The work described here provides a novel methodology combining ultrahigh-resolution microcomputed tomography for the interrogation of interior locations of lung specimens. Image-processing methods allow for the objective quantification of three-dimensional acinar morphology, such that one can begin to understand species differences along with changes occurring in acinar structure associated with, for instance, aging. With the comprehension of structural differences at the lung periphery, one can begin to understand better the interaction between structure and function.
Keywords: acinus, acinar morphometry, Micro-CT, high-resolution imaging, quantitative CT
Abstract
We seek to establish a method using interior tomographic techniques (Xradia MicroXCT-400) for acinar morphometric analysis using the pathway center lines from micro X-ray computed tomographic (Micro-CT) images as the road map. Through the application of these techniques, we present a method to extend the atlas of murine lungs to acinar levels and present a comparison between two age groups of the C57BL/6 strain. Lungs fixed via vascular perfusion were scanned using high-resolution Micro-CT protocols. Individual acini were segmented, and skeletonized paths to alveolar sacs from the entrance to the acinus were formed. Morphometric parameters, including branch lengths, diameters, and branching angles, were generated. Six mice each, at two age groups (∼20 and ∼90 wk of age), were studied. Additive Gaussian noise (0 mean and SD 1, 2, 5, and 10) was used to test the robustness of the analytical method. Noise-based variations were within ±6 μm for branch lengths and ±5 μm for diameters. At a noise level of 10, errors increased. Branch diameters were less susceptible to noise than lengths. There was >95% center line overlap across all noise levels. The measurements obtained using the center lines as a road map were not affected by added noise. Acini from younger mice had smaller branch diameters and lengths at all generations without significant differences in branching angles. The relative distribution of volume in the alveolar ducts was similar across both age groups. The method has been demonstrated to be repeatable and robust to image noise and provides a new, nondestructive technique to assess and compare acinar morphometry quantitatively.
NEW & NOTEWORTHY
The work described here provides a novel methodology combining ultrahigh-resolution microcomputed tomography for the interrogation of interior locations of lung specimens. Image-processing methods allow for the objective quantification of three-dimensional acinar morphology, such that one can begin to understand species differences along with changes occurring in acinar structure associated with, for instance, aging. With the comprehension of structural differences at the lung periphery, one can begin to understand better the interaction between structure and function.
the pulmonary acinus, defined as a region of the lung distal to a terminal bronchiole (1, 6, 12, 21), functions as the site of gas exchange in the lung. Accurate quantitative assessment of the acinus (8, 26) is therefore an essential component of improved understanding of the physiology of gas exchange. Most acinar models use geometric approximations of the acinus based on available anatomical data. These models are continually improved upon by additionally acquired, accurate morphometric data. By extension, morphometric analysis of the pulmonary acinus is a critical component in characterizing the acinar structure, allowing for improvements in modeling. Such improved models find important applications in areas of airflow, particle transport, alveolar mechanics (2), and understanding of disease progression.
Traditionally, morphometric analysis of the acinus has been carried out by the use of classical techniques, such as serial histological sections (11, 16) and casting techniques (2, 6, 23, 25). The traditional approaches have a number of significant disadvantages, including preparation-induced distortions, inability to resample or reorient a stack of serial sections, and the loss of contextual information regarding the location of sampled portions of the lung. Micro X-ray computed tomographic (Micro-CT) imaging (7, 19, 20) is becoming a powerful tool to overcome some of these limitations. Perfusion-fixation methods (28) have been developed, which provide an in vivo-like preservation of the lung structure, as well as the radiologic qualities of the tissue, to ensure that the ex vivo specimens can be studied reliably and the measurements extrapolated to in vivo images (28).
The advent of high-resolution (HRES) Micro-CT scanners that enable imaging of the lung on the order of 1-2 μm voxel sizes allows us to assess the three-dimensional (3D) anatomy of the lung microstructures at the acinar and alveolar levels (26, 27). In addition, the nondestructive nature of Micro-CT imaging protocols provides the flexibility of resampling, reorientation, and 3D visualization of the anatomy. The anatomical information from HRES Micro-CT images of the acinus has already been applied to develop realistic models of alveolar mechanics (13, 14).
Analysis of 3D tubular segments, such as the branches within an acinus, is simplified by the representation of the structures using a 1D skeleton tree. Such skeleton trees provide a guidance tool for exploration of the entire tree and facilitate quantitative analysis of measurements along the center line. There are multiple studies that perform analysis of airway morphometry using airway skeletonization (18, 29). The extraction of the center line has been researched extensively, and there are multiple algorithms that can be used for this purpose. An iterative thinning technique, suggested by Lee et al. (15), proves to be suitable for skeletonizing the acinar mask and has been applied to this workflow. With the application of these techniques to acinar structures, we developed a method to assess quantitatively and improve our understanding of the morphometry of the lung microstructures. This morphometry will serve as the basis for understanding the establishment of age- and disease-associated alterations in the micro structure of the lung.
METHODS
The workflow involved in the morphometric analysis is depicted in the series of images in Fig. 1. A total of 12 mice from a commonly used strain (C57BL/6; The Jackson Laboratory, Bar Harbor, ME) was used for this study, 6 each from 2 age groups (∼20 and ∼90 wk of age). All of the old mice and four of the young mice are the same mice reported previously by Vasilescu et al. (26). The analysis presented uses newly developed methodologies not previously reported. The animals were housed in an animal facility at the University of Iowa, and the protocols used were under the approval of the Institutional Animal Care and Use Committee at the University of Iowa. In vivo imaging and lung sample preparation, using a perfusion-fixation method, were performed, as previously outlined in Vasilescu et al. (28), and are discussed briefly below.
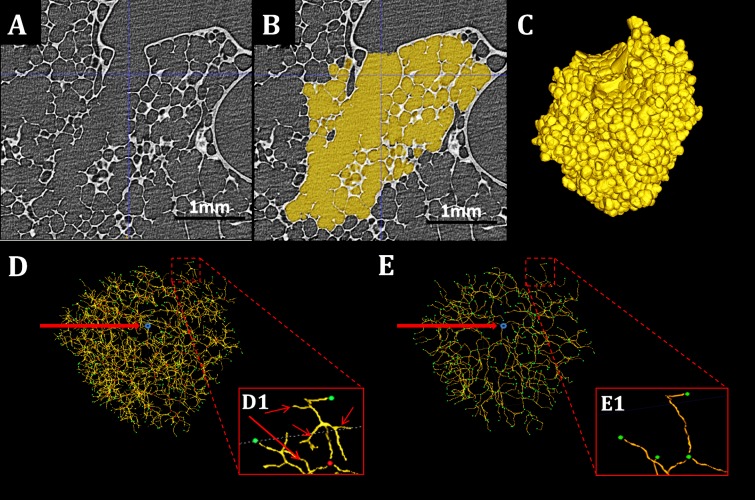
Fig. 1.
Workflow of morphometric analysis. A–C: sample slice of an HRES acinar image, the 2D acinar mask, and its 3D rendering, respectively. D: original skeleton with entrance (blue), branch points (red), and terminals (green). E: pruned skeleton with nodes (green) and entry point (blue). D1 and E1: examples of the pruning process. Branches indicated by the arrows are not in the path from the acinar entrance to the terminal node shown in green and are pruned and absent in Fig. 1E.
The animals were anesthetized before cannulation through a tracheotomy and were kept anesthetized throughout the study. A paralytic agent was administered, and ventilation was carried out through a mechanical ventilator. The animals were then imaged in vivo on a Siemens MicroCAT II scanner at an isotropic voxel size of 28 μm using an intermittent iso-pressure breath-hold technique (17), with imaging at 20 cmH2O pressures as the gold standard against which to evaluate adequacy of fixation. After image acquisition, the animals were euthanized, and the lungs were fixed via a perfusion-fixation technique and extracted and dried overnight at room temperature under tracheal pressure of 20 cmH2O. To verify structural integrity postfixation, the fixed lungs were scanned at the same resolution as used for the in vivo imaging, and total lung as well as lobe volumes were compared. Vasilescu et al. (28) have demonstrated, via serial Micro-CT scanning of similarly fixed lungs, that the lung geometry and density remain stable over 6 mo.
HRES imaging was carried out using the imaging protocols defined by Vasilescu et al. (28) and detailed below. Image-based segmentation of individual acini from the surrounding airspace was carried out using a multiscale topomorphologic approach (22). This approach has been applied previously for acinar segmentation (26), and the advantages of morphometric measurements using reconstructed acini over traditional casting techniques have been studied.
The acinar segmentation masks, thus created, were skeletonized using a 3D medial surface-thinning algorithm (15). The entry point into the acinus, the branch points, and terminal nodes was manually chosen to eliminate branches leading to individual alveoli, as well as loops in the skeleton that may arise due to deteriorations in the septal walls. Dijkstra's algorithm (3) was applied on the modified skeleton to identify the paths from each terminal to the entry points. The lengths, diameters, and branching angles for each branch were determined using the skeleton tree as a reference.
LFOV and HRES Micro-CT Imaging Protocol
MicroXCT-400: specifications.
The Xradia MicroXCT-400 scanner (Carl Zeiss X-ray Microscopy, Pleasanton, CA; 20–90 keV) was used for all HRES imaging. This scanner has five optical objectives installed in front of the charge-coupled device camera, allowing for continuous, adjustable magnification and resolution (pixel size). The combination of off-center scan capability and adjustable magnification of the camera allows us to image the whole lung, followed by focused imaging at particular spots of interest for higher-resolution scans.
A large field-of-view (LFOV) scan was obtained using the following parameters: 40 kV, 200 μA, 2,000 ms exposure time, detector binning of 1, step size of 0.125°/projection over 180° using a magnification lens of 0.5×. The datasets were reconstructed with a voxel size of ∼12 μm. At this resolution, the LFOV datasets allowed for identification of the terminal bronchioles that serve as the entry point for the acini and allowed us to select the locations of the HRES scans, such that each HRES scan contained one complete acinus. Two acini were chosen from each specimen: one each from the apical portion of the left lung and the caudal lobe of the right lung. These locations matched those used in Vasilescu et al. (26). Thus a total of 12 acini was evaluated. To enable a comparison of the acinar morphometry between each animal, we have manually inspected the airway tree in the LFOV scans to identify and replicate the anatomical location of the chosen acini across all specimens. The HRES scans were obtained with the same scanner using the following parameters: 40 kV, 200 μA, 8 s exposure time, detector binning of 2, and step size of 0.125°/projection over 184° using a magnification lens of 10×. The datasets were reconstructed with a voxel size of ∼2 μm, with an approximate resolution of 2.5 μm.
Image segmentation.
Acinar segmentation was performed using a semi-automatic 3D segmentation algorithm (4). Two sets of seed points were manually selected: one in the target acinus to be segmented and another in the surrounding airspaces. The segmentation algorithm itself (22) used an iterative, multiscale topo-morphologic opening to eliminate leakages iteratively at different scales and produces an isolated acinar volume at convergence. This isolated acinar mask image acts as the reference for all subsequent computations. The identification of the septal walls and other boundaries is achieved by the use of this acinar segmentation mask image. Figure 1, A–C, shows a single slice from the HRES image, the acinar segmentation mask, and the resulting 3D acinar volume, respectively.
Skeletonization.
The binary acinar mask was subjected to iterative 3D erosion (average of 7–8 erosions) to simulate increased wall thickness and eliminate septal wall leakages. A connected components algorithm was run on the mask to ensure that a single connected region was used for further analysis. These steps resulted in the removal of smaller alveoli from the segmentation mask but did not adversely affect the skeletonization or the morphometric analysis, which stopped at the level of alveolar ducts and sacs. The resulting mask was inspected after each erosion step to ensure that there was no loss of larger airspaces or loss of connectivity between branches of an acinus.
The eroded acinar mask was subjected to an iterative, 3D surface-thinning algorithm (15). The binary mask was iteratively and symmetrically eroded, guaranteeing the medial position of the skeleton line. Figure 1D shows a 3D rendering of a representative skeleton. The skeleton line was dilated to a diameter of five pixels, and the branch points dilated to seven pixels to improve visualization. The skeletonization was applied by means of Fiji, an ImageJ-based tool (23). Holes in the acinar mask led to the formation of loops in the skeleton structure and therefore needed to be corrected before the skeletonization step was applied. Furthermore, the generated skeleton tree had a number of short branches leading into individual alveoli. Therefore, the skeleton tree required postprocessing, which included pruning of very short terminal branches and elimination of loops. Subsequently, the entry point into the acinus, all branch points, and all terminal nodes were selected manually using 3D visualization software [ITK-SNAP (30)]. Branch points with trifurcations or higher-order branching appeared as a series of bifurcations due to the unit pixel width of the skeleton tree. A distance threshold of 10 pixels (∼20 μm) was chosen, such that branch points that occurred within this threshold of each other were merged into a single branching point with multiple child branches. The pruning step provided a simplified skeleton, which accurately represents the branching pattern of the entire acinus. Figure 1E shows the results of applying the pruning step to the skeleton in Fig. 1D. The nodes are enlarged and color coded for added visualization. Figure 1, D1 and E1, shows examples of pruning of loops that were not corrected during the previous steps and branches that lead to individual alveoli. The most distal points on the skeleton tree located in the center of the alveolar sacs were labeled as terminal nodes.
Morphometric analysis.
Dijkstra's algorithm (3) was applied to the skeleton tree to identify the path from each terminal node to the entrance of the acinus, thus allowing us to determine the path-length distribution. The branch points present on this path were recorded to analyze each individual branch.
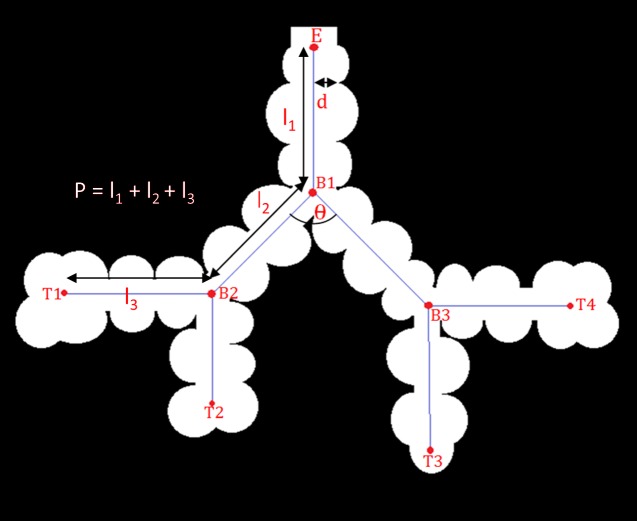
The morphometric analysis includes measurements of branch diameters, branching angles, and branch lengths for each segment and the overall path length from the entry point to each terminal node. The measurements are depicted in Fig. 2 and are defined as follows
Fig. 2.
Schematic representation of acinar measurements. E, entry point; B1–B3, branch points; T1–T4, terminal nodes; “d,” “l,” and “θ,” branch diameter, branch length, and branching angle, respectively.
Branch diameter (d): this is the inner diameter of each branch (i.e., the diameter of the acinar sleeve), as measured at the center of the branch. This measurement is defined to be the diameter of the largest sphere (centered at the midpoint of the branch) that will completely fit in the acinar sleeve. This measure is implemented using the distance transform map of the acinar mask, which provides a measure of the distance to the nearest septal wall. The diameter is measured at five pixel locations along the center of the branch skeleton (center ± 2 pixels), and the average of these diameters is chosen as the branch diameter.
Branching angle (θ): at every branch point, pairwise angles are measured between each pair of child branches. To reduce the influence of a small, localized deviation of the branch skeleton, the angles were computed between the parent node and the centers of the branches.
Branch length (l): this was computed as the sum of all points along the skeleton line between a parent node and a child node. The length computation takes into account the tortuosity of the branch.
Path length (p): this is the sum of branches between the entry point and a terminal node in the acinus.
The times for processing discussed above are as follows: 1) initial image preprocessing, 15 min technician time; 2) segmentation, 30 min technician time and 3 h computer run time; 3) skeletonization, 0 min technician time and 5 min computer run time; 4) branch point selection, 4–6 h technician time and 0 min computer run time; 5) pruning and morphometric assessment, 0 technician time and 5 min computer run time.
Validation
Because low-contrast objects, such as lungs, are more susceptible to noise artifacts, the polychromatic nature of X-ray photons, low kiloelectron volt ranges used, and Compton scattering are all contributors to noise. Additionally, the nature of interior tomography and the off-center location of HRES images lead to increased out-of-field artifacts. These can be resolved through the use of new reconstruction techniques, but these are yet to transition to Micro-CT systems. As a step toward understanding how robust our measures are in the presence of noise, we undertook evaluation of repeatability of our measures in the presence of noise.
Six acinar datasets were chosen, such that the inherent noise level in each HRES image covered the range of observed noise levels from all available datasets. The noise level was calculated as the SD (σ) of image intensity within the airspaces. In each acinus, the path from the entrance of the acinus to one terminal alveolar sac was computed and used for analysis.
Repeatability.
Each acinus was analyzed twice to test for repeatability. Initial preprocessing and segmentation steps were identically reproduced. Branch-point selection was performed by two separate users to check for user-induced variations.
Sensitivity to noise.
To test the sensitivity of the analysis technique to noise, 3D-additive Gaussian noise of mean 0 and multiple levels of SDs (σ = 0, 1, 2, 5, 10) were artificially introduced into the Micro-CT datasets, and the analysis was repeated and morphometry computed at each noise level. The original datasets were used as baseline measurements to estimate changes in branch measurements with increased noise levels.
RESULTS
Repeatability
Each acinus was analyzed twice (once each by 2 users) to determine repeatability of this method. Identical seed points were used for segmentation, followed by exact repetition of preprocessing steps and skeletonization. Manual selection of branch points and terminal nodes by the user provided the only source of variability in the workflow. Automated correction of branch points to the nearest point of divergence in the skeleton tree compensates for any minor errors and inconsistencies in user selection. The measurements were found to be a 100% match, indicating high repeatability of the workflow across multiple users.
Robustness to Noise
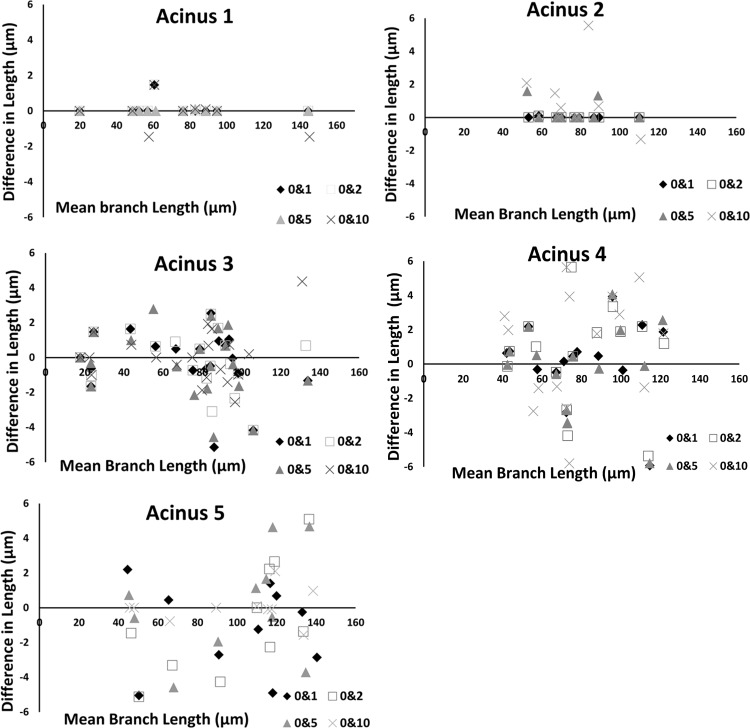
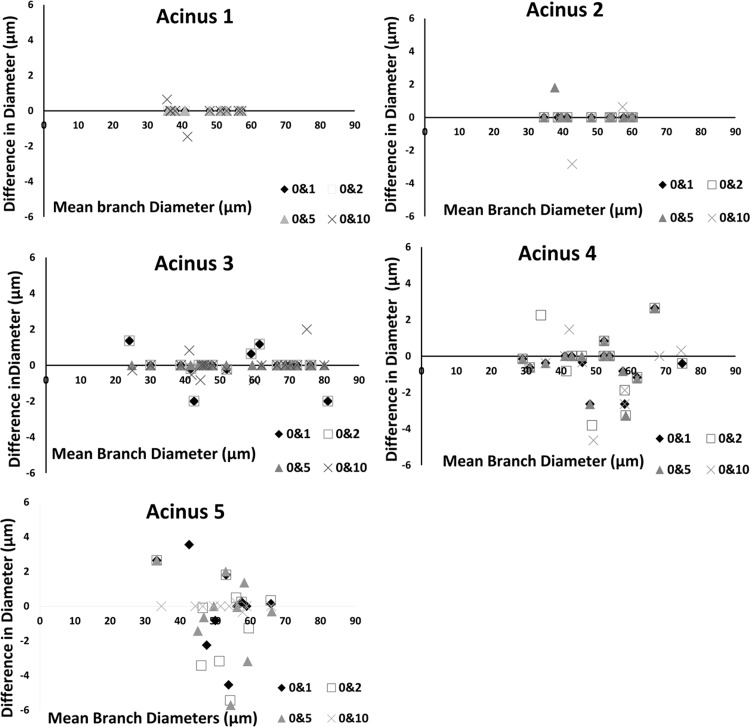
There was >95% overlap between center lines for each acinus across all four noise levels. At very high noise levels (σ = 25), the noise at the surface of the acinus was sufficient to cause variations of skeleton paths. At σ = 25, the center of the paths itself is displaced at points along the skeleton tree by a distance of ∼5 pixels (∼10 μm). One acinus (with least inherent noise) was found to match exactly with baseline measurements, even with additive noise of σ = 10. Because all differences are zero, we have not displayed this graph. On average, the branch diameters are less sensitive to change in noise levels compared with branch lengths. Average change in lengths and diameters across all branches is <5 μm. Bland-Altman plots, showing the pairwise changes in branch lengths across noise levels, are shown in Fig. 3. Changes in branch lengths did not exceed ±6 μm, and changes in branch diameters did not exceed ±5 μm. The use of preprocessing steps was found to minimize the effect of noise. In the case of one acinus, there was initially found to be a considerable change in branch length (∼18 μm) with no significant change in diameter for just one branch when noise was set to σ = 10. As a result of high noise, part of the branch leading to the alveolar sac was disconnected and eliminated during the initial preprocessing steps of morphological opening and closing. However, since the branches have a relatively consistent diameter, the branch diameter was not as susceptible to measurement error. A manual inspection of acinar volumes after each morphological operation was found to be sufficient to eliminate such errors, improving the accuracy and robustness of the method. The challenge of higher noise levels is more prominent in the image segmentation stage than at the measurement stage. Therefore, for a given acinus, the measurements can be reliably estimated as long as the segmentation mask itself can be created without appreciable error. There were no statistically significant differences in the measurements, indicating that the current method is insensitive to noise, up to an additive noise of σ = 10, matching the maximal noise inherent with the scanning modality used here. Similar Bland-Altman plots for branch diameters are shown in Fig. 4.
Fig. 3.
Bland-Altman plots showing pairwise comparison of effect of noise levels on branch lengths for 5 acini.
Fig. 4.
Bland-Altman plots showing pairwise comparison of the effect of noise levels on branch diameters for 5 acini.
Morphometric Changes across Age Groups
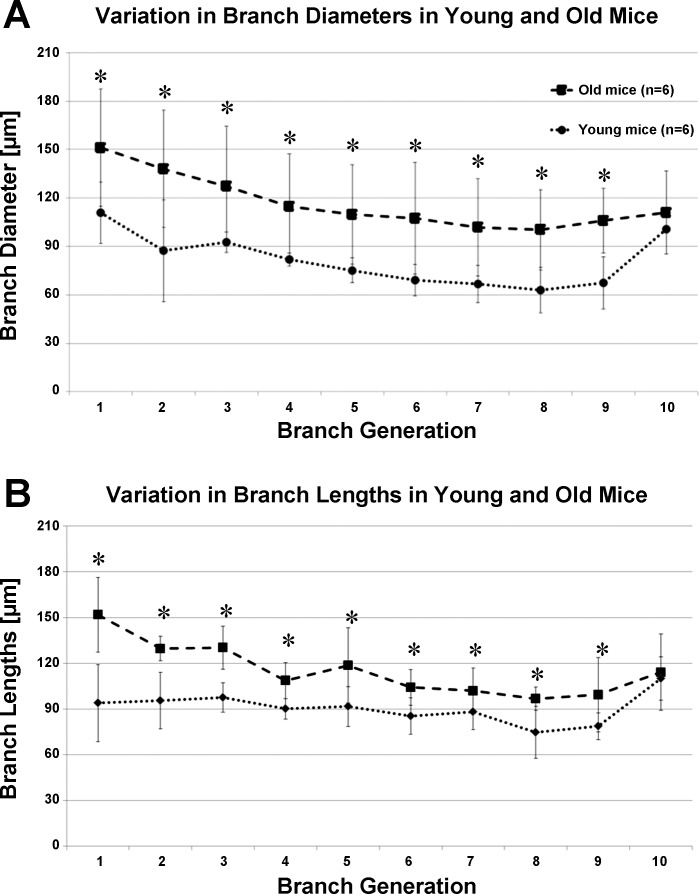
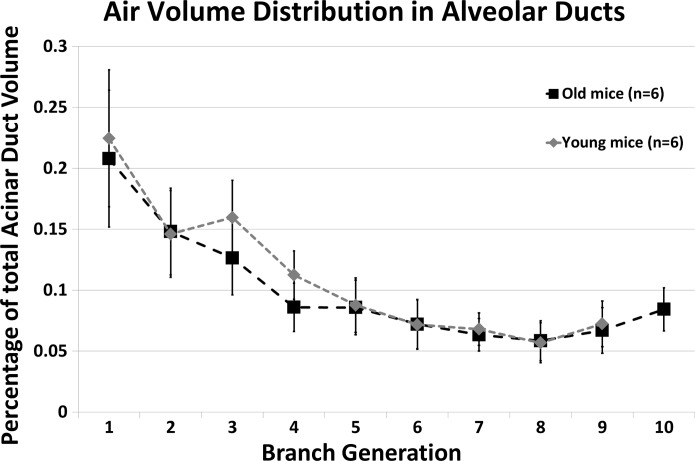
With the use of the above-validated metrics, morphometric measurements in each acinus have been averaged over the generation count (estimated from the acinar entrance) and presented in Fig. 5. Pairwise statistical comparison was performed at each generation. Acini from the older mice were found to have significantly larger branch diameters and branch lengths at every generation (P < 0.03 for diameters; P < 0.01 for lengths), as shown in Fig. 5. Acini from younger mice were found to have fewer generations, on average, with only 1 acinus in the younger mice reaching generation 10. In older mice, the segments extended to the 10th generation. Because of a limited number of data points in the younger mice, the 10th generation was omitted from statistical comparisons between the two age groups. Change in branch diameters remains relatively constant across generations, whereas change in branch lengths is greatest near the entrance to the acinus and diminished near the alveolar sacs. There were no significant differences in branching angles, but acini from the old age group were found occasionally to have a greater number of generations. On average. there were 8.714 ± 0.76 generations per acinus in the young mice vs. 9.286 ± 0.76 in the old mice. However, these differences were not significant. The alveolar duct volumes (of the alveolar sleeve, excluding surrounding alveoli), expressed as a percentage of total duct volume, are shown in Fig. 6. The average branching angle in acini across young and old mice, averaged by the generation, as measured from entrance to the acinus, is provided in Table 1.
Fig. 5.
Variations in acinar morphometry in young and old mice, averaged by generation number, as measured from entrance to the acinus. Generations 1–9 show a statistically significant difference [*P < 0.01 for diameters (A); *P < 0.01 for branch lengths (B)].
Fig. 6.
Percentage distribution of acinar duct volume at each generation in young-adult and old mice.
Table 1.
Average branching angle in young and old mice by generation
| Generation from Entrance to Acinus | Young Mice | Old Mice |
|---|---|---|
| Mean (±SD), ° | Mean (±SD), ° | |
| 1 | 107.11 (26.68) | 116.09 (16.37) |
| 2 | 99.24 (24.62) | 107.256 (19.72) |
| 3 | 96.83 (29.46) | 105.13 (32.52) |
| 4 | 91.12 (23.34) | 102.55 (31.95) |
| 5 | 92.75 (26.25) | 103.68 (27.74) |
| 6 | 100.83 (27.14) | 106.32 (28.13) |
| 7 | 105.19 (24.81) | 102.81 (26.95) |
| 8 | 106.91 (21.89) | 106.37 (15.36) |
| 9 | 103.25 (20.17) | 99.92 (11.82) |
| 10* | 87.78 (3.9) | 100.99 (17.46) |
Generation 10 occurred only in 1 acinus of the young mice, thus explaining the low SD.
Statistical Analysis
Two-way ANOVA testing (with generation number and age as the independent variables) was used on the morphometric measurements to look for statistically significant variations between the age groups and between generation numbers. As previously mentioned, the 10th generation was not considered for analysis due to lack of data points. We found that there were statistically significant differences in both branch diameters and branch lengths between the two age groups with P < 0.01. The differences between generations were less significant, with P = 0.06. We found that when considering only the first five generations, the differences in measurements between generations were more significant, with P = 0.05 for both diameters and lengths.
To account for changes in morphometric measurements as a result of increased lung size, we normalized our measurements with total lung height as the reference. Two-way ANOVA testing was performed on the normalized data and yielded similar results. There was statistically significant differences in normalized branch diameters and branch lengths across the two age groups, with P < 0.01. The generational dependency was less significant.
DISCUSSION
In both age groups, branch lengths and branch diameters progressively decrease from the terminal bronchiole up until the terminal alveolar sacs. The change in branch diameters is relatively constant across generations, whereas change in branch lengths is observed to diminish closer to the terminals. The decrease in branch lengths and diameters with increasing generation number is in agreement with previous studies in mice, rats, and humans (6, 21, 25). The 10th generations in the old mice, which only include alveolar sacs, were found to have increased branch lengths. The alveolar sacs start appearing near the sixth generation (as measured from the terminal bronchiole), on average. As seen in Fig. 5B, the effect of including terminal sacs in the sample pool for averaging might account for the reduced branch length changes at more distal generations. In addition, this inclusion may account for the increased levels of statistical significance observed within the first few generations, as opposed to the distal generations. Because we include the alveolar sacs in the terminal generations, there is a sharp increase in size when only alveolar sacs remain in the average, as is the case in generation 10 of the old mice. These observations were also seen in studies in the rat acinus (23).
The comparison across the young-adult and old age groups revealed significant changes in the size and the structure of acini. The data indicate that when comparing these two age groups, the alveolar ducts are larger by way of larger branch diameters and lengths without significant differences in acinar branching patterns. The greater size of alveolar ducts contributes to the greater lung volumes observed in the old mice. The duct volume in each generation, expressed as a percentage of the total volume of the alveolar duct spaces (shown in Fig. 6), is consistent between young and old mice. This indicates that whereas each alveolar duct has an isotropic difference in branch morphometry, the contribution of each generation to the total acinar volume remains unaffected.
The C57BL/6 strain and its mutant derivative strains are commonly used in pulmonary research, especially in investigations of genetic emphysema and early-onset emphysema (5, 9, 10). Some of these strains have shown increases in alveolar duct spaces not accompanied by destruction of the septa (10), whereas other strains have shown destruction with age. To understand better the nature and onset of emphysema vs. the age-related anatomic changes, it becomes important to decouple the changes in the acinar airspaces that indicate nondestructive, age-related anatomic changes and destruction due to pathology.
Acinar development in the mouse lung begins in the fetal stage with formation of the terminal sacs and expansion of airspaces, culminating in alveolarization and formation of septa within 2 wk of postnatal development. The lung is generally considered to be fully developed within 4 wk of postnatal growth, and growth continues until adulthood (∼8 wk of age) (24). However, Micro-CT-based evaluation of the acinus and the alveolar septa indicates that there are changes beyond these accepted timelines (26). Our group has previously shown that the number of alveoli per acinus is significantly increased between young-adult and old mice (26). Data from the current study suggest that a higher number of generations within an acinus may also contribute to increased lung volumes. We see 10 generation ducts in the old mice, but the differences between the average number of ducts when comparing the two age groups are not significant. These observations warrant further study. Because of the study design—whereby we have young mice and very old mice without intervening ages—we cannot state whether these changes represent a continuous architectural alteration or represent a process occurring more in association with older age. A better understanding of the time course of the observed differences will come with the study of additional age groups. What these observations do is to demonstrate the ability of HRES 3D Micro-CT imaging and image processing to provide a tool, whereby the structure of the pulmonary acinus can be more fully explored within the intact lung in the context of acinar spatial location.
What we have provided here is a framework, based on ultra-HRES Micro-CT and a set of image-processing tools, that can facilitate future investigations.
Summary
A comprehensive set of analysis tools for performing acinar morphometry has been presented in association with HRES interior tomographic imaging protocols used for the visualization of the peripheral airspaces of the mouse lung. The analysis tools were tested for susceptibility to noise and have not resulted in deterioration in performance, except at the highest levels of added noise. The semi-automated methods provide a simple and easy-to-use alternative to traditional methods, such as serial histological sectioning, and allow for nondestructive visualization and analysis of large datasets. The manual branch-point selection is the only step in the workflow requiring time and expertise and automatically corrects for minor errors in user selection without an effect on morphometric measurements. Further improvements in automating the branch-point selection and inclusion of the outer diameter of alveolar ducts will further reduce analysis time and provide more morphometric detail about the acinar structure. Age-related changes in acinar morphometry have also been presented, proving the capability of this method to study lung microstructure computationally in great detail. The analysis of the morphometric relationship between parent-child branches and sibling branches may allow classification of acinar branch morphometry and in conjunction with traditional analysis techniques, such as stereology, provide a greater understanding of acinar development and the mechanics of gas exchange.
GRANTS
Funds for this project were provided, in part, by the National Heart, Lung, and Blood Institute (R01-HL-080285) and National Center for Research Resources (1-S10-RR019242-01) and by the National Science Foundation/Major Research Instrumentation (Grant 0923297), awarded for “MRI: Development of the Next-Generation Nano-CT System for ROI-Focused Scanning and Exact Interior Reconstruction.”
DISCLOSURES
E. A. Hoffman is a founder and shareholder in VIDA Diagnostics, a company commercializing lung imaging analysis software, developed, in part, at the University of Iowa.
AUTHOR CONTRIBUTIONS
A.S.K.P., D.M.V., K.S.S., G.W., and E.A.H. conception and design of research; A.S.K.P., D.M.V., and K.S.S. performed experiments; A.S.K.P., D.M.V., and E.A.H. analyzed data; A.S.K.P., D.M.V., and E.A.H. interpreted results of experiments; A.S.K.P. and E.A.H. prepared figures; A.S.K.P. drafted manuscript; A.S.K.P., D.M.V., K.S.S., G.W., and E.A.H. edited and revised manuscript; A.S.K.P., D.M.V., G.W., and E.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the efforts of our collaborators at the University of Iowa and Virginia Polytechnic Institute, especially Tim Eggleston and Sourav Mishra, for their help in acquiring and analyzing data in this study.
Present address of D. M. Vasilescu: Dept. of Pathology, University of British Columbia, Vancouver, BC, Canada.
Present address of K. Sen Sharma: Paradigm4, Waltham, Massachusetts 02451.
Present address of G. Wang: Rensselaer Polytechnic Institute, Troy, New York 12180.
REFERENCES
- 1.Terminology, definitions, and classification of chronic pulmonary emphysema and related conditions: A report of the conclusions of a Ciba Guest Symposium. Thorax 14: 286–299, 1959. [Google Scholar]
- 2.Boyden E. The structure of the pulmonary acinus in a child of six years and eight months. Am J Anat 132: 275–300, 1971. [DOI] [PubMed] [Google Scholar]
- 3.Dijkstra EW. A note on two problems in connexion with graphs. Numer Math 1: 269–271, 1959. [Google Scholar]
- 4.Gao Z, Vasilescu DM, Hoffman EA, Saha PK. A multi-scale topo-morphologic opening approach for segmenting the pulmonary acinus in high resolution micro-CT images of fixed murine lungs (Abstract). Am J Respir Crit Care Med 181: A3630, 2010. [Google Scholar]
- 5.Gardi C, Martorana PA, de Santi MM, van Even P, Lungarella G. A biochemical and morphological investigation of the early development of genetic emphysema in tight-skin mice. Exp Mol Pathol 50: 398–410, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Haefeli-Bleuer B, Weibel ER. Morphometry of the human pulmonary acinus. Anat Rec 220: 401–414, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC, McDonough JE, Sanchez PG, Cooper JD, Coxson HO, Elliott WM, Naiman D, Pochettino M, Horng D, Gefter WB, Wright AC. Micro-computed tomography measurements of peripheral lung pathology in chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 546–549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsia CC, Hyde DM, Ochs M, Weibel ER; ATS/ERS Joint Task Force on the Quantitative Assessment of Lung Structure . An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, Bartolak-Suki E, Shipley JM, Parameswaran H, Majumdar A, Suki B. Early emphysema in the tight skin and pallid mice: roles of microfibril-associated glycoproteins, collagen, and mechanical forces. Am J Respir Cell Mol Biol 34: 688–694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keil M, Lungarella G, Cavarra E, van Even P, Martorana PA. A scanning electron microscopic investigation of genetic emphysema in tight-skin, pallid, and beige mice, three different C57 BL/6J mutants. Lab Invest 74: 353–362, 1996. [PubMed] [Google Scholar]
- 11.Kitaoka H, Itoh H. Computer-assisted three-dimensional volumetry of the human pulmonary acini. Tohoku J Exp Med 167: 1–12, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen L, Weibel ER, Gundersen HJ, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol 108: 412–421, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Tawhai MH, Hoffman EA, Lin CL. The effects of geometry on airflow in the acinar region of the human lung. J Biomech 42: 1635–1642, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar H, Vasilescu DM, Yin Y, Hoffman EA, Tawhai MH, Lin CL. Multiscale imaging and registration-driven model for pulmonary acinar mechanics in the mouse. J Appl Physiol 114: 971–978, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TC, Kashyap RL, Chu CN. Building skeleton models via 3-D medial surface/axis thinning algorithms. CVGIP-Graph Model Im 56: 462–478, 1994. [Google Scholar]
- 16.Mercer RR, Crapo JD. Three dimensional reconstruction of the rat acinus. J Appl Physiol 63: 785–794, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Namati E, Chon D, Thiesse J, Hoffman EA, de Ryk J, Ross A, McLennan G. In vivo micro-CT lung imaging via a computer-controlled intermittent iso-pressure breath hold (IIBH) technique. Phys Med Biol 51: 6061–6075, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Palágyi K, Tschirren J, Hoffman EA, Sonka M. Quantitative analysis of pulmonary airway tree structures. Comput Biol Med 36: 974–996, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Paulus MJ, Gleason SS, Kennel SJ, Hunsicker PR, Johnson DK. High resolution X-ray computed tomography: an emerging tool for small animal cancer research. Neoplasia 2: 62–70, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritman EL. Micro-computed tomography of the lungs and pulmonary-vascular system. Proc Am Thorac Soc 2: 477–480, 501, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez MB, Bur S, Favre A, Weibel ER. Pulmonary acinus: geometry and morphometry of the peripheral airway system in rat and rabbit. Am J Anat 180: 143–155, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Saha PK, Gao Z, Alford SK, Sonka M, Hoffman EA. Topomorphologic separation of fused isointensity objects via multiscale opening: separating arteries and veins in 3-D pulmonary CT. IEEE Trans Med Imaging 29: 840–851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schittny JC, Burri PH. Development and growth of the lung. In: Fishman's Pulmonary Diseases and Disorders (4th ed), New York: McGraw-Hill, 2008, chapt. 5. [Google Scholar]
- 25.Schreider JP, Raabe OG. Structure of the human respiratory acinus. Am J Anat 162: 221–232, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Vasilescu DM, Gao Z, Saha PK, Yin L, Wang G, Haefeli-Bleuer B, Ochs M, Weibel ER, Hoffman EA. Assessment of morphometry of pulmonary acini in mouse lungs by nondestructive imaging using multiscale microcomputed tomography. Proc Natl Acad Sci USA 109: 17105–17110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasilescu DM, Klinge C, Knudsen L, Yin L, Wang G, Weibel ER, Ochs M, Hoffman EA. Stereological assessment of mouse lung parenchyma via nondestructive, multiscale micro-CT imaging validated by light microscopic histology. J Appl Physiol 114: 716–724, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasilescu DM, Knudsen L, Ochs M, Weibel ER, Hoffman EA. Optimized murine lung preparation for detailed structural evaluation via micro-computed tomography. J Appl Physiol 112: 159–166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood SA, Zerhouni EA, Hoford JD, Hoffman EA, Mitzner W. Measurement of three-dimensional lung tree structures by using computed tomography. J Appl Physiol 79: 1687–1697, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31: 1116–1128, 2006. [DOI] [PubMed] [Google Scholar]