Abstract
Background
Workers in pesticide manufacturing industries are constantly exposed to pesticides. Genetic biomonitoring provides an early identification of potential cancer and genetic diseases in exposed populations. The objectives of this biomonitoring study were to assess DNA damage through comet assay in blood samples collected from industry workers and compare these results with those of classical analytical techniques used for complete blood count analysis.
Methods
Samples from controls (n = 20) and exposed workers (n = 38) from an industrial area in Multan, Pakistan, were subjected to various tests. Malathion residues in blood samples were measured by gas chromatography.
Results
The exposed workers who were employed in the pesticide manufacturing industry for a longer period (i.e., 13–25 years) had significantly higher DNA tail length (7.04 μm) than the controls (0.94 μm). Workers in the exposed group also had higher white blood cell and red blood cell counts, and lower levels of mean corpuscular hemoglobin (MCH), MCH concentration, and mean corpuscular volume in comparison with normal levels for these parameters. Malathion was not detected in the control group. However, in the exposed group, 72% of whole blood samples had malathion with a mean value of 0.14 mg/L (range 0.01–0.31 mg/L).
Conclusion
We found a strong correlation (R2 = 0.91) between DNA damage in terms of tail length and malathion concentration in blood. Intensive efforts and trainings are thus required to build awareness about safety practices and to change industrial workers' attitude to prevent harmful environmental and anthropogenic effects.
Keywords: comet assay, DNA damage, genotoxicity, hematological tests, malathion
1. Introduction
Pakistan's agricultural sector holds a significant position in its financial system and accounts for approximately 21% of the country's gross domestic product [1]. A major portion of crop production is lost annually because of the problems associated with growth of weeds, pests, and diseases [2]. This productivity loss coupled with population expansions have led to the increase in use of pesticides. The pesticides used must be toxic to the targeted organisms only. However, various studies have demonstrated the potential hazards of organophosphates, organochlorines, and carbamates for the environment and nontarget species [3]. To reduce their toxic effects, detoxification and biodegradation processes are available, which get activated upon entry of these substances into various compartments of the environment [4], [5]. However, inhalation of pesticides remains a serious threat and an important cause of health deterioration in human beings. Exposure to organophosphates could lead to abnormal sperms, fetal death, birth defects, hormonal changes, DNA damage, and changes in ovaries and eggs. Studies in literature have reported that workers exposed to pesticides are more prone to develop leukemia and prostrate, skin, and brain cancers compared with the general population [6], [7].
In general, biomonitoring tools complement the classical methods of monitoring. Genetic biomonitoring provides an early identification of potential cancer and genetic diseases in exposed populations [8]. Many techniques and approaches have been reported for human populations' monitoring [9]. Cytogenetic assays have been used by many researchers to evaluate the potential genotoxic effects of pesticides' exposures in occupationally exposed populations from different countries [10], [11]. Interestingly, in populations exposed to pesticides, both positive genotoxic effects [12] and negative findings [13], [14] have been reported. The contradictions in the data might depend on the type of pesticide used, protective measures adopted, and/or the cytogenetic end points considered. Comet assay or single-cell gel electrophoresis has been regularly used in biomonitoring studies. It has also been used to test the residual toxicities of biodegradation products [15]. Comet assay is a fast, low-cost, and sensitive tool for assessment of DNA damage (strand break) in single-cell organisms [16]. Generally, human populations have been exposed to different kinds of pesticides almost every day through different routes, including direct or indirect residues in food. However, pesticide workers in manufacturing units and the farmers responsible for spraying pesticides on crops have a high degree of exposure risk and may face health complications. Despite this high risk, only a limited number of studies focusing on the genotoxic effects of exposure to pesticide have been reported [16], [17], [18].
Pakistan is an agricultural country, and thus pesticides are produced throughout the year. Consequently, workers in pesticide manufacturing industries are constantly exposed to pesticides. However, only limited work regarding DNA damage at the cell level in exposed workers had been reported in literature [18]. In this context, the purpose of this study was to evaluate DNA damage at the cell level in workers at production units who are constantly exposed to pesticides and highlight the possible risks to the workers. The data generated would help the policy makers to have stringent rules to reduce the exposure risks during occupational activity. The industrial units selected for this study were involved in the production of carbamates, organophosphates, and pyrethroids. The specific objectives of this biomonitoring study were to (1) assess DNA damage through comet assay in blood samples collected from industry workers and (2) compare these results with those of classical analytical techniques used for complete blood count analysis.
2. Materials and methods
2.1. Study groups
A preliminary survey was conducted to identify target groups. The study included 58 individuals. A detailed questionnaire was developed, which was completed through face-to-face interaction. The questionnaire covered very basic questions such as medical history (vaccinations, exposure to X-rays, current treatment if any), demographic data (sex and age), lifestyle habits, working years for exposure period calculations and use of any protective equipment. All the individuals were divided into two groups, namely, control and exposed groups. Twenty healthy individuals (faculty and university students) were grouped into a control group. Through a questionnaire, it was ensured that the selected individuals have not been exposed to any kind of pesticide or other similar agent that can cause serious DNA damage. Thirty-eight individuals from the pesticide industry located in Multan, Pakistan, were included in the exposed group. Based on preliminary data from the questionnaire, smokers were excluded from the sample count. Moreover, individuals with hepatitis C infection were not considered for sampling. The exposure period linked to their employment varied from 6 months to 25 years. During production, all these individuals were simultaneously exposed to a complex mixture of pesticides. All the study participants in the control and exposed groups were informed of the study objectives. The research procedure followed in this study was approved by the Departmental Ethical Committee, Institute of Environmental Sciences and Engineering, National University of Sciences and Technology, Islamabad, Pakistan.
2.2. Blood sample collection
For the study of DNA damage, 5 mL of blood was collected in heparinized tubes containing EDTA as anticoagulant. The samples were collected in the morning at the time when workers logged in at the production plant. This was done to minimize the generation of false-positive results in the comet assay. All the samples were transported in cold chain to the laboratory. Samples were stored at 4°C in clean plastic vials for DNA damage assessments and pesticides analysis. A similar protocol was adapted for the control samples. To avoid interval-based interferences, blood samples from both groups were stored for equal periods, and then processed for further analysis.
2.3. Hematological analysis
Using an automatic analyzer (Medonic, Kobe, Japan), hematological analysis was performed on the freshly collected blood samples. The following hematological parameters were tested in whole blood samples: white blood cells (WBCs), red blood cells (RBCs), and hemoglobin (Hb). In addition, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and MCH concentration (MCHC) were also calculated from the data obtained. MCH gives the average weight of Hb in a single RBC. MCV reflects the size of RBCs by expressing the volume occupied by a single RBC. MCHC measures the average concentration of Hb in RBCs.
2.4. DNA damage analysis using the comet assay
The alkaline comet assay was performed as described by Singh et al [19], but with some minor adjustments. Slides were prepared in duplicate for each sample. Fully frosted microscope slides were covered with 50 μL of 2% normal-melting-point agarose (40–42°C). After application of a coverslip, the slides were allowed to gel at 4°C for 10 minutes. The coverslips were carefully removed, and an aliquot of 10 μL of whole blood and 65 μL of 1% normal-melting-point agarose (37°C) was pipetted onto the precoated slides and allowed to solidify at 4°C for 10 minutes after the application of coverslips. The slides were cleaned and dried, and then a final third low-melting-point agarose layer (75 μL) was applied. Coated slides were kept in a refrigerator at 4°C for 10–15 minutes to let the agarose solidify. Each comet assay run contained randomly selected samples from both controls and workers.
The slides without coverslips were immersed in cold, freshly prepared lysing solution (2.5M NaCl, 100mM EDTA, 10mM Trizma base, 1% Triton X-100, and 10% dimethyl sulfoxide added just before use), and refrigerated at 4°C for 10 hours. The slides were then placed in an alkaline buffer (300mM NaOH and 1mM EDTA, pH > 13) for 20 minutes at room temperature to allow DNA unwinding. Using chilled unwinding TBE buffer (Tris base, boric acid, and EDTA), electrophoresis was conducted for 45 minutes at 25 V (0.74 V/cm), adjusted to 300 mA in a horizontal electrophoresis apparatus (Mupid One, Japan). The slides were then neutralized three times (3 minutes/neutralization) with Tris–HCl buffer (pH 7.5). Slides were stained with 50 μL of 1× ethidium bromide and after placing cover slips, they were oven dried at 37°C for 1–2 hours before scoring. The whole procedure was carried out in dim light to minimize artifactual DNA damage. Analysis was performed using a 100× objective on a trinocular fluorescent microscope (OPTIKA B-353FL, Ponteranica, Italy). The microscope was equipped with an ocular micrometer of 10 μm, camera (AHD-Z600; AIPTEK, Willich, Viersen, Germany), and white LED/12-V 20-W illuminator. Only one person performed comet scoring to eliminate chances of personal variability.
Fifty cells were scored for each sample (i.e., 25 cells/slide). Normal cells have spherical or semispherical intact nucleus. By contrast, the damaged cells appear like comets [20]. Evaluation of the DNA “comet” tail shape and migration pattern allows for assessment of DNA damage [21], and the tail shape was observed and measured (tail length) using an ocular micrometer. Tail length was calculated using the following formula [22]:
| Comet tail length (μm) = maximum total length − head diameter comet tail length |
2.5. Pesticide concentration analysis
We analyzed the pesticide residues using gas chromatography (GC). The adapted methodology was developed by the United States Environmental Protection Agency (method 8141A) for evaluating organophosphorous compounds [23]. Pesticide analysis was performed on a gas chromatograph with a flame ionization detector (Shimadzu series 2010; Shimadzu, Tokyo, Japan) at Faculty of Biological Sciences, Quaid-e-Azam University, Islamabad, Pakistan. Pesticides standards for organophosphates pesticide and malathion were obtained from Sigma-Aldrich, Taufkirchen, Munich, Germany. Steam distillation using acetone and diethyl ether was performed just before use to separate the various compounds in the samples. All the solvents (i.e., hydrochloric acid, anhydrous sodium sulfate, and n-hexane) and glassware used were of GC grade. Standard malathion stock solution (20 mL) was prepared with a 1 mg/mL concentration in acetone (GC grade). Effective solutions were prepared in n-hexane. The pesticide stock solution was stored at 4°C (stable for 1 month). Working solutions of malathion pesticide were injected into the chromatograph and concentrations were calculated from the peaks obtained.
2.6. Statistical analysis
Statistical analysis of data was carried out using rules for normally distributed data. Correlation was also used to represent the linkage between pesticide concentration in blood and DNA damage (comet tail length).
3. Results
3.1. Hematology analysis
The blood samples were tested for complete blood count to correlate the genotoxicity with different human blood parameters. The test results were studied according to the guidelines and normal ranges of all parameters followed in Pakistan. Some hematological parameters were found to have unusual levels. Data on WBCs, RBCs, MCHC, and MCV are presented in Table 1. The WBC count significantly increased with increase in exposure period among workers. The exposed group had higher values for WBCs and RBCs and lower values for MCH, MCHC, and MCV than normal ranges (Table 1) prescribed by the World Health Organization (WHO). High WBC count can be a cause of leukemia, which is a cancer of the blood cells that have an abnormal increase of immature WBCs, a condition termed “blasts.”
Table 1.
Hematological analysis of blood samples from the study groups
| Exposure time (y) | n | White blood cells (x103/μL) | Red blood cells (M/μL) | Mean corpuscular hemoglobin (pg/cell) | Mean corpuscular hemoglobin concentration (g/dL) | Mean corpuscular volume (fL/cell) |
|---|---|---|---|---|---|---|
| Permissible range∗ | 3.5–9.5 | 3.5–5.5 | 25–35 | 31–38 | 75–100 | |
| Control | 20 | 7.5 ± 1.3 | 4.6 ± 0.8 | 29.5 ± 0.8 | 33.8 ± 0.9 | 86.8 ± 6.7 |
| ≤ 1 | 14 | 6.2 ± 2.1 | 4.9 ± 0.7 | 29.1 ± 1.7 | 33.6 ± 1.4 | 86.7 ± 5.4 |
| 2–4 | 10 | 7.1 ± 1.8 | 5.0 ± 0.3 | 28.7 ± 1.1 | 32.9 ± 1.0 | 85.8 ± 5.5 |
| 5–12 | 8 | 10.1 ± 0.9 | 4.9 ± 0.7 | 28.2 ± 0.9 | 31.8 ± 2.1 | 81.2 ± 6.4 |
| 13–25 | 6 | 10.8 ± 0.6 | 5.2 ± 0.2 | 24.7 ± 1.3 | 29.6 ± 1.5 | 77.6 ± 2.8 |
As recommended by the World Health Organization (Geneva, Switzerland).
3.2. DNA damage assessments
The mean age of the individuals in the control group and in the group exposed to pesticides was almost similar (27 ± 6.1 years vs. 32 ± 14.7 years). The mean comet lengths in blood cells of exposed workers and the individuals in the control group estimated using the comet assay are summarized in Table 2. Overall, the workers had a significantly greater mean DNA tail length than the controls (0.94 ± 0.2 μm vs. 7.04 ± 0.21 μm; Table 2). For analyzing age effects, the individuals in both the groups were divided into two subgroups: age < 27 years and age > 27 years. There was a significant effect of age on mean comet tail length for the exposed workers compared with the controls [0.94 ± 0.2 μm (controls) vs. 2.78 ± 0.81 μm (aged < 27 years) and 4.62 ± 2.14 μm (aged > 27 years)].
Table 2.
Mean comet tail length in the study groups according to the age and exposure time
| Parameters | n | Tail length (μm) |
|
|---|---|---|---|
| Control | Exposed | ||
| Age (y) | |||
| < 27 | 13, 19∗ | 0.94 ± 0.2 | 2.78 ± 0.81 |
| > 27 | 7, 19∗ | 0.94 ± 0.2 | 4.62 ± 2.14 |
| Exposure years | |||
| ≤ 1 | 14 | – | 2.29 ± 0.3 |
| 2–4 | 10 | – | 3.02 ± 0.28 |
| 5–12 | 8 | – | 4.07 ± 0.07 |
| 13–25 | 6 | – | 7.04 ± 0.21 |
Number of individuals (n) exposed in each (age-based worker) group when there were 13 and 7 individuals, respectively, in the control group.
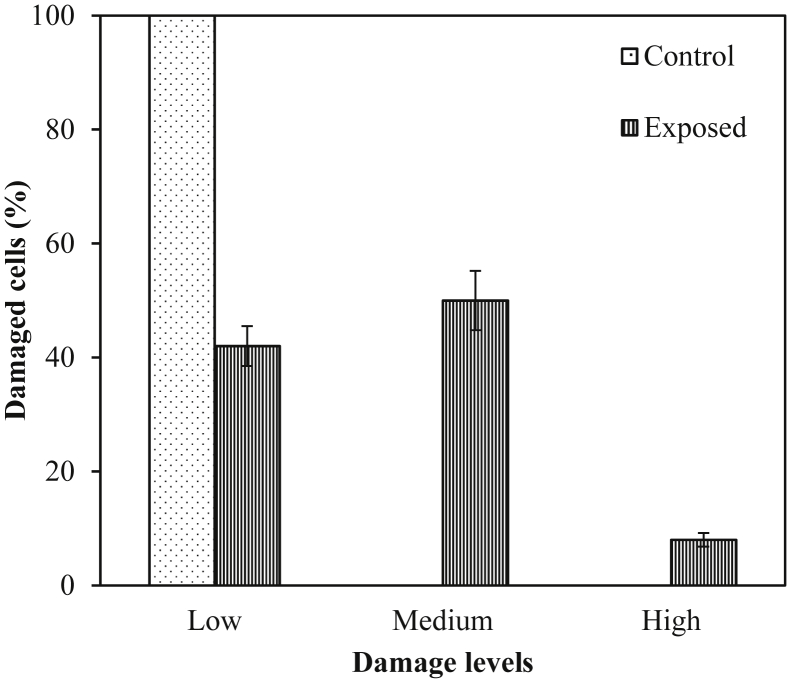
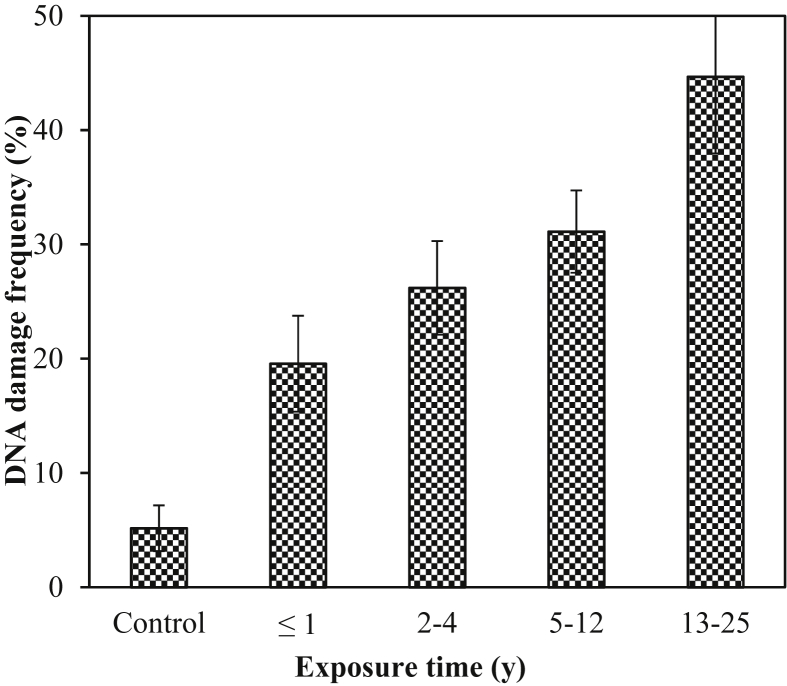
Fig. 1 shows the DNA damage levels in the control and exposed groups as the percentage of damaged cells in each category. The blood cells with comet appearance were further divided into three groups (low-, medium-, and high-level damage groups) according to the tail length. The low-level DNA damage group included those blood cells in which the length of DNA tail measured 0–3 μm; for the medium group, it measured between 3 μm and 6 μm and for the high-damage group, it was from 6 μm to 10 μm. All the control cells belonged to the low-level damage group. In exposed group, 50% of the damaged blood cells had low-level damage, 42% had medium-level damage and 8% had high-level damage. The mean DNA damage frequency increased with the increase in pesticides' exposure time (Fig. 2). The workers who had longer working years in pesticides industry had larger tail lengths and more DNA damage percentages compared with the new workers.
Fig. 1.
DNA damage levels in the control and exposed groups mentioned as percentage of damaged cells in each category. All the damaged cells in the control group had low-level damages. However, all the three categories were found in exposed ones. The tail length for low-, medium-, and high-level DNA damage varied from 0.1 μm to 3.0 μm, 3.1 μm to 6.0 μm, and 6.1 μm to 10 μm, respectively.
Fig. 2.
Exposure period and DNA damage frequency, which was calculated using the following formula: number of damaged cells/total number of cells scored × 100.
3.3. Concentrations of pesticides in blood and genotoxicity
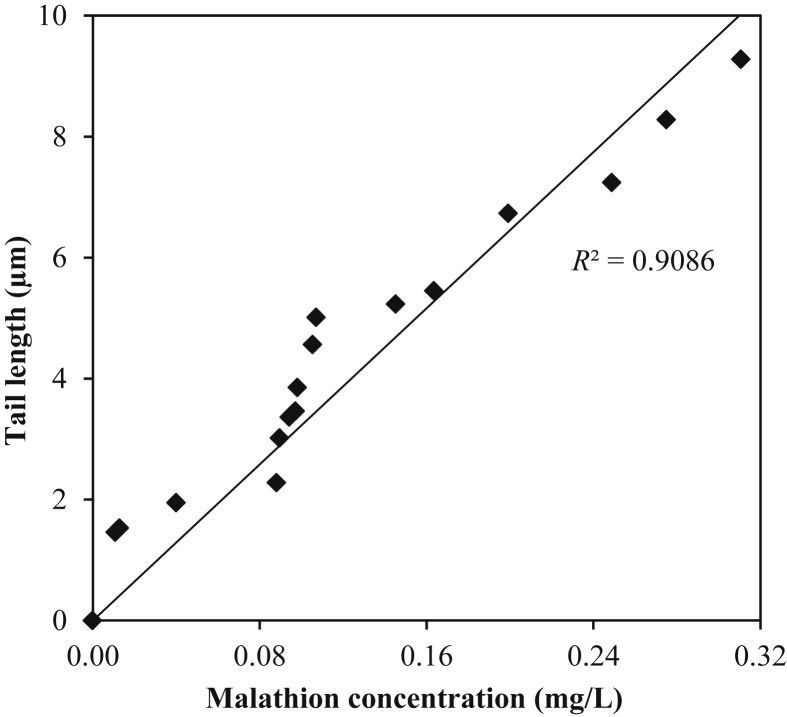
Workers in the pesticides industry have constant exposure to a number of pesticides, so as a representative of these chemicals, we determined the malathion levels in the blood samples of individuals from both study groups. GC was used to detect the concentration of pesticide residues in exposed blood samples and the results were compared with the control blood samples. An average adult human male body contains 4.7–5 L of blood. Malathion was detected in 72% of the exposed blood samples with an average value of 0.14 mg/L (range 0.01–0.31 mg/L). Each value is the average of triplicate measurements; however, control samples had undetectable levels of these residues. The linear fitting curve in Fig. 3 shows the significant correlation (R2 = 0.91) of malathion concentration with the comet tail length. The linear correlation between malathion residues in blood and DNA damage is very alarming, and highlights the risks involved in direct exposure during the production activity.
Fig. 3.
Relationship between malathion concentrations in blood and comet tail length. Data from 17 individuals are presented here.
4. Discussion
This study is a recent addition to a limited number of in vivo investigations reported in the literature about pesticide exposure. Changes in Hb levels have been linked with high exposure to pesticides and chemicals [24]. Significant associations were also found for Hb, hematocrit, RBCs, WBCs, and platelet count [25]. However, it is not possible to draw a conclusive evidence of toxicity based on hematological measurements alone as a majority of the factors had values within the permissible limits specified by WHO [26]. Therefore, there is a need to develop a more sensitive method to highlight early DNA damages in the exposed individuals.
In a study reported in literature [27], comet assay was successfully used to quantify DNA damage levels in leukocytes from occupationally exposed French farmers. In that study, genetic damage was reported to be very high among the studied individuals [27]. Using the comet assay, Garaj-Vrhovac and Zeljezic [16] reported increased DNA damage in lymphocytes in workers of a production unit. Our results are in accordance with the aforementioned studies as well as with others in the literature [11], [28], [29]. The exposure period and increase in age of workers cause an increase in WBCs count, and this is a clear indicator of damage to DNA and blood cells. These modifications are reported as a cause of genotoxicity [30].
The malathion residues in blood samples showed the sensitivity of comet assay to measure genotoxicity. In this study, most of the pesticides industry workers had compound pesticide residues above the allowable daily intake in their blood, which is injurious to health. Although malathion generally does not produce mutations and genotoxic effects, its metabolite malaoxon was reported to cause genetic damages in mammalian cell mutation tests [31] as well as in humans [32].
In conclusion, exposure period and age positively affect DNA comet tail length. Based on information gathered from the hematological analysis, comet assay, and pesticide concentration analysis, it is clear that comet assay is an effective tool for biomonitoring and toxicological studies. Insecure practices in industrial workers resulted in significant increase in DNA damage and increased WBC count. Furthermore, the pesticide industry workers had little or no knowledge about the health and safety measures. Intensive efforts and trainings are thus required to build awareness about safety practices and to change industrial workers' attitude to prevent harmful environmental and anthropogenic effects.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research work was funded by the Higher Education Commission of Pakistan (Grant No. PM-IPFP/HRD/HEC/2010/1501). We are especially grateful to Dr Niaz Ahmad, Assistant Chemist, Pesticides Quality Control Laboratory, Government of Punjab, Multan, Pakistan, for helping us in getting access to pesticide production units and sampling. We are also thankful to production managers of the pesticide industries for their kind cooperation during sample collections.
References
- 1.Pakistan Economic Survey: 2011–12. Pakistan Bureau of Statistics; Islamabad (Pakistan): 2012. [Google Scholar]
- 2.Food and Agriculture Organization (FAO) of the United Nations . FAO of the United Nations; Rome (Italy): 2009. How to feed the world in 2050? [Google Scholar]
- 3.Huang Y., Liu J., Li L., Pang T., Zhang L. Efficacy of binary combinations of botanical pesticides for rotifer elimination in microalgal cultivation. Bioresour Technol. 2014;154:67–73. doi: 10.1016/j.biortech.2013.11.098. [DOI] [PubMed] [Google Scholar]
- 4.Ntougias S., Melidis P., Navrozidou E., Tzegkas F. Diversity and efficiency of anthracene-degrading bacteria isolated from a denitrifying activated sludge system treating municipal wastewater. Int Biodeterior Biodegrad. 2015;97:151–158. [Google Scholar]
- 5.Dong W., Hou Y., Xi X., Wang F., Li Z., Ye X., Huang Y., Cui Z. Biodegradation of fenoxaprop-ethyl by an enriched consortium and its proposed metabolic pathway. Int Biodeterior Biodegrad. 2015;97:159–167. [Google Scholar]
- 6.Cabello G., Valenzuela M., Vilaxa A., Durán V., Rudolph I., Hrepic N., Calaf G. A rat mammary tumor model induced by the organophosphorus pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environ Health Perspect. 2001;109:471–479. doi: 10.1289/ehp.01109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merhi M., Raynal H., Cahuzac E., Vinson F., Cravedi J.P., Gamet-Payrastre L. Occupational exposure to pesticides and risk of hematopoietic cancers: meta-analysis of case-control studies. Cancer Causes Control. 2007;18:1209–1226. doi: 10.1007/s10552-007-9061-1. [DOI] [PubMed] [Google Scholar]
- 8.Kvitko K., Bandinelli E., Henriques J.A., Heuser V.D., Rohr P., da Silva F.R., Schneider N.B., Fernandes S., Ancines C., da Silva J. Susceptibility to DNA damage in workers occupationally exposed to pesticides, to tannery chemicals and to coal dust during mining. Genet Mol Biol. 2012;35:1060–1068. doi: 10.1590/s1415-47572012000600022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira T.S., Beltrami L.S., Rocha J.A., Broto F.P., Comellas L.R., Salvadori D.M., Vargas V.M. Toxicogenetic monitoring in urban cities exposed to different airborne contaminants. Ecotoxicol Environ Saf. 2013;90:174–182. doi: 10.1016/j.ecoenv.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Zeljezic D., Garaj-Vrhovac V. Chromosomal aberration and single cell gel electrophoresis (comet) assay in the longitudinal risk assessment of occupational exposure to pesticides. Mutagenesis. 2011;16:359–363. doi: 10.1093/mutage/16.4.359. [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Arroyo S., Díaz-Sánchez Y., Meneses-Pérez M.A., Villalobos-Pietrini R., De León-Rodríguez J. Cytogenetic biomonitoring in a Mexican floriculture worker group exposed to pesticides. Mutat Res. 2000;466:117–124. doi: 10.1016/s1383-5718(99)00231-4. [DOI] [PubMed] [Google Scholar]
- 12.Bolognesi C., Parrini M., Bonassi S., Ianello G., Salanitto A. Cytogenetic analysis of a human population occupationally exposed to pesticides. Mutat Res. 1993;285:239–249. doi: 10.1016/0027-5107(93)90112-s. [DOI] [PubMed] [Google Scholar]
- 13.Lucero L., Pastor S., Suárez S., Durbán R., Gómez C., Parrón T., Creus A., Marcos R. Cytogenetic biomonitoring of Spanish greenhouse workers exposed to pesticides: micronuclei analysis in peripheral blood lymphocytes and buccal epithelial cells. Mutat Res. 2000;464:255–262. doi: 10.1016/s1383-5718(99)00200-4. [DOI] [PubMed] [Google Scholar]
- 14.Pastor S., Gutiérrez S., Creus A., Xamena N., Piperakis S., Marcos R. Cytogenetic analysis of Greek farmers using the micronucleus assay in peripheral lymphocytes and buccal cells. Mutagenesis. 2001;16:539–545. doi: 10.1093/mutage/16.6.539. [DOI] [PubMed] [Google Scholar]
- 15.Swati P.G., Das M.T., Thakur I.S. In vitro toxicity evaluation of organic extract of landfill soil and its detoxification by indigenous pyrene-degrading Bacillus sp. ISTPY1. Int Biodeterior Biodegrad. 2014;90:145–151. [Google Scholar]
- 16.Garaj-Vrhovac V., Zeljezic D. Evaluation of DNA damage in workers occupationally exposed to pesticides using single-cell gel electrophoresis (SCGE) assay. Pesticide genotoxicity revealed by comet assay. Mutat Res. 2000;469:279–285. doi: 10.1016/s1383-5718(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 17.Padmavathi P., Aruna Prabhavathi P., Reddy P.P. Frequencies of SCEs in peripheral blood lymphocytes of pesticides workers. Bull Environ Contam Toxicol. 2000;64:155–160. doi: 10.1007/s001289910024. [DOI] [PubMed] [Google Scholar]
- 18.Bhalli J.A., Khan Q.M., Nasim A. DNA damage in Pakistani pesticide-manufacturing workers assayed using the comet assay. Environ Mol Mutagen. 2006;47:587–593. doi: 10.1002/em.20232. [DOI] [PubMed] [Google Scholar]
- 19.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 20.Danadevi K., Rozati R., Saleha Banu B., Hanumanth Rao P., Grover P. DNA damage in workers exposed to lead using comet assay. Toxicology. 2003;187:183–193. doi: 10.1016/s0300-483x(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 21.Grover P., Danadevi K., Mahboob M., Rozati R., Banu S.B., Rahman M.F. Evaluation of genetic damage in workers employed in pesticide production utilizing the comet assay. Mutagenesis. 2003;18:201–205. doi: 10.1093/mutage/18.2.201. [DOI] [PubMed] [Google Scholar]
- 22.Heepchantree W., Paratasilpin T., Kangwanpong D. A biological evaluation of DNA damage detected by comet assay in healthy populations residing in areas that differ in lung cancer incidence. J Toxicol Environ Health A. 2006;69:1071–1082. doi: 10.1080/15287390500360257. [DOI] [PubMed] [Google Scholar]
- 23.United States Environmental Protection Agency (USEPA) Office of Pesticides Programs. Annual report [Internet]. Washington DC (WA): USEPA; 2004 [cited 24 Nov 2015]. Available from: http://www.epa.gov/oppfead/annual/2002/2022.
- 24.Srivastava A.K., Gupta B.N., Bihari V., Mathur N., Pangtey B.S., Bharti R.S. Chronic effects of hexachlorocyclohexane exposure: clinical, hematologic and electrocardiographic studies. Vet Hum Toxicol. 1995;37:302–305. [PubMed] [Google Scholar]
- 25.Del Prado-Lu J.L. Pesticide exposure, risk factors and health problems among cutflower farmers: a cross sectional study. J Occup Med Toxicol. 2007;2:9. doi: 10.1186/1745-6673-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO) WHO; Geneva (Switzerland): 2010. Exposure to malathion: a major public health concern. Preventing disease through healthy environments. [Google Scholar]
- 27.Lebailly P., Vigreux C., Lechevrel C., Ledemeney D., Godard T., Sichel F., LeTalaër J.Y., Henry-Amar M., Gauduchon P. DNA damage in mononuclear leukocytes of farmers measured using the alkaline comet assay: discussion of critical parameters and evaluation of seasonal variations in relation to pesticide exposure. Cancer Epidemiol Biomarkers Prev. 1998;7:917–927. [PubMed] [Google Scholar]
- 28.Antonucci G.A., de Syllos Cólus I.M. Chromosomal aberrations analysis in a Brazilian population exposed to pesticides. Teratog Carcinog Mutagen. 2000;20:265–272. [PubMed] [Google Scholar]
- 29.Shaham J., Kaufman Z., Gurvich R., Levi Z. Frequency of sister-chromatid exchange among greenhouse farmers exposed to pesticides. Mutat Res. 2001;491:71–80. doi: 10.1016/s1383-5718(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 30.Khalaf-Allah S.S. Effect of pesticide water pollution on some haematological, biochemical and immunological parameters in Tilapia nilotica fish. Dtsch Tierarztl Wochenschr. 1999;106:67–71. [PubMed] [Google Scholar]
- 31.Flessel P., Quintana P.J., Hooper K. Genetic toxicity of malathion: a review. Environ Mol Mutagen. 1993;22:7–17. doi: 10.1002/em.2850220104. [DOI] [PubMed] [Google Scholar]
- 32.Foureman P., Mason J.M., Valencia R., Zimmering S. Chemical mutagenesis testing in Drosophila. X. Results of 70 coded chemicals tested for the National Toxicology Program. Environ Mol Mutagen. 1994;23:208–227. doi: 10.1002/em.2850230310. [DOI] [PubMed] [Google Scholar]