Abstract
Immune reconstitution inflammatory syndrome (IRIS) can complicate antifungal treatment of cryptococcosis. There are limited data on managing cryptococcal-associated IRIS. We describe an immunocompetent patient who developed IRIS complicating Cryptococcus neoformans meningitis, successfully treated with thalidomide following failure of corticosteroid therapy. Data on thalidomide use in cryptococcal IRIS are awaited.
Keywords: Thalidomide, Cryptococcus neoformans, Immune reconstitution
1. Introduction
Cryptococcal meningitis is the commonest form of fungal meningitis. Infection caused by two main species, Cryptococcus neoformans and Cryptococus gattii, affects both immunocompromised and immunocompetent hosts [1]. It commonly results in permanent neurologic deficits and disability and mortality is high [1].
During antifungal therapy of cryptococcal meningitis, clinical deterioration may occur despite evidence of mycological response. Known as cryptococcal-immune reconstitution inflammatory syndrome (c-IRIS), this paradoxical heightened immune response was initially observed in HIV-infected patients following antifungal therapy and reversal of immune deficiency with antiretroviral therapy [2]. However, it is increasingly recognized in immunocompetent hosts [3], [4]. Manifestations include worsening, or relapse, of clinical symptoms and/or radiological findings.
Optimal management of c-IRIS is not well defined. The lack of consensus case definitions and standardized diagnostic criterion (particularly in non-immuncompromised individuals) make comparison of available therapies difficult [2]. Corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), interferon-γ (IFN-γ) and tumor necrosis factor (TNF) antagonists have been previously used with varying success [1], [5]. Thalidomide has been prescribed in a number of immunocompromised persons with c-IRIS, but its efficacy in immunocompetent hosts is unclear [6], [7].
Herein, we report for the first time, thalidomide use in c-IRIS occurring during antifungal treatment of C. neoformans meningitis in an immunocompetent host who was clinically deteriorating despite corticosteroid therapy.
2. Case
A 36-year-old previously well female presented with headaches, ataxia and blurred vision. Two months earlier, a diagnosis of tension headache was made when she noticed headache, neck stiffness, nausea and vomiting; there was no improvement with benzodiazepines and anti-depressants. She had no known co-morbidities and was not receiving immunosuppressants. Neurological examination revealed dysarthria, incoordination and partial left abducens nerve palsy. Non-contrast computed tomography (CT) imaging of the brain revealed a small low-density lesion in the left caudate head of indeterminate age.
Cerebrospinal fluid (CSF) analysis showed protein level of 0.37 g/L (normal range 0.15–0.45), glucose of 2.2 mmol/L (2.2–3.9), 91×106/L mononuclear cells, 89×106/L erythrocytes; opening pressure was 35 cm H2O (normal range 10–20). Encapsulated yeasts were seen on Indian ink stain, but gram stain and bacterial cultures were negative. C. neoformans, subsequently identified on canavanine-glycine-bromothymol blue agar, was cultured. The minimum inhibitory concentrations of the isolate to amphotericin B, fluconazole and 5-flucytosine (5-FC) were 0.50 mg/L, 8 mg/L and 4 mg/L respectively (Sensititre® YeastOne®, TREK Diagnostic Systems, West Sussex, England). CSF and serum cryptococcal antigen test (CALAS®, Meridian Bioscience, Cincinnati, OH, USA) was positive with titers of 4096 and ≥8192 respectively. HIV serology was negative and her CD4 count was 609 cells/µL (corresponding to 42%). Quantitative β-human chorionic gonadotropin levels were not indicative of pregnancy. Intravenous liposomal amphotericin (l-AMB) 3 mg/kg daily and 5-FC 25 mg/kg 6-hourly were commenced.
Repeat lumbar puncture on day three of admission revealed opening pressure of 60 cm H2O. Despite insertion of an external ventricular drain the next day, bilateral sensorineural hearing loss, followed by impaired consciousness developed by day seven of therapy. On day 14, the ventricular drain was changed; no clinical improvement was observed. Magnetic resonance imaging (MRI) of the brain on day 19 revealed cryptococcomas in the left caudate nucleus and right cerebellar hemisphere, with extensive supra-and infra-tentorial leptomeningeal enhancement. Dexamethasone 4 mg thrice daily was commenced for raised intracranial pressure.
Due to clinical deterioration with fevers, increasing headache, vomiting and agitation, she underwent repeat lumbar puncture on day 21. CSF examination again showed encapsulated yeasts and 39×106/L leukocytes (100% mononuclear cells), but cultures were negative for C. neoformans. There was no focal seizure activity on electroencephalogram and CT venogram did not show intracranial thrombosis. Empirical meropenem and vancomycin were commenced for suspected device-related ventriculomeningitis without clinical improvement. Intracranial pressure (ICP) continued to fluctuate up to 73 cm H2O.
Five weeks into therapy, further neurological deterioration was observed and intubation was required for airway protection. ICP was measured at 45–50 cm H2O. Repeat cerebral MRI showed widespread new parenchymal contrast-enhancing nodules, enlargement of the left caudate nucleus cryptococcoma with mass effect and persistent leptomeningeal enhancement (Fig. 1). A clinical diagnosis of c-IRIS was made. Given the lack of clinical response to dexamethasone, thalidomide 100 mg daily was added. Defervescence and significant neurological improvement were observed within 3–7 days of commencing thalidomide. Repeat cerebral MRI two weeks later showed reduction in leptomeningeal enhancement and in the size of the caudate nucleus cryptococcoma, but not the parenchymal nodules (Fig. 2).
Fig. 1.
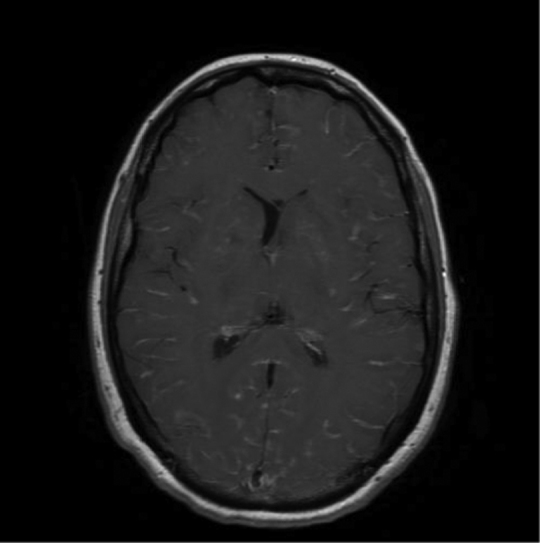
Axial T1 FLAIR with contrast showing meningeal and basal ganglia enhancement.
Fig. 2.
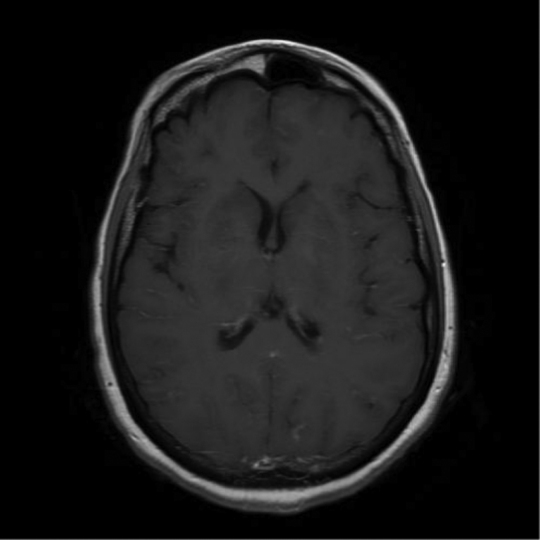
Marked improvement following thalidomide treatment.
Thalidomide therapy was continued for one month and dexamethasone was weaned over the same period. Antifungal therapy was changed to fluconazole 800 mg daily after eight weeks of combined l-AMB and 5-FC. The patient was discharged after eight months and remains on fluconazole at 18 months. She walks with a single-point stick, but still has persistent right leg spasticity, requiring intermittent botulinum toxin injections.
3. Discussion
Thalidomide is a barbiturate-like medication with immunomodulatory and anti-inflammatory effects. It acts by modulating inflammatory cytokines, including TNF-α, IFN, interleukin (IL)-10, IL-12 and cyclooxygenase 2 (COX-2). It also has anti-angiogenic properties, mediated by the depletion of vascular endothelial growth factor (VEGF) receptors [8]. Licensed as an anti-emetic in the 1950s, its use was discontinued by the early 1960s due to its serious teratogenic potential. However it is now used in treating hematologic and solid organ malignancies, and also for infections, including Mycobacterium leprae, M. tuberculosis, HIV-associated aphthous ulcers, wasting syndrome and Kaposi's sarcoma. To our knowledge, this is the first reported case of thalidomide use for the treatment of steroid-refractory IRIS associated with C. neoformans meningitis in an immunocompetent person.
In non-immunosuppressed hosts, outcomes from central nervous system cryptococcosis are typically poor, with reported mortality rates of 16–44% [9], [10], [11]. Late diagnosis of infection, suboptimal management of raised ICP and the exuberance of host responses in c-IRIS all contribute to the high morbidity. c-IRIS has been described in C. neoformans and C. gattii infections in immunocompromised patients, but there is a paucity of reports in immunocompetent hosts. The pathogenesis of c-IRIS in immunocompetent hosts parallels IRIS in HIV-infected persons, in that successful antifungal therapy induces a change from the initial Th2 to a Th1 cytokine response [4].
The diagnosis of IRIS in cryptococcal infections remains challenging due to the absence of specific diagnostic tests. In HIV-infected persons, Haddow et al. propose diagnostic criteria for two distinct types of c-IRIS; paradoxical c-IRIS and antiretroviral-associated IRIS [2], based on antecedent factors, strict clinical criteria and exclusion of alternative causes of clinical deterioration. By contrast, no consensus criteria for the diagnosis of IRIS are available in non-HIV-infected persons. Other investigators have identified clinical, mycological and cytokine profile risk factors to predict development of IRIS in HIV-infected persons [11], [12]. Low CD4 counts, persistent cryptococcal growth, paucity of inflammatory response with low CSF leukocytes, protein, and cytokines including TNF-α, IFN-γ, IL-6 and IL-8 have all been observed more often in HIV-infected patients who develop c-IRIS.
In the HIV population, paradoxical c-IRIS typically occurs after 4 weeks – 10 months of antifungal therapy, but can occur as early as a few days. In our patient, significant clinical and radiological deterioration was noted after five weeks of therapy, despite corticosteroids and appropriate antifungal therapy. c-IRIS was strongly suspected after exclusion of other potential explanations for her deterioration. CSF cytokines were not measured at initial diagnosis or when c-IRIS was suspected, but may have been useful in predicting the risk of or identifying c-IRIS, respectively.
In severe c-IRIS, corticosteroids are generally used to reduce the inflammatory response and raised ICP [1]. The dose and duration of therapy with corticosteroids is not defined, and is commonly individualized, according to the clinical response. Interferon-γ is not recommended, as it may exacerbate c-IRIS through its pro-inflammatory effect [4]. Other agents, including NSAIDs and thalidomide have also been used in treating c-IRIS, but data are limited to anecdotal experience and small numbers.
Brunel et al. previously reported successful use of thalidomide in two HIV-infected patients with cryptococcal meningitis who developed paradoxical IRIS after initiation of antifungal and antiretroviral therapy [6]. Both patients improved after commencement of corticosteroids and thalidomide was used for up to 15 months to facilitate the weaning of steroids. Of note, the investigators were not able to demonstrate the immunomodulatory effects of thalidomide by measuring serum cytokines including TNF-α, IFN-γ, IL-6 and IL-8. By contrast, thalidomide was used in our patient as her c-IRIS was steroid-refractory. Lortholary et al. also successfully used thalidomide in another HIV-infected patient with c-IRIS that developed enlarging lymphadenopathy following antifungal and antiretroviral therapy [7]. Thalidomide was prescribed for four months in total, and the lymphadenopathy resolved.
Given the lack of data guiding the duration of thalidomide therapy in immunocompetent patients with c-IRIS, our patient's considerable improvement and the potential for adverse effects including irreversible peripheral neuropathy, we treated her for four weeks only. Our patient tolerated thalidomide well with no significant adverse effects.
Thalidomide represents one of the few therapeutic options for patients with steroid-refractory c-IRIS. Further reports of its use in this situation and as primary therapy for c-IRIS in both immunocompromised and immunocompetent patients are awaited.
Conflict of interest
There are none.
Acknowledgements
No acknowledgments.
References
- 1.Perfect J.R., Dismukes W.E., Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddow L.J., Colebunders R., Meintjes G. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1 infected individuals: proposed clinical case definitions. Lancet Infect. Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ecevit I.Z., Clancy C.J., Schmalfuss I.M., Nguyen H. The poor prognosis of central nervous system cryptococcosis among nonimmunosuppressed patients: a call for better disease recognition and evaluation of adjuncts to antifungal therapy. Clin. Infect. Dis. 2006;42:1443–1447. doi: 10.1086/503570. [DOI] [PubMed] [Google Scholar]
- 4.Chen S.C., Meyer W., Sorrell T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014;27:980–1024. doi: 10.1128/CMR.00126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitapati A.M., Kao C.L., Cachay E.R. Treatment of HIV-related inflammatory cerebral cryptococcoma with adalimumab. Clin. Infect. Dis. 2010;50 doi: 10.1086/649553. (e7-10) [DOI] [PubMed] [Google Scholar]
- 6.Brunel A., Reynes J., Tuaillon E. Thalidomide for steroid-dependent immune reconstitution inflammatory syndromes during AIDS. AIDS. 2012;26:2110–2112. doi: 10.1097/QAD.0b013e328358daea. [DOI] [PubMed] [Google Scholar]
- 7.Lotholary O., Fontanet A., Memain N. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 8.Franks M.E., Macpherson G.R., Figg W.D. Thalidomide. Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 9.Dromer F., Mathoulin-Pélissier S., Launay O., Lortholary O. the French Cryptococcosis Study Group, Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4:e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappas P.G., Perfect J.R., Cloud C.A. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 11.Boulware D.R., Bonham S.C., Meya D.B. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J. Infect. Dis. 2010;202:962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C.C., Dorasamy A.A., Gosnell B.I. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS) AIDS. 2013;27:2089–2099. doi: 10.1097/QAD.0b013e3283614a8d. [DOI] [PubMed] [Google Scholar]


