ABSTRACT
Anterior signaling centers help specify and pattern the early anterior neuroectoderm (ANE) in many deuterostomes. In sea urchin the ANE is restricted to the anterior of the late blastula stage embryo, where it forms a simple neural territory comprising several types of neurons as well as the apical tuft. Here, we show that during early development, the sea urchin ANE territory separates into inner and outer regulatory domains that express the cardinal ANE transcriptional regulators FoxQ2 and Six3, respectively. FoxQ2 drives this patterning process, which is required to eliminate six3 expression from the inner domain and activate the expression of Dkk3 and sFRP1/5, two secreted Wnt modulators. Dkk3 and low expression levels of sFRP1/5 act additively to potentiate the Wnt/JNK signaling pathway governing the positioning of the ANE territory around the anterior pole, whereas high expression levels of sFRP1/5 antagonize Wnt/JNK signaling. sFRP1/5 and Dkk3 levels are rigidly maintained via autorepressive and cross-repressive interactions with Wnt signaling components and additional ANE transcription factors. Together, these data support a model in which FoxQ2 initiates an anterior patterning center that implements correct size and positions of ANE structures. Comparisons of functional and expression studies in sea urchin, hemichordate and chordate embryos reveal striking similarities among deuterostome ANE regulatory networks and the molecular mechanism that positions and defines ANE borders. These data strongly support the idea that the sea urchin embryo uses an ancient anterior patterning system that was present in the common ambulacrarian/chordate ancestor.
KEY WORDS: Neuroectoderm patterning, Wnt signal transduction, Anterior-posterior, Deuterostome evolution, Gene regulatory networks, Dkk3, sFRP1/5, Strongylocentrotus purpuratus
Summary: FoxQ2 activates an anterior signaling center that expresses the secreted Wnt modulators sFRP1/5 and Dkk3 – essential components of the Wnt network governing anterior-posterior neuroectoderm patterning.
INTRODUCTION
The embryonic anterior neuroectoderm (ANE) territory of deuterostomes develops into a variety of neurosensory organs, from the simple apical tuft of sea urchin embryos to the complex vertebrate forebrain and eye field. Although there are significant differences in the nervous systems created from this early territory among the deuterostomes, the ANE initially arises from a relatively simple, flat neuroepithelium during the early stages of development and many of the cell types that this territory produces are remarkably conserved (Burke et al., 2014; Castro et al., 2015; Cavodeassi, 2013; Garner et al., 2015; Pani et al., 2012; Range, 2014). Based on the spatial and temporal expression of ANE regulatory genes in several species, it appears that the ANE gene regulatory network (GRN) hierarchy is also remarkably conserved among many deuterostome embryos, suggesting that these could be homologous territories (Range, 2014). Strengthening this view, it appears that aspects of the mechanism used to position the ANE territory around the anterior pole might also be shared among deuterostomes (Range, 2014). For example, early ANE genes, such as six3, are broadly expressed throughout the presumptive ectoderm in most invertebrate deuterostomes and the presumptive neuroectoderm in vertebrates (Darras et al., 2011; Kozmik et al., 2007; Lagutin et al., 2003; Posnien et al., 2011; Range, 2014; Wei et al., 2009). Then, a molecular mechanism that depends on a posterior-to-anterior cascade of Wnt/β-catenin signaling activity downregulates ANE factors from the more posterior ectoderm so that their activities are restricted to a region around the anterior pole (Darras et al., 2011; Kiecker and Niehrs, 2001; Nordström et al., 2002; Range et al., 2013; Yaguchi et al., 2008).
In the sea urchin embryo, six3 and foxq2 are the first ANE regulatory genes expressed in the presumptive ectoderm and both transcription factors act as cardinal regulators of ANE territory specification (Wei et al., 2009; Yaguchi et al., 2008, 2011, 2010). Similar to other deuterostome embryos, six3 and foxq2 initially exhibit pan-ectodermal expression, but then their expression is progressively restricted to a territory around the anterior pole (Wei et al., 2009; Yaguchi et al., 2008). Based on functional and expression experiments it appears that these cardinal regulators are expressed in two concentric domains around this pole, with six3 expressed in the outer ANE and foxq2 expressed around the central ANE territory (Li et al., 2014; Wei et al., 2009; Yaguchi et al., 2008). Six3 sits at or near the top of the entire ANE GRN and antagonizes posterior-to-anterior Wnt signaling (Wei et al., 2009). FoxQ2 is necessary for serotonergic neural specification and acts as a cardinal regulator of apical tuft specification in the central ANE subdomain (Yaguchi et al., 2012, 2010). Remarkably, the ANE positioning mechanism involves much more than the Wnt/β-catenin signaling pathway in the sea urchin. It depends on the ability to integrate information from at least three different Wnt signaling branches, namely Wnt/β-catenin, Wnt/JNK and Wnt/PKC (see Fig. 8A) (Range et al., 2013), representing one of the few examples of all three being integrated into an overall Wnt signaling network that impinges on the same developmental process. In addition, comparative functional and expression data from vertebrates, amphioxus, hemichordates and sea star suggest that aspects of this Wnt network could be widely shared among deuterostome embryos (Range, 2014).
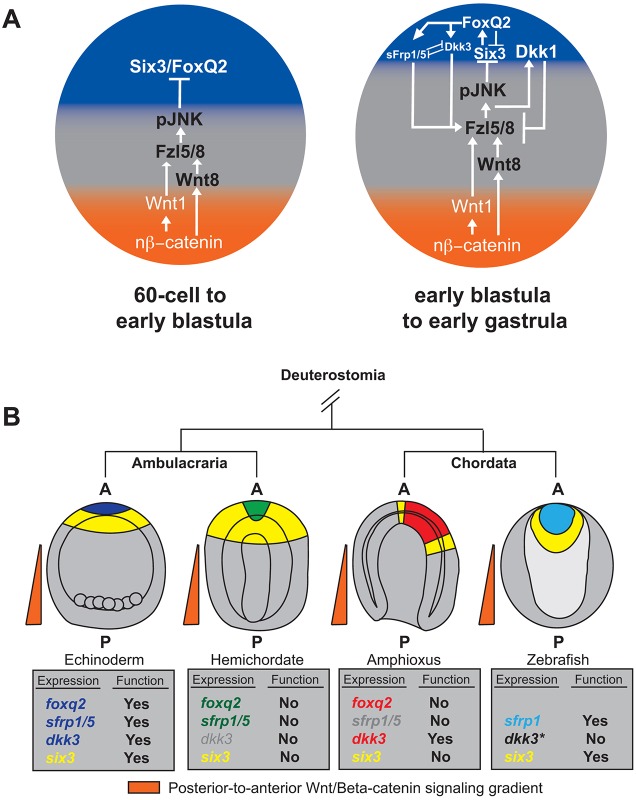
Fig. 8.
An extended model for ANE restriction in the sea urchin embryo and the conservation of anterior signaling centers among deuterostome embryos. (A) In sea urchin early development, the Wnt/β-catenin, Wnt/JNK and Fzl1/2/7-PKC pathways all converge on the same developmental process: ANE restriction. Illustrated is an extended model for sea urchin ANE restriction that integrates the FoxQ2, sFRP1/5 and Dkk3 signaling center (see main text for details). nβ-catenin, nuclear β-catenin. (B) The territorial expression of foxq2, six3, sfrp1/5 and dkk3 orthologs in ANE territories during the gastrula stages of several deuterostome model species. The embryos are colored to indicate the location of six3 and foxq2 in relation to orthologs of sea urchin sfrp1/5 and dkk3; colors match the regulatory factors in the boxes beneath (no expression data are available for genes in gray). The boxes also indicate whether expression or functional studies have been performed on each regulatory factor in each deuterostome model species (data are from Darras et al., 2011; Fritzenwanker et al., 2014; Hsu et al., 2010; Kozmik et al., 2007; Onai et al., 2012; Pani et al., 2012; Range, 2014; Seo et al., 1998; Tendeng and Houart, 2006; Yu et al., 2003). *Expression studies on dkk3 have been performed in zebrafish, but it is not clear from the available data where dkk3 is expressed at the late gastrula stage. At the early shield stage (6 hpf) dkk3 appears to be expressed broadly throughout the embryo, and expression appears to be restricted to the ANE region by 24 hpf (∼26-somite stage) (Hsu et al., 2010). A, anterior; P, posterior.
In vertebrate embryos, specific territories within the presumptive ANE (forebrain and eye field) activate signaling centers that function to subdivide the territory into the telencephalon, diencephalon, and eye fields, once the ANE is positioned around the anterior pole of the early neural plate (Houart et al., 2002; Wilson and Houart, 2004). One of the most important of these signaling centers forms at the anteriormost region of the neural plate, termed the anterior neural ridge (ANR) (Cavodeassi and Houart, 2012; Echevarría et al., 2003). Secreted Frizzled-related proteins (sFRPs) are secreted from the ANR and play key roles in establishing a posterior-to-anterior Wnt signaling gradient within the early anterior neural plate, which is necessary to establish structurally and functionally distinct subregions within the ANE (Cavodeassi and Houart, 2012). sFRPs comprise the largest family of Wnt modulators in the animal kingdom (Bovolenta et al., 2008). They contain a cysteine-rich domain (CRD) that is almost identical to the CRD of the Wnt receptor Frizzled (Fzl), and several studies suggest that sFRPs could bind Wnt via this domain and inhibit the ability of Wnt to activate the Fzl receptor (Bafico et al., 1999; Bhat et al., 2007; Lin et al., 1997). However, recent data inconsistent with this model for sFRP function suggest, alternatively, that they can act as Wnt signaling agonists. For example, several studies suggest that sFRP may augment Wnt signaling by facilitating interactions between the Fzl receptor and Wnt or by activating Wnt signaling independently of Wnt ligands, since sFRPs can form complexes with Fzl receptors (Bafico et al., 1999; Dufourcq et al., 2008; Esteve et al., 2011; Mii and Taira, 2009, 2011; Rodriguez et al., 2005). Based on these apparently conflicting results, it has been proposed that the functions of sFRPs depend on their expression levels as well as the molecular and cellular context (Bovolenta et al., 2008; Mii and Taira, 2011).
Dickkopf family members are expressed in ANE territories in many deuterostome embryos from sea urchins to vertebrates and appear to play conserved roles in establishing the ANE. The family is divided into two subclasses, with one branch including Dkk1, Dkk2 and Dkk4 and the other Dkk3. Many studies indicate that the most common function for Dkk1, Dkk2 and Dkk4 is to antagonize Wnt signaling by interfering with the Fzl co-receptors Lrp5/6 and Kremen (Bafico et al., 2001; Cruciat and Niehrs, 2013; Mao et al., 2001; Semënov et al., 2001). By contrast, secreted Dkk3 does not appear to bind either Fzl receptors or Wnt signaling co-receptors, and studies focusing on its function do not point to a clear role for Dkk3 in modulating Wnt signaling (Veeck and Dahl, 2012).
Here, we show that as the ANE begins to be established around the anterior pole of the sea urchin embryo, the cardinal ANE regulatory factor FoxQ2 establishes the anteriormost ANE territory, separating the ANE into two concentric domains by the beginning of gastrulation. We also found that FoxQ2 activates an anterior signaling center that secretes the Wnt modulators sFRP1/5 and Dkk3, which work additively through the Fzl5/8-JNK-mediated ANE positioning mechanism to establish the correct size of the entire ANE territory. In addition, our data indicate that sFRP1/5 and Dkk3 tightly control one another's expression while at the same time regulating their own. Finally, comparison of functional and expression data from several deuterostomes with the data from this study suggests that anterior signaling centers necessary for ANE patterning existed in the last common ancestor of ambulacrarians and chordates.
RESULTS
Two concentric ANE territories
The first ANE genes known to be expressed in the sea urchin embryo are six3 and foxq2 (Wei et al., 2009; Yaguchi et al., 2008). Their expression domains appear to be congruent in the same anterior ectoderm cells during the initial stages of ANE specification, when they are expressed throughout the anterior half of the 32- to 60-cell stage embryo (Fig. 1A) (Range et al., 2013; Wei et al., 2009; Yaguchi et al., 2008). As the Wnt/JNK-mediated ANE positioning mechanism downregulates six3 and foxq2 transcripts in the more posterior ectoderm cells, expression of both transcripts remains very similar within the anterior ectoderm through the blastula stages [as late as 18-20 hours post fertilization (hpf) in S. purpuratus] (Fig. 1A, middle) (Range et al., 2013; Wei et al., 2009; Yaguchi et al., 2008). Then, beginning in late blastula stages, their transcripts appear to accumulate in separate ANE territories (Fig. 1A, right; Fig. 2) (Li et al., 2014; Wei et al., 2009; Yaguchi et al., 2008).
Fig. 1.
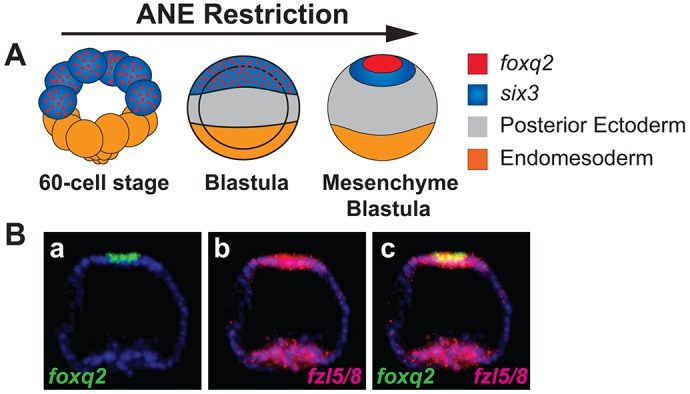
The ANE territory is segregated into two concentric domains. (A) Schematic model showing the progressive positioning of expression of the cardinal ANE regulators foxq2 and six3 around the anterior pole of early S. purpuratus embryos from the 60-cell to the mesenchyme blastula stage (data from Li et al., 2014; Wei et al., 2009; Yaguchi et al., 2008). (Ba-c) foxq2 expression is nested within the fzl5/8 expression domain and is surrounded by six3 expression. Two-color in situ hybridization for foxq2 (green) and fzl5/8 (red) transcript expression at the mesenchyme blastula stage; overlap appears in yellow.
Fig. 2.
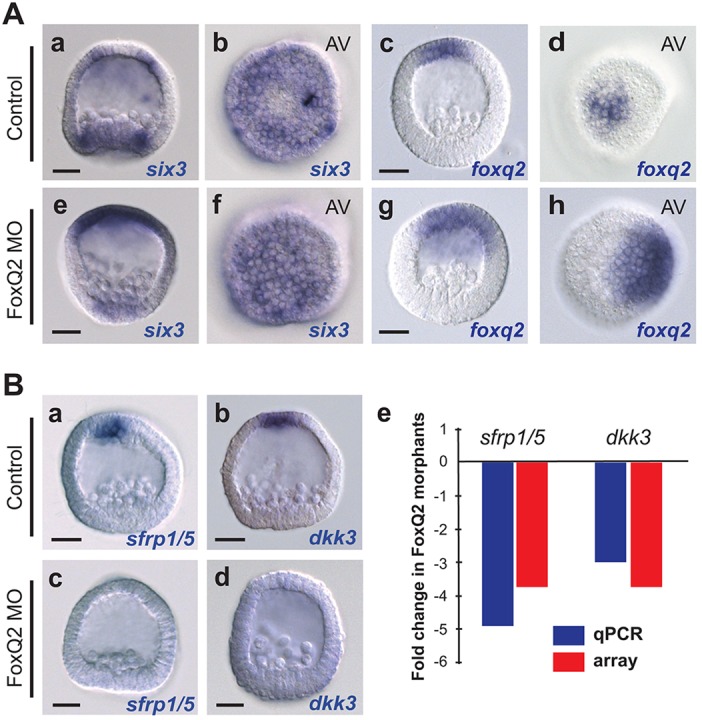
FoxQ2 defines the central ANE territory, where it activates sFRP1/5 and Dkk3. (A) (a,b) six3 is expressed in a ring of ANE cells surrounding the central anterior pole of the 24 hpf embryo. (c,d) foxq2 expression around the anterior pole of the embryo. (e-h) Embryos injected with a FoxQ2 morpholino express six3 throughout the ANE territory (compare e,f with a,b). six3 and foxq2 (g,h) expression is expanded towards the vegetal/posterior pole in FoxQ2 morphants. AV, anterior view. (B) (a-d) Injecting a FoxQ2 morpholino into embryos completely eliminates sfrp1/5 and dkk3 expression. (e) qPCR analysis confirms downregulation of sfrp1/5 and dkk3 transcripts in FoxQ2 morphants. Microarray results are shown for comparison. MO, morpholino. Scale bars: 20 µm.
In a previous study, we showed that Fzl5/8 signaling activates the secreted Wnt signaling antagonist Dkk1 around the anterior pole during the final stages of ANE positioning and, in a negative-feedback loop, Dkk1 blocks further ANE GRN downregulation by Fzl5/8-JNK signaling, establishing the ANE territory. As a result of this negative-feedback loop fzl5/8 expression is maintained in the ANE territory, and the functional data indicate that, along with dkk1, it marks the entire presumptive ANE territory at the mesenchyme blastula stage (24 hpf) (Range et al., 2013). In the current study, double-label in situ hybridization with fzl5/8 and foxq2 probes revealed that foxq2 is restricted to a central territory around the anterior pole, nested within the fzl5/8 expression domain (Fig. 1Ba-c), consistent with the idea that there are at least two concentric ANE regulatory domains.
FoxQ2 determines the inner ANE territory and activates the secreted signaling modulators sFRP1/5 and Dkk3
six3 transcripts are expressed broadly and uniformly around the anterior pole from early- to mid-blastula stages (Wei et al., 2009). Then, during later blastula stages, six3 transcripts are downregulated in the central anterior pole region (Wei et al., 2009), resulting in a ring of six3 expression by mesenchyme blastula stages (24 hpf; Fig. 2Aa,b), which appears to extend to the outer edges of the ANE territory. Interestingly, foxq2 is expressed in the anteriormost territory where six3 expression was downregulated (Fig. 2Aa-d). These observations indicate that, although six3 and foxq2 are both initially restricted to the anterior pole by the Wnt-mediated anteroposterior positioning mechanism, they subsequently respond differently to this and/or some other signaling mechanism, resulting in two concentric domains of expression.
Based on the expression patterns of six3 and foxq2 at the mesenchyme blastula stage (24 hpf) and the fact that FoxQ2 acts as a cardinal regulator of many cell fates in the central ANE territory (Yaguchi et al., 2012, 2011, 2010), we reasoned that FoxQ2 might play a crucial role in establishing the inner and outer ANE domains. To test this hypothesis, we monitored the expression of six3 at the mesenchyme blastula stage in embryos injected with FoxQ2 morpholino oligonucleotides. In the absence of FoxQ2, six3 transcripts were no longer expressed in a ring around the anterior pole, but were instead expressed throughout an expanded ANE territory (Fig. 2Ae,f). This indicates that FoxQ2 activity is necessary for establishing a distinct regulatory domain in the central animal pole region, confirming similar data previously reported in S. purpuratus (Li et al., 2014). Interestingly, the expression domains of both foxq2 and six3 also expanded towards the posterior pole of the embryo (Fig. 2Aa,c,e,g). This surprising result suggests that FoxQ2 directly or indirectly limits the range of its own expression and is necessary for sizing the ANE.
We have shown previously that the size of the ANE territory depends on a remarkable balance between Wnt potentiation and Wnt antagonism along the anteroposterior axis of the sea urchin embryo (Range et al., 2013). Thus, we hypothesized that FoxQ2 might activate the expression of secreted Wnt modulators, the absence of which would cause the ANE expansion observed in the absence of FoxQ2. We identified from a differential microarray screen two potential Wnt modulators, namely sFRP1/5 and Dkk3, that were severely downregulated in the absence of FoxQ2. To confirm this result we analyzed the effect of FoxQ2 knockdown by qPCR (Fig. 2Be) and in situ hybridization (Fig. 2Ba-d) assays, which showed that both sfrp1/5 and dkk3 are severely downregulated in the absence of FoxQ2 (Fig. 2B).
sfrp1/5, dkk3 and foxq2 expression overlap during ANE restriction
We performed in situ hybridization with sfrp1/5, dkk3 and foxq2 probes to determine the developmental time course of their expression. sfrp1/5 and dkk3 transcripts were first detectable after the initial expression of foxq2, which is initiated at the 32-cell stage and extends throughout the presumptive ectoderm by the 32- to 60-cell stage (7-9 hpf) (Fig. 3A). sfrp1/5 expression was first detected at the 120-cell stage (12 hpf) and dkk3 expression at the hatched blastula stage (15 hpf) (Fig. 3). From the onset of expression, sfrp1/5 and dkk3 transcripts were progressively downregulated from posterior ectoderm to a region that approximates the central ANE domain, mimicking foxq2 expression. These data suggest that the sfrp1/5 and dkk3 expression domains overlap exactly with that of foxq2 at later stages. To test this hypothesis we performed double-label in situ hybridization with probes for these signaling modulators and foxq2 at the mesenchyme blastula stage (24 hpf) and confirmed that their expression is congruent with that of foxq2 transcripts at the anterior pole of the embryo (Fig. S1A,B).
Fig. 3.
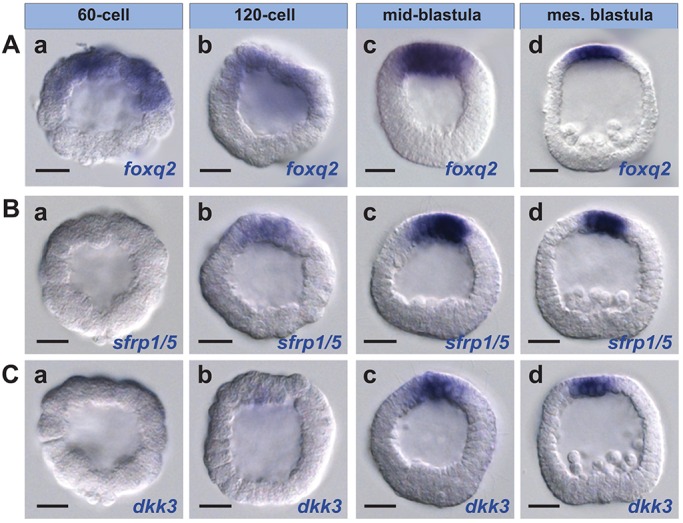
Expression of sfrp1/5, dkk3 and foxq2 transcripts overlaps during ANE restriction. (Aa-d) foxq2 expression is first detected at the 32- to 60-cell stage in anterior/animal blastomeres (Aa shows a 60-cell embryo). (Ba-d) sfrp1/5 expression is first detected at the 120-cell stage within the anterior/animal half of the embryo. (Ca-d) dkk3 expression is first detected in 15-16 hpf blastula embryos within the anterior/animal half of the embryo. mes., mesenchyme. Scale bars: 20 µm.
FoxQ2 activates an anterior signaling center
FoxQ2 activates sfrp1/5 and dkk3 within the central ANE territory during the middle to late stages of ANE restriction, consistent with a role for these genes in mediating the function of FoxQ2 in sizing the ANE. If this hypothesis is true, then the ANE territory should expand in the absence of sFRP1/5 and/or Dkk3, as observed in FoxQ2 morphants (Fig. 2Ac,g). We tested this by morpholino knockdown and measured the expansion and/or upregulation of several ANE factors by in situ hybridization and qPCR at the end of ANE positioning [mesenchyme blastula stage (24-26 hpf) in S. purpuratus]. Embryos injected with either of two morpholinos targeting sfrp1/5 (sFRP1/5 MO1 and MO2) or two targeting dkk3 (Dkk3 MO1 and MO2) showed posterior expansion of ANE markers (Fig. 4A,B, Fig. S2A,B). In contrast to FoxQ2 morphants, in which six3 was expressed uniformly throughout the ANE territory, six3 was expressed in a thin ring of ectoderm cells near the embryonic equator, presumably because it was downregulated by FoxQ2 throughout the expanded central territory (Fig. 2Ae, Fig. 4Ag,k). Moreover, at the mesenchyme blastula stage (24-26 hpf), the expression of several ANE regulatory factors that depend on Six3 and/or FoxQ2 was upregulated in the absence of either secreted signaling modulator (Fig. 4C).
Fig. 4.
sFRP1/5 and Dkk3 size the sea urchin ANE. (A) foxq2, nkx3.2 and six3 expression at mesenchyme blastula stages in control (a-c), sFRP1/5 morpholino knockdown (e-g) and Dkk3 morpholino knockdown (i-k) embryos. (d,h,l) Serotonergic neurons (green) in control, sFRP1/5 and Dkk3 knockdown 80 hpf pluteus larvae. The white line indicates the territory occupied by the serotonergic neurons (arrowheads). (B) The angle α shown in A was measured for the most posterior boundaries of the selected ANE territory markers for ∼24 embryos from three batches using ImageJ. Solid lines indicate the posterior boundaries of each ANE marker used to measure α; the dashed lines show the most anterior boundary of six3 expression. Volume=0.5(1−cos α/2) was used to calculate the percentage of the surface area (±s.e.m.) occupied by the inner (foxq2, nkx3.2) and outer (six3) ANE territories in control, sFRP1/5 and Dkk3 morphants. (C) qPCR analysis showing that the expression of ANE regulatory genes is upregulated in sFRP1/5 and Dkk3 morphants. The y-axis shows the fold change in gene expression level in sFRP1/5 and Dkk3 morphants. The bars represent data using two different morpholinos. MO, morpholino. Scale bars: 20 µm.
Specification of serotonergic neurons requires both Six3 and FoxQ2 activity. By 72 hpf, control plutei normally develop three to five serotonergic neurons (control averaged 3.7 serotonergic neurons, n=23; Fig. 4Ad). In the absence of either sFRP1/5 or Dkk3 there was a 3- to 4-fold increase in the number of serotonergic neurons and these were distributed over a broader territory (sFRP1/5 morphant, 12.3 serotonergic neurons, n=32; Dkk3 morphant, 11.7 serotonergic neurons, n=27; Fig. 4Ah,l, Fig. S2B). Taken together, these results indicate that sFRP1/5 and Dkk3 are part of an anterior signaling center activated by FoxQ2. This signaling center is essential for the establishment of a correctly sized ANE territory and appears to be subsequently required for the correct pattern and number of serotonergic neurons within the ANE.
sFRP1/5 and Dkk3 are required to potentiate the Fzl5/8-JNK-mediated ANE positioning mechanism
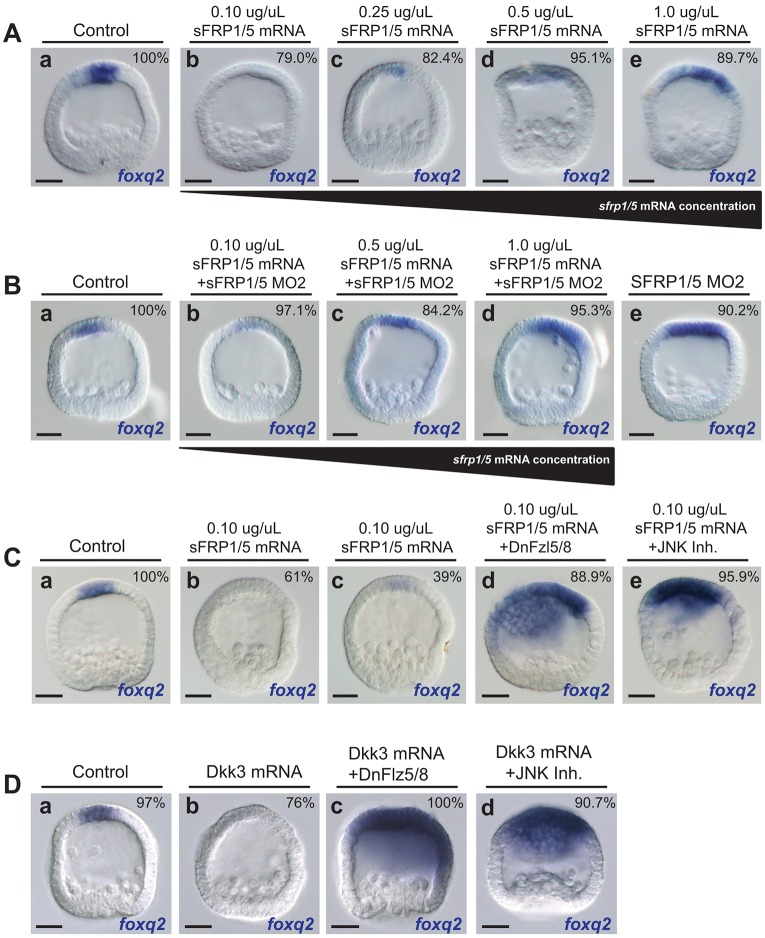
FoxQ2 activates dkk3 and sfrp1/5 expression during the later stages of ANE positioning (15-24 hpf; Fig. 2Bb). Both ligands have presumptive roles as Wnt signaling modulators, and both morphant phenotypes at the end of ANE positioning (mesenchyme blastula stage, 24 hpf) are similar to the phenotype observed when signaling via the Fzl5/8-JNK pathway is inhibited: expansion of the ANE territory (Range et al., 2013). Based on this evidence, we hypothesized that the anterior signaling center secretes Dkk3 and sFRP1/5, forming an anterior-to-posterior diffusion gradient that potentiates downregulation of ANE gene expression by Fzl5/8-JNK signaling. To test this hypothesis, we overexpressed Dkk3, which resulted in the complete elimination of expression of the ANE regulatory factors foxq2, fzl5/8 and nkx3.2 (Fig. 5Db, Fig. S2D). Similarly, low levels of sFRP1/5 overexpression reduced foxq2 expression (Fig. 5Aa-c), but, surprisingly, foxq2 expression expanded in embryos that were injected with progressively higher concentrations of sfrp1/5 mRNA (Fig. 5Ad,e, Fig. S2C). Furthermore, injection of relatively low levels of sfrp1/5 mRNA rescued the sFRP1/5 morpholino phenotype (three different batches of embryos; 84.2% rescue of sFRP1/5 MO2; n=67; Fig. 5Ba-c), whereas foxq2 was expanded in embryos co-injected with sFRP1/5 morpholinos and higher levels of sfrp1/5 mRNA (three different batches of embryos; 95.3% foxq2 posterior expansion; n=69; Fig. 5Bd).
Fig. 5.
The roles of sFRP1/5 and Dkk3 in Fzl5/8-JNK-mediated ANE positioning. The percentage of embryos examined that show the representative phenotypes depicted is indicated in each panel. (A) Compared with the control (a), the expression of foxq2 is downregulated in embryos injected with lower concentrations of sfrp1/5 mRNA (0.10 μg/μl, n=81; 0.25 μg/μl, n=86) (b,c) and expands in embryos injected with higher concentrations of sfrp1/5 mRNA (0.50 μg/μl, n=77; 1.0 μg/μl, n=75) (d,e). (B) Low levels of sFRP1/5 expression rescue embryos injected with sFRP1/5 MO2 (compare a,b with e) but increasing concentrations do not (c,d). sFRP1/5 MO2 does not bind to exogenous sfrp1/5 mRNA. (C) foxq2 expression is completely eliminated in two-thirds of embryos injected with relatively low levels of sfrp1/5 mRNA (b,c compared with a). The sFRP1/5-mediated inhibition of foxq2 expression requires functional Fzl5/8 (d); the sFRP1/5-mediated inhibition of foxq2 expression requires functional JNK activity (e). (D) Dkk3 overexpression downregulates foxq2 expression (b compared with a). The Dkk3-mediated inhibition of foxq2 expression requires functional Fzl5/8 (c); the Dkk3-mediated inhibition of foxq2 expression requires functional JNK activity (d). MO, morpholino. Scale bars: 20 µm.
The Dkk3 and low-level sFRP1/5 overexpression phenotypes are identical to those observed when the Fzl-5/8-JNK pathway is upregulated in sea urchin embryos (Range et al., 2013), suggesting that they might potentiate signaling through this pathway. To test this, we determined whether Dkk3 and/or sFRP1/5 could downregulate foxq2 expression in the absence of a functional Fzl5/8-JNK signaling pathway. Overexpression of either Dkk3 or sFRP1/5 (at low concentrations) no longer downregulated foxq2 expression when the function of Fzl5/8 was blocked with a dominant-negative construct (ΔFzl5/8) [three different batches of embryos; 88.9% rescue of sFRP1/5 (n=57) and 100% rescue of Dkk3 (n=71) misexpression phenotypes; Fig. 5Cd,Dc]. Similarly, Dkk3 and sFRP1/5 overexpression did not suppress foxq2 expression in embryos treated with JNK inhibitor [three different batches of embryos; 95.9% rescue of sFRP1/5 (n=65) and 90.7% rescue of Dkk3 (n=61) misexpression phenotypes; Fig. 5Ce,Dd]. These data strongly suggest that Dkk3 and low levels of sFRP1/5 are required for Fzl5/8-JNK signaling to fully downregulate ANE gene expression.
sFRP1/5 and Dkk3 act additively to restrict the ANE territory
To gain a more detailed understanding of the role of sFRP1/5 and Dkk3 in sizing the ANE territory, we carefully measured the size of the inner ANE territory (i.e. the region expressing foxq2) in each morphant at the completion of the ANE positioning process (24 hpf; Fig. 6). Injection of either sFRP1/5 or Dkk3 morpholino oligonucleotides caused similar large increases in the area of the inner ANE discoidal domain when compared with the control (Fig. 6Aa-c,e-g), and the expanded ANE territory was approximately the same size in both morphants (Fig. 6B). Next, we co-injected embryos with Dkk3 and sFRP1/5 morpholino oligonucleotides (Fig. 6d,h). Strikingly, in these double-knockdown embryos the area containing the inner ANE territory increased ∼2-fold when compared with either morphant alone (Fig. 6B). We conclude that sFRP1/5 and Dkk3 are likely to work additively to promote downregulation of ANE gene expression by Fzl5/8-JNK signaling during the later stages of the positioning mechanism, resulting in an appropriately sized ANE territory.
Fig. 6.
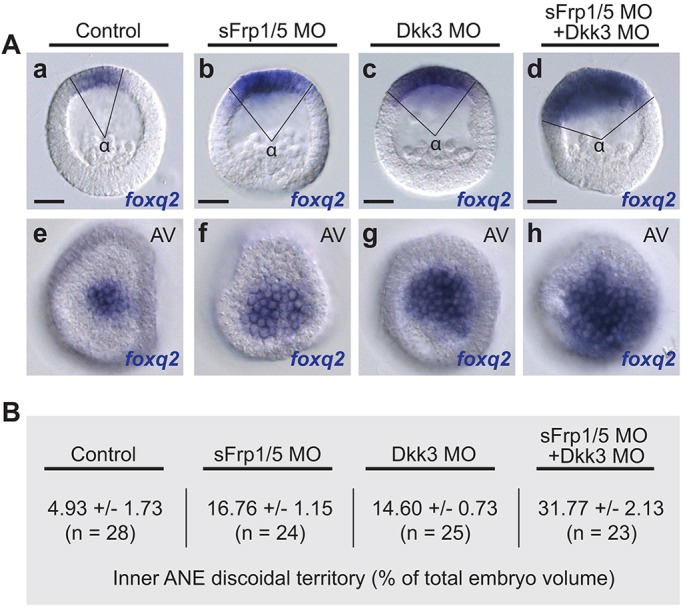
ANE restriction depends on additive inputs from sFRP1/5 and Dkk3. (A) foxq2 mRNA expression marks the inner ANE territory in control (a,e), sFRP1/5 morphants (b,f), Dkk3 morphants (c,g), and sFRP1/5 plus Dkk3 double morphants (d,h). (a-d) Representative images showing the distribution of foxq2 mRNA expression in inner ANE of control and knockdown embryos. Scale bars: 20 µm. (B) The angle α shown in Aa-d was measured for ∼24 embryos from three batches using ImageJ. Volume=0.5(1–cos α/2) was used to calculate the percentage of the surface area (±s.e.m.) occupied by the inner ANE territory in control, sFRP1/5 morphants, Dkk3 morphants, and sFRP1/5 plus Dkk3 double morphants. MO, morpholino.
Negative regulatory interactions control sfrp1/5 and dkk3 expression
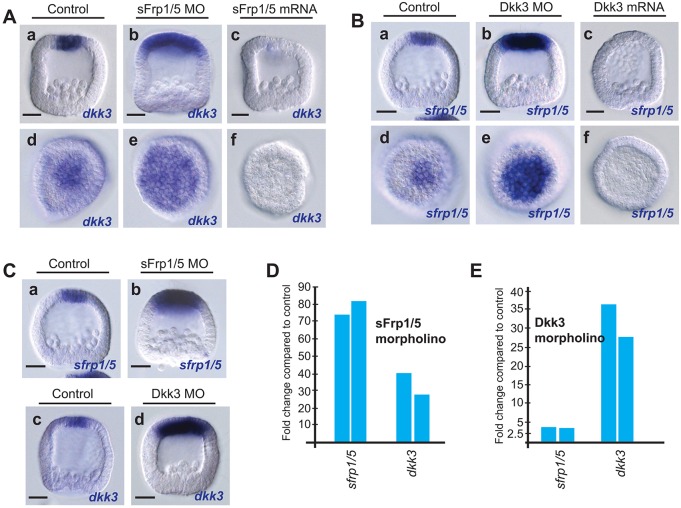
During the course of this study, we uncovered two additional regulatory interactions: anterior dkk3 expression expanded in sFRP1/5 morphants (Fig. 7Ab,e) and sfrp1/5 expression expanded in Dkk3 morphants (Fig. 7Bb,e). These results suggested that sFRP1/5 and Dkk3 negatively regulate one another's expression. To test this we overexpressed sFRP1/5 at high levels (1.0-1.5 µg/µl), which severely downregulated dkk3 expression in the ANE (Fig. 7Ac,f); conversely, when we overexpressed Dkk3, sfrp1/5 expression was completely eliminated (Fig. 7Bc,f). To quantitate these effects we measured the expression levels of these factors by qPCR. As expected, the level of sfrp1/5 transcripts increased significantly (4-fold) in Dkk3 morphants (Fig. 7E) and dkk3 expression increased ∼35-fold in sFRP1/5 morphants (Fig. 7D).
Fig. 7.
Autorepressive and cross-repressive regulatory interactions between sFRP1/5 and Dkk3 refine the size of the ANE. (A,D) ANE expression of dkk3 is expanded towards the posterior pole (Ab,e) and is upregulated (D) in sFRP1/5 morpholino-injected embryos. dkk3 expression is severely downregulated in embryos injected with sfrp1/5 mRNA (Ac,f). (B,E) Embryos injected with Dkk3 morpholinos fail to completely restrict the sfrp1/5 expression domain to the anterior pole (Bb,e) and the level of sfrp1/5 is strongly upregulated (E). Expression of sfrp1/5 is eliminated in embryos injected with dkk3 mRNA (Bc,f). (C,D) The anterior sfrp1/5 expression domain is expanded in sFRP1/5 morphants (Ca,b) and is strongly upregulated (D). Similarly, Dkk3 morpholino-injected embryos show expanded dkk3 expression (Cc,d) and severe upregulation of dkk3 transcripts (E). (D,E) qPCR analysis showing that sfrp1/5 and dkk3 expression is strongly upregulated in sFRP1/5 (D) and Dkk3 (E) morpholino-injected embryos. MO, morpholino. Scale bars: 20 µm.
We next tested the expression of dkk3 in a Dkk3 morphant background and sfrp1/5 in an sFRP1/5 morphant background. In situ hybridization showed that the domains of both transcripts expanded in their respective morphant backgrounds and that their expression levels were also markedly upregulated (Fig. 7Ca-d). Again, we confirmed these results with qPCR analysis, which showed that dkk3 expression increased by an average of 32-fold in Dkk3 morphants (Fig. 7E) and that in sFRP1/5 morphants sfrp1/5 expression increased by a remarkable 78-fold on average (Fig. 7D). Collectively, these results show that sFRP1/5 and Dkk3 negatively regulate both their own and each other's expression.
DISCUSSION
We have characterized the roles of the secreted signaling modulators sFRP1/5 and Dkk3 in the specification and patterning of subdomains of the ANE territory in the sea urchin embryo. Our functional analyses indicate that the cardinal ANE transcriptional regulator FoxQ2 is necessary to subdivide the ANE into two nested concentric domains of gene expression and to activate the expression of sFRP1/5 and Dkk3 within the anteriormost discoidal domain. We show that Dkk3 and low levels of sFRP1/5 expression are both required and act additively to stimulate the Fzl5/8-JNK signaling-mediated downregulation of ANE gene expression during ANE positioning. Interestingly, higher levels of sFRP1/5 expression appear to antagonize Fzl5/8-JNK signaling. In addition, our data reveal that remarkable negative autoregulatory and cross-regulatory interactions of sFRP1/5 and Dkk3 are necessary to reproducibly establish an appropriately sized ANE around the anterior pole. This is also the first study in deuterostomes outside of the chordate phylum to define the functions of sFRP1/5 and Dkk3.
Based on our findings we propose an expanded model for ANE positioning in the sea urchin embryo (Fig. 8A). During the early cleavage stages (32- to 60-cell stage) to early- to mid-blastula stage, Wnt/β-catenin signaling activates posterior-to-anterior gradients of Wnt1 and Wnt8 that downregulate ANE regulatory factors in the posterior ectoderm via activation of the Fzl5/8-JNK signaling pathway. Then, during the mid-blastula stages, Fzl5/8 signaling in the regressing ANE territory activates Dkk1 expression. At this time, Dkk1 antagonizes the posterior-to-anterior repression of ANE cell fate in a classical negative-feedback loop (Range et al., 2013). Our new data suggest that at around the same time FoxQ2 – now under the control of Six3 (Wei et al., 2009) – drives expression of sFRP1/5 and Dkk3, which diffuse in an anterior-to-posterior gradient from the inner ANE territory, reinforcing the Wnt1/8-Fzl5/8-JNK-mediated downregulation of ANE factors at the outer limits of their diffusion gradient. Conversely, high concentrations of secreted sFRP1/5 around the anteriormost pole can antagonize Fzl5/8-JNK signaling, possibly assisting Dkk1 in defining domains within the ANE territory. Finally, our data show that sFRP1/5 and Dkk3 (directly or indirectly) negatively regulate one another's expression, which is also essential to achieve the proper size of the final ANE territory. Thus, the signaling pathways that mediate ANE positioning depend on positive inputs from both primary poles of the embryo. It is the elegant balancing act between the potentiation of the Fzl5/8-JNK positioning mechanism by posteriorly expressed Wnt ligands and anteriorly expressed sFRP1/5 and Dkk3 secreted modulators opposed to the antagonism of Dkk1 that reproducibly establishes a correctly sized ANE territory in the early sea urchin embryo.
FoxQ2 transcriptional activity is necessary for the specification of several cell types within the ANE territory in the sea urchin embryo, including the apical tuft cells and serotonergic neurons (Yaguchi et al., 2012, 2008, 2010). The formation of serotonergic neurons begins during the early pluteus larval stages on the dorsal side of the embryo in a territory around the interface of the inner anterior discoidal domain marked by foxq2 expression and the outer ANE domain marked by the ring of six3 expression (Fig. S3). In the absence of either sFRP1/5 or Dkk3, both the inner and outer ANE domains expand towards the posterior pole (see Fig. 4), resulting in a larger territory around the border between the two domains on the dorsal side of the embryo. Thus, the increased size of the foxq2/six3 dorsal border territory correlates with the greater number of serotonergic neurons that can be specified. The simplest interpretation of these observations is that the pattern of the ANE is proportionately expanded in sFRP1/5 and Dkk3 morphants, including the area within which serotonergic neurons can form, thus increasing the number of serotonergic neurons there. Alternatively, it is possible that a function of sFRP1/5 and Dkk3 is to antagonize the specification of serotonergic neurons at the foxq2/six3 interface.
Our functional studies demonstrate that the sea urchin ANE is expanded in sFRP1/5 morphants and embryos overexpressing high levels of sFRP1/5, whereas ANE gene expression is downregulated in embryos overexpressing sFRP1/5 at low levels. These data suggest a biphasic function for sFRP1/5, similar to a model proposed by Uren et al. (2000) based on cell culture studies, which showed that low levels of sFRP1 promote nuclear localization of β-catenin, whereas high levels block nuclear localization. We propose that the higher concentration of sFRP1/5 protein around the anterior pole works with increasing levels of Dkk1 protein to antagonize Fzl5/8-JNK signal-mediated downregulation of ANE genes in this anteriormost regulatory domain. Then, as sFRP1/5 protein diffuses into the extracellular space, lower concentrations of sFRP1/5 proteins further from the anterior pole potentiate Fzl5/8-JNK signaling. The exact mechanism by which sFRP1/5 either promotes or antagonizes Fzl5/8-JNK signaling is unclear. It is possible that sFRP1/5 physically interacts with the Fzl5/8 receptor, Wnt ligands and/or other uncharacterized extracellular proteins to directly promote and/or antagonize Fzl5/8 signaling (see Bovolenta et al., 2008). Alternatively, sFRP1/5 could act indirectly by promoting the diffusion of Wnt1 and Wnt8 ligands by interfering with their interactions with extracellular matrix proteins and Fzl5/8 receptor (see Mii and Taira, 2009, 2011).
We also demonstrate that the ANE territory also expands in the absence of Dkk3, suggesting that Dkk3 and sFRP1/5 diffusing from the anterior signaling center work additively to promote Fzl5/8-JNK signaling. However, some available data suggest that Dkk3 might not function in the Wnt signaling pathway. For example, cell culture studies provide evidence that Dkk3 secreted into the extracellular space does not bind Lrp6 or extracellular Kremen (Mao et al., 2002; Nakamura and Hackam, 2010), two extracellular co-receptors that are necessary for canonical and/or non-canonical Wnt signaling, depending on the context. By contrast, a recent study has shown that Dkk3 can bind to Kremen in intracellular compartments, where it can potentiate Wnt3a signaling (Nakamura and Hackam, 2010), representing a potential molecular mechanism by which Dkk3 may stimulate Wnt signaling during the ANE restriction process. Interestingly, blocking the function of the Fzl5/8 receptor or JNK has a more severe effect on ANE restriction than does knockdown of either Wnt1 and Wnt8 (Range et al., 2013) or sFRP1/5 and Dkk3 (this study). Taken together, these data are consistent with a model in which sFRP1/5 and Dkk3 diffuse extracellularly in an anterior-to-posterior gradient and Wnt1 and Wnt8 in a posterior-to-anterior gradient and both gradients work in concert to restrict the ANE around the anterior pole.
For many years it was thought that anterior signaling centers were a vertebrate invention that led to the more complex territorial specification and morphogenetic processes of vertebrate forebrain/eye field development (Holland and Short, 2008; Pani et al., 2012). This study, as well as recent studies in invertebrate chordate amphioxus and ambulacrarian hemichordate embryos (Onai et al., 2012; Pani et al., 2012), challenge this idea. Similar to early sea urchin embryos, amphioxus and hemichordate embryos express foxq2 in a territory within the broader fzl5/8 and six3 expression domains after these regulatory factors are restricted to a territory around the anterior pole by a Wnt/β-catenin-dependent positioning mechanism (Fig. 8B). In amphioxus, the expression pattern of sfrp1/5 has not been determined; however, another sFRP family member, sfrp2-like, as well as dkk3 are expressed at the anterior pole in a pattern similar to that of foxq2 (Kong et al., 2012), suggesting that FoxQ2 might activate an anterior signaling center, as in sea urchin embryos. Interestingly, knocking down Dkk3 protein expression causes loss of ANE specification in amphioxus, whereas overexpression causes an expansion of the ANE territory (Onai et al., 2012), presumably because Dkk3 antagonizes the Wnt/β-catenin-driven restriction mechanism. In hemichordates no functional or expression data are available for Dkk3, but sfrp1/5 and foxq2 transcripts overlap in the ANE towards the end of gastrulation (Pani et al., 2012). The function of sFRP1/5 has not been determined in hemichordates, but Pani et al. (2012) showed that expression of another signaling factor, hedgehog, appears to overlap with that of foxq2 in Saccoglossus kowalevskii and is necessary for ANE pattering, suggesting that FoxQ2 might also activate an anterior signaling center in these embryos. Our limited understanding of the signaling landscape (ligands, co-receptors and intracellular modulators) makes it difficult to interpret how the modulators function within the regulatory network that specifies and patterns the ANE in any deuterostome embryo. Yet, collectively the available information strongly suggests that anterior signaling centers are a common feature of deuterostome ANE specification and patterning, advocating that studies in non-vertebrate deuterostomes can continue to help inform studies in vertebrate embryos.
The above comparisons among deuterostomes raise the question of when anterior signaling centers arose in the evolution of metazoans. In animals outside of the deuterostome superphylum, FoxQ2 and Six3 appear to play important regulatory roles during the patterning of anterior/apical territories that develop into sensory organs (Posnien et al., 2011; Sinigaglia et al., 2013; Steinmetz et al., 2010). In cnidarian embryos, which form the sister group to bilaterians, foxq2 and six3 are expressed in the ectoderm around the aboral pole of the embryo, opposite to high levels of Wnt/β-catenin signaling that specify endoderm within the oral territory and pattern the embryo along its primary oral-aboral axis (Marlow et al., 2013; Momose and Houliston, 2007; Röttinger et al., 2012; Wikramanayake et al., 2003). Similar to its role in several deuterostome embryos Six3 antagonizes Wnt/β-catenin signaling, and both Six3 and FoxQ2 are necessary for specification and patterning of the apical organ, which is thought to have a sensory function (Rentzsch et al., 2008; Sinigaglia et al., 2013). Although the functions of sFRPs and Dkk3 remain unclear in cnidarian embryos, it is interesting that foxq2 activates a signaling factor, FGFA, which is necessary for patterning the territory that forms the apical organ around the aboral pole (Sinigaglia et al., 2013). In addition to these similarities in cnidarians, a recent study on the bilaterian lophotrochozoan annelid Platynereis dumerilii showed that a remarkably conserved set of ANE/apical sensory organ transcription factors and signaling modulators, including fzl5/8, six3, foxq2 and sfrp1/5, is expressed in the head. Similar to sea urchins, the expression domains of foxq2 and sfrp1/5 overlap and are nested within the larger domains of fzl5/8 and six3. Also, ectopic overactivation of the posteriorly localized Wnt/β-catenin pathway severely downregulated the anterior apical sensory organ in these embryos (Marlow et al., 2014), suggesting that control of Wnt signaling could be important for establishing the ANE in these embryos.
Taken together, these studies make it tempting to speculate that signaling centers that are active around the opposite pole to that of high Wnt/β-catenin signaling along the primary axis might be an ancient developmental mechanism essential for eumetazoan (cnidarians and bilaterians) body axis specification and patterning.
MATERIALS AND METHODS
Animals and embryos
Strongylocentrotus purpuratus sea urchins were obtained from Point Loma Marine Invertebrate Lab (Lakeside, CA, USA), The Cultured Abalone (Goleta, CA, USA) or Marinus Scientific (Garden Grove, CA, USA). Embryos were cultured in artificial seawater at 15°C.
Microarray
S. purpuratus genome sequence information was used to prepare the microarray (Wei et al., 2006). mRNA from control and FoxQ2 morphants was isolated and used to synthesize cDNAs that were labeled, hybridized and scanned by Roche Nimblegen microarray services. Data analysis was described previously (Wei et al., 2006).
Preparation of cDNA clones
cDNA from 12-24 hpf blastula stage embryos was used to obtain full-length clones for sfrp1/5 and dkk3. The following primers (forward and reverse, 5′-3′; bold indicates the translational start codon) were based on the sea urchin genome sequence: Sp-dkk3, AGAATGGCGGCTCCTTCTGC and TCATAATACAGTTAACTGGC; Sp-sfrp1/5, AGAATGGCTGCCTTCAGTGGAAC and TCACACCTGTACATTTGGTA.
mRNA and morpholino injections
Misexpression studies were performed with full-length sfrp1/5 and dkk3 cDNA sequences inserted into the pCS2+ vector. pCS2 constructs were linearized with NotI and mRNA was synthesized with the mMessage mMachine Kit (Ambion), purified by LiCl precipitation and injected at the following concentrations: sfrp1/5 mRNA, 0.1-1.5 µg/µl; dkk3 mRNA, 0.75-1.0 µg/µl.
S. purpuratus EST sequences for sfrp1/5 and dkk3 were used to generate translation-blocking morpholino oligonucleotides (Gene Tools). Sequences (5′-3) and injection concentrations were as follows: sFRP1/5 MO1, ACACCACCAACACTCGCTCAATCAT, 1.0 mM; sFRP1/5 MO2, AAAGCACAGTAATTGATTCCTCACC, 1.2 mM; Dkk3 MO1, AGGGAGATGCTTACCTAGTGTTCTT, 1.0 mM; Dkk3 MO2, CGGTGTCCATAATCCGAACCATCTC, 1.5 mM.
Zygotes were injected immediately after fertilization with solutions containing fluorescein isothiocyanate (FITC), 20% glycerol and mRNA and/or morpholino oligonucleotides. Injected embryos were cultured at 15°C. Microinjection experiments were performed using at least three different batches of embryos, and each experiment consisted of 50-250 embryos. Only representative phenotypes present in at least 80% of the injected embryos are presented unless otherwise stated.
Quantitative PCR (qPCR)
qPCR was performed as previously described (Wei et al., 2009). Embryos from at least three different mating pairs were used for each experiment and each PCR reaction was carried out in triplicate. Primer set information for ANE GRN genes, including dkk3 and sfrp1/5, is given in Range et al. (2013).
Whole-mount in situ hybridization
Full-length cDNA sequence was used to generate antisense probes for each gene analyzed. Alkaline phosphatase and two-color fluorescent in situ hybridization were carried out as previously described (Sethi et al., 2012; Wei et al., 2009). For the two-color in situ hybridization, foxq2 was labeled with fluorescein and detected with Cy5-TSA and sfrp1/5 was labeled with DIG and detected with fluorescein-TSA in Fig. S1A. In Fig. S1B, sfrp1/5 was labeled with DIG and detected with fluorescein-TSA and dkk3 was labeled with fluorescein and detected with Cy5-TSA.
Immunohistochemistry
Embryos were fixed in 2-4% paraformaldehyde in artificial seawater at room temperature for 20 min and washed five times in phosphate-buffered saline containing 0.1% Tween 20 (PBST). Embryos were incubated at 4°C overnight with primary antibodies (1:1000) against serotonin (Sigma, S5545) and synaptotagmin B/1e11 (Nakajima et al., 2004) in PBST and 4% normal goat serum. Primary antibodies were detected by incubating embryos for 1 h at room temperature with 1:2000 Alexa Fluor-coupled secondary antibodies (Thermo Fisher Scientific). Nuclei were stained with DAPI.
Acknowledgements
We thank Dr Lynne Angerer for mentorship and many insightful discussions; Dr Robert Angerer for careful editing of the manuscript; and Dr David McClay as well as past members of the Angerer lab, Dr Aditya Sethi and Dr Diane Adams, for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
R.C.R. and Z.W. planned, performed and analyzed experiments. R.C.R. prepared the manuscript.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIDCR) [grant number ZO1DE000712], as well as startup funding from Mississippi State University. Deposited in PMC for release after 12 months.
Data availability
The microarray expression data have been deposited at Gene Expression Omnibus with accession number GSE80012.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.128165/-/DC1
References
- Bafico A., Gazit A., Pramila T., Finch P. W., Yaniv A. and Aaronson S. A. (1999). Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J. Biol. Chem. 274, 16180-16187. 10.1074/jbc.274.23.16180 [DOI] [PubMed] [Google Scholar]
- Bafico A., Liu G., Yaniv A., Gazit A. and Aaronson S. A. (2001). Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3, 683-686. 10.1038/35083081 [DOI] [PubMed] [Google Scholar]
- Bhat R. A., Stauffer B., Komm B. S. and Bodine P. V. (2007). Structure–function analysis of secreted frizzled-related protein-1 for its Wnt antagonist function. J. Cell. Biochem. 102, 1519-1528. 10.1002/jcb.21372 [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Esteve P., Ruiz J. M., Cisneros E. and Lopez-Rios J. (2008). Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121, 737-746. 10.1242/jcs.026096 [DOI] [PubMed] [Google Scholar]
- Burke R. D., Moller D. J., Krupke O. A. and Taylor V. J. (2014). Sea urchin neural development and the metazoan paradigm of neurogenesis. Genesis 52, 208-221. 10.1002/dvg.22750 [DOI] [PubMed] [Google Scholar]
- Castro A., Becerra M., Manso M. J. and Anadón R. (2015). Neuronal organization of the brain in the adult amphioxus (Branchiostoma lanceolatum): a study with acetylated tubulin immunohistochemistry. J. Comp. Neurol. 523, 2211-2232. 10.1002/cne.23785 [DOI] [PubMed] [Google Scholar]
- Cavodeassi F. (2013). Integration of anterior neural plate patterning and morphogenesis by the Wnt signaling pathway. Dev. Neurobiol. 74, 759-771. doi: 10.1002/dneu.22135 [DOI] [PubMed] [Google Scholar]
- Cavodeassi F. and Houart C. (2012). Brain regionalization: of signaling centers and boundaries. Dev. Neurobiol. 72, 218-233. 10.1002/dneu.20938 [DOI] [PubMed] [Google Scholar]
- Cruciat C.-M. and Niehrs C. (2013). Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 5, a015081 10.1101/cshperspect.a015081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras S., Gerhart J., Terasaki M., Kirschner M. and Lowe C. J. (2011). beta-catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development 138, 959-970. 10.1242/dev.059493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq P., Leroux L., Ezan J., Descamps B., Lamazière J.-M. D., Costet P., Basoni C., Moreau C., Deutsch U., Couffinhal T. et al. (2008). Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: role in neovessel formation. Am. J. Pathol. 172, 37-49. 10.2353/ajpath.2008.070130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarría D., Vieira C., Gimeno L. and Martínez S. (2003). Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res. Rev. 43, 179-191. 10.1016/j.brainresrev.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Esteve P., Sandonis A., Ibanez C., Shimono A., Guerrero I. and Bovolenta P. (2011). Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development 138, 4179-4184. 10.1242/dev.065839 [DOI] [PubMed] [Google Scholar]
- Fritzenwanker J. H., Gerhart J., Freeman R. M. Jr. and Lowe C. J. (2014). The Fox/Forkhead transcription factor family of the hemichordate Saccoglossus kowalevskii. Evodevo 5, 17 10.1186/2041-9139-5-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner S., Zysk I., Byrne G., Kramer M., Moller D., Taylor V. and Burke R. D. (2015). Neurogenesis in sea urchin embryos and the diversity of deuterostome neurogenic mechanisms. Development 143, 286-297. doi: 10.1242/dev.124503 [DOI] [PubMed] [Google Scholar]
- Holland L. Z. and Short S. (2008). Gene duplication, co-option and recruitment during the origin of the vertebrate brain from the invertebrate chordate brain. Brain Behav. Evol. 72, 91-105. 10.1159/000151470 [DOI] [PubMed] [Google Scholar]
- Houart C., Caneparo L., Heisenberg C.-P., Barth K. A., Take-Uchi M. and Wilson S. W. (2002). Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron 35, 255-265. 10.1016/S0896-6273(02)00751-1 [DOI] [PubMed] [Google Scholar]
- Hsu R.-J., Lin C.-Y., Hoi H.-S., Zheng S.-K., Lin C.-C. and Tsai H.-J. (2010). Novel intronic microRNA represses zebrafish myf5 promoter activity through silencing dickkopf-3 gene. Nucleic Acids Res. 38, 4384-4393. 10.1093/nar/gkq148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C. and Niehrs C. (2001). A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189-4201. [DOI] [PubMed] [Google Scholar]
- Kong W., Yang Y., Zhang T., Shi D.-L. and Zhang Y. (2012). Characterization of sFRP2-like in amphioxus: insights into the evolutionary conservation of Wnt antagonizing function. Evol. Dev. 14, 168-177. 10.1111/j.1525-142X.2012.00533.x [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Holland N. D., Kreslova J., Oliveri D., Schubert M., Jonasova K., Holland L. Z., Pestarino M., Benes V. and Candiani S. (2007). Pax–Six–Eya–Dach network during amphioxus development: conservation in vitro but context specificity in vivo. Dev. Biol. 306, 143-159. 10.1016/j.ydbio.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Lagutin O. V., Zhu C. C., Kobayashi D., Topczewski J., Shimamura K., Puelles L., Russell H. R., McKinnon P. J., Solnica-Krezel L. and Oliver G. (2003). Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 17, 368-379. 10.1101/gad.1059403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Cui M., Peter I. S. and Davidson E. H. (2014). Encoding regulatory state boundaries in the pregastrular oral ectoderm of the sea urchin embryo. Proc. Natl. Acad. Sci. USA 111, E906-E913. 10.1073/pnas.1323105111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Wang S., Julius M. A., Kitajewski J., Moos M. Jr. and Luyten F. P. (1997). The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc. Natl. Acad. Sci. USA 94, 11196-11200. 10.1073/pnas.94.21.11196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A. and Niehrs C. (2001). LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321-325. 10.1038/35077108 [DOI] [PubMed] [Google Scholar]
- Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B. M., Delius H., Hoppe D., Stannek P., Walter C. et al. (2002). Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417, 664-667. 10.1038/nature756 [DOI] [PubMed] [Google Scholar]
- Marlow H., Matus D. Q. and Martindale M. Q. (2013). Ectopic activation of the canonical wnt signaling pathway affects ectodermal patterning along the primary axis during larval development in the anthozoan Nematostella vectensis. Dev. Biol. 380, 324-334. 10.1016/j.ydbio.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow H., Tosches M. A., Tomer R., Steinmetz P. R., Lauri A., Larsson T. and Arendt D. (2014). Larval body patterning and apical organs are conserved in animal evolution. BMC Biol. 12, 7 10.1186/1741-7007-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mii Y. and Taira M. (2009). Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 136, 4083-4088. 10.1242/dev.032524 [DOI] [PubMed] [Google Scholar]
- Mii Y. and Taira M. (2011). Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev. Growth Differ. 53, 911-923. 10.1111/j.1440-169X.2011.01299.x [DOI] [PubMed] [Google Scholar]
- Momose T. and Houliston E. (2007). Two oppositely localised frizzled RNAs as axis determinants in a cnidarian embryo. PLoS Biol. 5, e70 10.1371/journal.pbio.0050070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Kaneko H., Murray G. and Burke R. D. (2004). Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 6, 95-104. 10.1111/j.1525-142X.2004.04011.x [DOI] [PubMed] [Google Scholar]
- Nakamura R. E. I. and Hackam A. S. (2010). Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors 28, 232-242. 10.3109/08977191003738832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström U., Jessell T. M. and Edlund T. (2002). Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 5, 525-532. 10.1038/nn0602-854 [DOI] [PubMed] [Google Scholar]
- Onai T., Akira Takai T., Setiamarga D. H. E. and Holland L. Z. (2012). Essential role of Dkk3 for head formation by inhibiting Wnt/beta-catenin and Nodal/Vg1 signaling pathways in the basal chordate amphioxus. Evol. Dev. 14, 338-350. 10.1111/j.1525-142X.2012.00552.x [DOI] [PubMed] [Google Scholar]
- Pani A. M., Mullarkey E. E., Aronowicz J., Assimacopoulos S., Grove E. A. and Lowe C. J. (2012). Ancient deuterostome origins of vertebrate brain signalling centres. Nature 483, 289-294. 10.1038/nature10838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnien N., Koniszewski N. D. B., Hein H. J. and Bucher G. (2011). Candidate gene screen in the red flour beetle Tribolium reveals six3 as ancient regulator of anterior median head and central complex development. PLoS Genet. 7, e1002416 10.1371/journal.pgen.1002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range R. (2014). Specification and positioning of the anterior neuroectoderm in deuterostome embryos. Genesis 52, 222-234. 10.1002/dvg.22759 [DOI] [PubMed] [Google Scholar]
- Range R. C., Angerer R. C. and Angerer L. M. (2013). Integration of canonical and noncanonical Wnt signaling pathways patterns the neuroectoderm along the anterior–posterior axis of sea urchin embryos. PLoS Biol. 11, e1001467 10.1371/journal.pbio.1001467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F., Fritzenwanker J. H., Scholz C. B. and Technau U. (2008). FGF signalling controls formation of the apical sensory organ in the cnidarian Nematostella vectensis. Development 135, 1761-1769. 10.1242/dev.020784 [DOI] [PubMed] [Google Scholar]
- Rodriguez J., Esteve P., Weinl C., Ruiz J. M., Fermin Y., Trousse F., Dwivedy A., Holt C. and Bovolenta P. (2005). SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat. Neurosci. 8, 1301-1309. 10.1038/nn1547 [DOI] [PubMed] [Google Scholar]
- Röttinger E., Dahlin P. and Martindale M. Q. (2012). A framework for the establishment of a cnidarian gene regulatory network for “endomesoderm” specification: the inputs of β-catenin/TCF signaling. PLoS Genet. 8, e1003164 10.1371/journal.pgen.1003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semënov M. V., Tamai K., Brott B. K., Kühl M., Sokol S. and He X. (2001). Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11, 951-961. 10.1016/S0960-9822(01)00290-1 [DOI] [PubMed] [Google Scholar]
- Seo H.-C., Drivenes Ø., Ellingsen S. and Fjose A. (1998). Expression of two zebrafish homologues of the murine Six3 gene demarcates the initial eye primordia. Mech. Dev. 73, 45-57. 10.1016/S0925-4773(98)00028-8 [DOI] [PubMed] [Google Scholar]
- Sethi A., Angerer R. C. and Angerer L. M. (2012). Multi-color labeling in developmental gene regulatory network analysis. Methods Mol. Biol. 1128, 249-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia C., Busengdal H., Leclère L., Technau U. and Rentzsch F. (2013). The bilaterian head patterning gene six3/6 controls aboral domain development in a cnidarian. PLoS Biol. 11, e1001488 10.1371/journal.pbio.1001488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R. H., Urbach R., Posnien N., Eriksson J., Kostyuchenko R. P., Brena C., Guy K., Akam M., Bucher G. and Arendt D. (2010). Six3 demarcates the anterior-most developing brain region in bilaterian animals. Evodevo 1, 14 10.1186/2041-9139-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendeng C. and Houart C. (2006). Cloning and embryonic expression of five distinct sfrp genes in the zebrafish Danio rerio. Gene Expr. Patterns 6, 761-771. 10.1016/j.modgep.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Uren A., Reichsman F., Anest V., Taylor W. G., Muraiso K., Bottaro D. P., Cumberledge S. and Rubin J. S. (2000). Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 275, 4374-4382. 10.1074/jbc.275.6.4374 [DOI] [PubMed] [Google Scholar]
- Veeck J. and Dahl E. (2012). Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochim. Biophys. Acta 1825, 18-28. 10.1016/j.bbcan.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Wei Z., Angerer R. C. and Angerer L. M. (2006). A database of mRNA expression patterns for the sea urchin embryo. Dev. Biol. 300, 476-484. 10.1016/j.ydbio.2006.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Yaguchi J., Yaguchi S., Angerer R. C. and Angerer L. M. (2009). The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development 136, 1179-1189. 10.1242/dev.032300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikramanayake A. H., Hong M., Lee P. N., Pang K., Byrum C. A., Bince J. M., Xu R. and Martindale M. Q. (2003). An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature 426, 446-450. 10.1038/nature02113 [DOI] [PubMed] [Google Scholar]
- Wilson S. W. and Houart C. (2004). Early steps in the development of the forebrain. Dev. Cell 6, 167-181. 10.1016/S1534-5807(04)00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Angerer R. C. and Angerer L. M. (2008). A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev. Cell 14, 97-107. 10.1016/j.devcel.2007.10.012 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Wei Z., Shiba K., Angerer L. M. and Inaba K. (2010). ankAT-1 is a novel gene mediating the apical tuft formation in the sea urchin embryo. Dev. Biol. 348, 67-75. 10.1016/j.ydbio.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Wei Z., Jin Y., Angerer L. M. and Inaba K. (2011). Fez function is required to maintain the size of the animal plate in the sea urchin embryo. Development 138, 4233-4243. 10.1242/dev.069856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi J., Angerer L. M., Inaba K. and Yaguchi S. (2012). Zinc finger homeobox is required for the differentiation of serotonergic neurons in the sea urchin embryo. Dev. Biol. 363, 74-83. 10.1016/j.ydbio.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. K., Holland N. D. and Holland L. Z. (2003). AmphiFoxQ2, a novel winged helix/forkhead gene, exclusively marks the anterior end of the amphioxus embryo. Dev. Genes Evol. 213, 102-105. [DOI] [PubMed] [Google Scholar]