Hajcak G. Beyond the broken error-related negativity: functional and diagnostic correlates of error processing in psychosis. Biol. Psychiatry. 2012;71:864–872. doi: 10.1016/j.biopsych.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D. The cognitive abnormalities underlying the symptomatology and the disability of patients with schizophrenia. Int. Clin. Psychopharmacol. 1995;10(Suppl. 3):87–98. [PubMed] [Google Scholar]
- Frith C.D., Done D.J. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol. Med. 1989;19:359–363. doi: 10.1017/s003329170001240x. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Knight R.T. Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H. A neural system for error detection and compensation. Psychol. Sci. 1993;4:385–390. [Google Scholar]
- Holroyd C.B., Coles M.G.H. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hong L.E., Moran L.V., Du X. Mismatch negativity and low frequency oscillations in schizophrenia families. Clin. Neurophysiol. 2012;123:1980–1988. doi: 10.1016/j.clinph.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthoofd S., Morrens M., Sabbe B. Trait and state aspects of internal and external performance monitoring in schizophrenia. Int. J. Psychophysiol. 2013;87:42–51. doi: 10.1016/j.ijpsycho.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Howes O.D., Montgomery A.J., Asselin M.-C. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Howes O.D., Williams M., Ibrahim K. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain J. Neurol. 2013;136:3242–3251. doi: 10.1093/brain/awt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S., Zipursky R.B., Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am. J. Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Kessler R.M., Woodward N.D., Riccardi P. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol. Psychiatry. 2009;65:1024–1031. doi: 10.1016/j.biopsych.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-H., Jang K.-M., Kim M.-S. Deficits in error-monitoring by college students with schizotypal traits: an event-related potential study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Reynolds D., Goodman M. Risperidone in the treatment of schizotypal personality disorder. J. Clin. Psychiatry. 2003;64:628–634. doi: 10.4088/jcp.v64n0602. [DOI] [PubMed] [Google Scholar]
- Luu P., Tucker D.M. Regulating action: alternating activation of midline frontal and motor cortical networks. Clin. Neurophysiol. 2001;112:1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Manoach D.S., Agam Y. Neural markers of errors as endophenotypes in neuropsychiatric disorders. Front. Hum. Neurosci. 2013;7:350. doi: 10.3389/fnhum.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon D.H., Fedor M., Faustman W.O. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J. Abnorm. Psychol. 2002;111:22–41. [PubMed] [Google Scholar]
- Mohr C., Ettinger U. An overview of the association between schizotypy and dopamine. Front. Psychiatry. 2014;5:184. doi: 10.3389/fpsyt.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.E., Holroyd C.B., Mann-Wrobel M.C., Gold J.M. Dissociation of response and feedback negativity in schizophrenia: electrophysiological and computational evidence for a deficit in the representation of value. Front. Hum. Neurosci. 2011 doi: 10.3389/fnhum.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S., Ridderinkhof K.R., Blom J. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu. Rev. Clin. Psychol. 2006;2:291–326. doi: 10.1146/annurev.clinpsy.2.022305.095318. [DOI] [PubMed] [Google Scholar]
- Raine A., Lencz T., Mednick S.A., editors. Schizotypal Personality. Cambridge University Press; New York: 1995. [Google Scholar]
- Riba J., Rodríguez-Fornells A., Morte A. Noradrenergic stimulation enhances human action monitoring. J. Neurosci. 2005;25:4370–4374. doi: 10.1523/JNEUROSCI.4437-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Riesel A., Endrass T., Kaufmann C., Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am. J. Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Rosell D.R., Futterman S.E., McMaster A., Siever L.J. Schizotypal personality disorder: a current review. Curr. Psychiatry Rep. 2014;16:452. doi: 10.1007/s11920-014-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnáková S., Daniel P., Chládek J. The executive functions in frontal and temporal lobes: a flanker task intracerebral recording study. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2011;28:30–35. doi: 10.1097/WNP.0b013e31820512d4. [DOI] [PubMed] [Google Scholar]
- Sarpal D.K., Robinson D.G., Lencz T. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.-W., Krishnan G., Hetrick W.P. Increased temporal variability of auditory event-related potentials in schizophrenia and schizotypal personality disorder. Schizophr. Res. 2010;124:110–118. doi: 10.1016/j.schres.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever L.J., Koenigsberg H.W., Harvey P. Cognitive and brain function in schizotypal personality disorder. Schizophr. Res. 2002;54:157–167. doi: 10.1016/s0920-9964(01)00363-2. [DOI] [PubMed] [Google Scholar]
- Simmonite M., Bates A.T., Groom M.J. Error processing-associated event-related potentials in schizophrenia and unaffected siblings. Int. J. Psychophysiol. 2012;84:74–79. doi: 10.1016/j.ijpsycho.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T., Okubo Y., Yasuno F. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch. Gen. Psychiatry. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- Turken A.U., Vuilleumier P., Mathalon D.H. Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am. J. Psychiatry. 2003;160:1881–1883. doi: 10.1176/appi.ajp.160.10.1881. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Danielmeier C., Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- Wang K., Wang Y., Yan C. Semantic processing impairment in individuals with schizotypal personality disorder features: a preliminary event-related potential study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;40:93–102. doi: 10.1016/j.pnpbp.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Woodward N.D., Cowan R.L., Park S. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am. J. Psychiatry. 2011;168:418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnheld P.J., Carroll C.A., Kieffaber P.D. Haloperidol impairs learning and error-related negativity in humans. J. Cogn. Neurosci. 2004;16:1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.