Abstract
Amla is one of the most important plants in Indian traditional medicine and has been shown to improve various age-related disorders while decreasing oxidative stress. Mitochondrial dysfunction is a proposed cause of aging through elevated oxidative stress. In this study, we investigated the effects of Amla on mitochondrial function in C2C12 myotubes, a murine skeletal muscle cell model with abundant mitochondria. Based on cell flux analysis, treatment with an extract of Amla fruit enhanced mitochondrial spare respiratory capacity, which enables cells to overcome various stresses. To further explore the mechanisms underlying these effects on mitochondrial function, we analyzed mitochondrial biogenesis and antioxidant systems, both proposed regulators of mitochondrial spare respiratory capacity. We found that Amla treatment stimulated both systems accompanied by AMPK and Nrf2 activation. Furthermore, we found that Amla treatment exhibited cytoprotective effects and lowered reactive oxygen species (ROS) levels in cells subjected to t-BHP-induced oxidative stress. These effects were accompanied by increased oxygen consumption, suggesting that Amla protected cells against oxidative stress by using enhanced spare respiratory capacity to produce more energy. Thus we identified protective effects of Amla, involving activation of mitochondrial function, which potentially explain its various effects on age-related disorders.
1. Introduction
Amla is one of the most important botanical materials in Indian traditional medicine, “Ayurveda.” It has been used for many diseases including diabetes, osteoporosis, liver dysfunction, and anemia, not only in India but also in other countries [1, 2]. Recently, the chemical composition of Amla was analyzed, showing relatively high levels of phenolic compounds [2]. A recent clinical study showed that Amla extract improved endothelial function in patients with type 2 diabetes mellitus [3]. Furthermore, its antioxidant [4], hepatoprotective [5], nephroprotective [6], hypolipidemic [7, 8], cardioprotective [9, 10], and antidiabetic effects [11] were demonstrated in in vivo animal models. One potential mechanism for these effects was reduction of oxidative stress, based on observations of decreased oxidative stress with Amla treatment. However, the detailed mechanisms have not yet been fully identified.
Accumulating evidence suggested a central role for mitochondria in aging through production of both energy and reactive oxygen species (ROS). Mitochondria can produce substantial energy through aerobic metabolism, though they also produce ROS as unwanted byproducts, causing serious damage to various cellular components. Because mitochondria are the major ROS producing organelle, they are also vulnerable to injury by ROS. Damaged mitochondria produce more ROS, leading to further mitochondrial damage [12]. These defective mitochondria exhibit impaired energy production and increased oxidative stress, both negatively impacting cellular function. Numerous reports implicated mitochondrial dysfunction in many pathologies or disorders related to the aging process [13, 14]. Mitochondrial spare respiratory capacity is regarded as an important aspect of mitochondrial function and is defined as the difference between basal ATP production and its maximal activity. When cells are subjected to stress, energy demand increases, with more ATP required to maintain cellular functions. A cell with a larger spare respiratory capacity can produce more ATP and overcome more stress, including oxidative stress [15]. Therefore, we hypothesized that Amla could improve mitochondrial function, especially spare respiratory capacity, and exert positively effects on various disorders related to oxidative stress.
To explore the effects of Amla on mitochondrial function, we used a skeletal muscle cell known to contain abundant mitochondria. Using this model, we investigated the molecular mechanisms of Amla's beneficial effects.
2. Materials and Methods
2.1. Plant Materials
A commercial product of Amla fruit juice extract (SunAmla) was obtained from Taiyo Kagaku Co., Ltd. (Mie, Japan). It is a dried powder of water extract from fresh Amla fruit and was previously shown to contain ~30% polyphenols and 2% vitamin C [6]. To confirm the equivalence of Amla used in this study, total polyphenol was analyzed by a colorimetric method using gallic acid as a standard [6], and vitamin C content was analyzed by high-performance liquid chromatography (HPLC) as previously reported [16]. Glucose and fructose in Amla were analyzed by HPLC as previously reported [17]. To prepare Amla extract stock solution, the extract was dissolved in distilled water at a concentration of 200 mg/mL and then sterilized using a polyvinylidene difluoride membrane filter (Merck Millipore, Darmstadt, Germany).
2.2. Reagents
Oligomycin, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), rotenone, and antimycin A were obtained from Seahorse Bioscience (North Billerica, MA, USA) in XF Cell Mito Stress Test kit (#103015-100). Water soluble tetrazolium salts for the MTT assay were from Kishida Chemical Co., Ltd. (Osaka, Japan). 2′,7′-Dichlorofluorescein diacetate (D6883) and tert-butyl hydroperoxide (t-BHP; 458139) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.3. Cell Culture
C2C12 myoblasts were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 units/mL penicillin, and 100 mg/mL streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C. At confluence, myoblasts were induced to differentiate in DMEM with 2% FBS, 100 units/mL penicillin, and 100 mg/mL streptomycin. Differentiation medium was replaced every 48 h. Human embryonic kidney 293 (HEK293) cells were obtained from the Japanese Collection of Research Bioresources cell bank (Osaka, Japan) and grown in Eagle's minimum essential medium with 10% (v/v) FBS, 100 units/mL penicillin, and 100 mg/mL streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C. For Amla treatment, Amla stock solution was diluted with medium to final concentrations of 100 μg/mL and 200 μg/mL.
2.4. Measurement of Mitochondrial Function
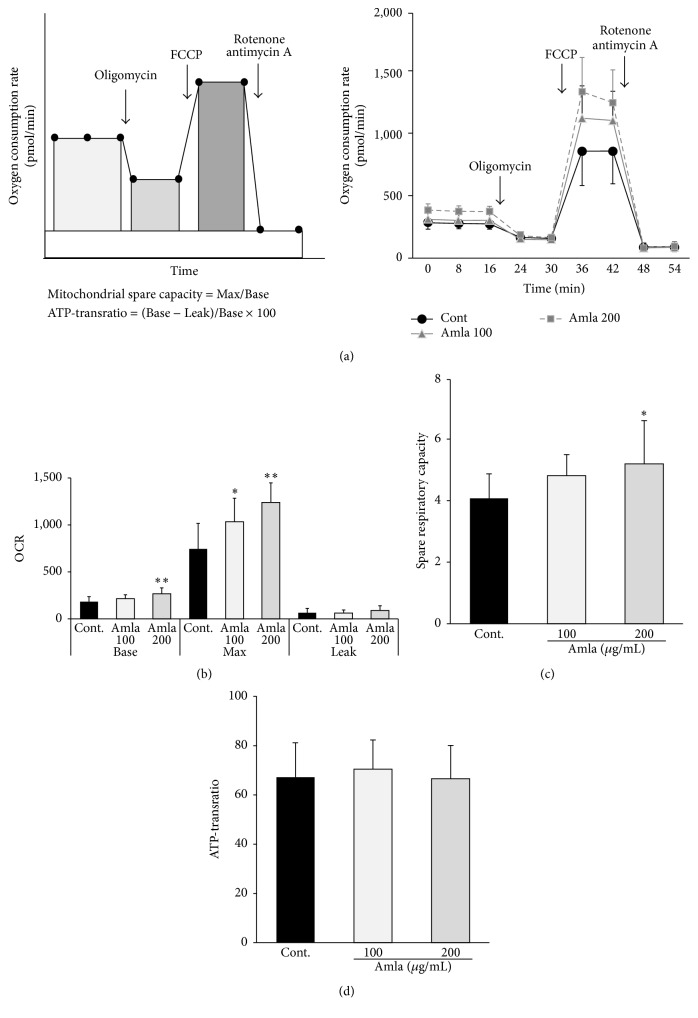
Mitochondrial function in C2C12 myotubes was analyzed using the XFe24 flux analyzer with XF Cell Mito Stress Test kit according to manufacturer instructions (Seahorse Bioscience). Briefly, differentiated C2C12 myotubes were prepared on Seahorse 24-well plates and treated with or without Amla (100 μg/mL or 200 μg/mL) for 48 h. The culture medium was changed at least 40 min prior to the assay to unbuffered DMEM supplemented with 5 mM glucose. The oxygen consumption ratio (OCR, pmol/min) was monitored in real time, with sequential treatments with oligomycin (ATP synthase inhibitor), FCCP (mitochondrial uncoupler), and rotenone/antimycin A (respiration inhibitor) to evaluate OCR from proton leak, maximum respiration capacity, and nonmitochondrial respiration, respectively. OCR was measured multiple times at 8 min intervals at each stage, and average values were determined. Nonmitochondrial respiration was subtracted from OCR at each stage to calculate the net OCR for Basal, Leak, and Max values. Mitochondrial respiratory spare capacity and ATP-transratio were calculated by the formula shown in Figure 1(a), left panel.
Figure 1.
Amla treatment stimulated mitochondrial bioenergetic function. C2C12 myotubes were pretreated with two doses of Amla (100 μg/mL or 200 μg/mL) for 48 h and subjected to mitochondrial function analysis. ((a), left panel) Schematic figure analyzing mitochondrial function using an extracellular flux analyzer. After measuring basal OCR, oligomycin, FCCP, and rotenone/antimycin A were sequentially injected to measure OCR from proton leak, maximal respiratory capacity, and nonmitochondrial respiration, respectively. OCR from nonmitochondrial respiration was subtracted from OCR at each stage to calculate the net OCR for basal (Base), proton leak (Leak), and maximal respiratory capacity (Max) values. Mitochondrial spare respiratory capacity and ATP-transratio were calculated by the formula shown. ((a), right panel) OCR measurements over time (n = 10 or 11). (b) Basal, Max, and Leak OCRs represented as average values from multiple measurements. (c) Mitochondrial spare respiratory capacity was calculated from Max and Basal OCRs. (d) ATP-transratio was calculated from Basal and Leak OCRs. ∗ p < 0.05; ∗∗ p < 0.01 as compared with control (n = 10 or 11).
2.5. Nucleic Acid and Protein Isolation
Total DNA was isolated using a Qiamp DNA mini kit (Qiagen, Mississauga, ON, Canada) according to manufacturer instructions. RNA and total protein were isolated using a PARIS kit (AM1921; Thermo Fisher Scientific, Waltham, MA, USA). Nuclear lysates were isolated using a nuclear/cytosol-fractionation kit (K266; Biovision, Inc., Milpitas, CA, USA) according to manufacturer protocol. Isolated protein samples were denatured by boiling in sodium dodecyl sulfate (SDS) sample buffer.
2.6. Western Blot
Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. Immunoblotting was performed with primary antibodies against phosphorylated AMPKα (#2531; Cell Signaling Technology, Danvers, MA, USA), AMPKα (#2603; Cell Signaling Technology), Nrf2 (SC-722; Santa Cruz Biotechnology, Dallas, TX, USA), and YY-1 (ab109237; Abcam, Tokyo, Japan), with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare Japan, Tokyo, Japan). YY-1 was used as a nuclear-loading control. The intensity of protein bands was visualized using a chemiluminescence detection reagent (PerkinElmer, Inc., Waltham, MA, USA) and a WSE-6100 luminograph system (ATTO, Tokyo, Japan).
2.7. Quantitative PCR for Mitochondrial DNA (mtDNA) Content
mtDNA content was analyzed as previously described [18]. DNA primers were designed to detect cytochrome oxidase 2 (COX2) and uncoupling protein 2 (UCP2) for mtDNA and nuclear DNA, respectively (COX2-F: 5′-TTTTCAGGCTTCACCCTAGATGA-3′ COX2-R: 5′-GAAGAATGTTATGTTATGTTTACTCCTA-3′ UCP2-F: 5′-GCGACCAGCCCATTGTAGA-3′ UCP2-R: 5′-GCGTTCTGGGTACCATCCTAAC-3′). The ratio of COX2 to UCP2 within each sample was used to calculate mtDNA content.
2.8. mRNA Quantification
Complementary DNA was prepared using the PrimeScript2 1st strand cDNA synthesis kit (Takara, Otsu, Japan). RT-qPCR was performed in a StepOnePlus Real-Time PCR system (Thermo Fisher Scientific) using Fast SYBR Green Master Mix (Thermo Fisher Scientific) and primer pair sets described in Table 1. The 18S rRNA was used as a housekeeping gene and served as an endogenous control.
Table 1.
Primer sets used for RT-qPCR.
| Gene name | F/R | Sequence (5′ to 3′) |
|---|---|---|
| Internal standard | ||
| 18S rRNA | F | TTCCGATAACGAACGAGACTCT |
| R | TGGCTGAACGCCACTTGTC | |
| Mitochondrial biogenesis | ||
| PGC1α | F | CCAAACCCACAGAAAACAGG |
| PPAR-gamma coactivator 1-alpha | R | TGGGGTCATTTGGTGACTCT |
| NRF1 | F | GAACTGCCAACCACAGTCAC |
| Nuclear respiratory factor 1 | R | TCGTCTGGATGGTCATTTCA |
| mtTFA | F | CCGAAGTGTTTTTCCAGCAT |
| Mitochondrial transcriptional factor A | R | GGCTGCAATTTTCCTAACCA |
| Antioxidant system | ||
| Nrf2 | F | GGGAGAAAACGACAGAAACC |
| Nuclear factor erythroid 2 related factor 2 | R | TGGGAGAGTAAGGCTTTCCA |
| HO-1 | F | TGACACCTGAGGTCAAGCAC |
| Heme oxygenase-1 | R | TCCTCTGTCAGCATCACCTG |
| NQO1 | F | AAACGTCTGGAAACCGTCTG |
| Nadph dehydrogenase quinone 1 | R | TTCTGCTCCTCTTGAACTTCC |
| Cat | F | GGACGCTCAGCTTTTCATTC |
| Catalase | R | TTGTCCAGAAGAGCCTGGAT |
| GPx | F | CTCATGACCGACCCCAAGTA |
| Glutathione peroxidase | R | CCCACCAGGAACTTCTCAAA |
| Mn-SOD | F | TCTGTGGGAGTCCAAGGTTC |
| Manganese superoxide dismutase | R | TAAGGCCTGTTGTTCCTTGC |
| Cu-SOD | F | GAGACCTGGGCAATGTGACT |
| Copper superoxide dismutase | R | TCATGGACCACCATTGTACG |
2.9. Antioxidant Response Element (ARE) Luciferase Assay
The luciferase reporter plasmid (pGL4.26 luc2/minP/Hygro, E8441) and internal control plasmid (pRL-CMV, E2271) were from Promega (Madison, WI, USA). The DNA sequence for the ARE was obtained from the National Center for Biotechnology Information (GenBank: JQ858521.1), and a synthetic ARE polynucleotide was inserted into the HindΙΙΙ site in multicloning site of pGL4.26 using the In-Fusion HD cloning kit (Takara). The ARE-loaded reporter and internal control vectors were cotransfected into HEK293 cells using lipofectamine-transfection reagent (Thermo Fisher Scientific). After a 24 h incubation, cells were treated with Amla (200 μg/mL) for another 48 h, and luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega).
2.10. MTT Measurements of Cell Viability under Oxidative Stress
C2C12 cells were seeded on 96-well plates and differentiated to myotubes as previously described in Section 2.3. To evaluate the cytoprotective effects of Amla on t-BHP-induced oxidative stress, cells were pretreated with or without Amla (200 μg/mL) for 48 h, then with t-BHP (250 μM or 500 μM) for 6 h. Cell viability was determined using a cell-viability kit based on MTT reduction. Briefly, after treatment with t-BHP, cells were incubated with MTT assay reagents in culture medium for 20 min, and absorbance at 495 nm was measured using an ARVO SX 1420 multilabel counter (PerkinElmer, Inc., Waltham, MA, USA). Cell viability was expressed as the percentage of values obtained with control cells not treated with t-BHP.
2.11. ROS Measurement
Intracellular ROS levels were analyzed using the fluorescent probe 2′,7′-dichlorofluorescein diacetate. Differentiated C2C12 myotubes in 96-well culture plates were pretreated with Amla (200 μg/mL) for 48 h. Myotubes were incubated with 10 μM 2′,7′-dichlorofluorescein diacetate for 30 min following t-BHP treatment (0 μM, 250 μM, or 500 μM) for 2 h. The fluorescence in cells, which was increased by ROS, was analyzed at 485 nm/535 nm (excitation/emission) in an ARVO SX 1420 multilabel counter.
2.12. Oxygen Consumption Analysis in Response to Oxidative Stress
Amla (200 μg/mL) treated or untreated C2C12 myotubes in 24-well plates (Seahorse Bioscience) were prepared as described in Section 2.4. Basal OCR was measured three times at 8 min intervals prior to treatment. After administration of t-BHP (0 μM, 250 μM, or 500 μM), OCR was then measured at 8 min intervals for 160 min. Data are expressed as relative-OCR values normalized to basal OCR values.
2.13. Statistical Analysis
Results are means ± standard deviation. Significance was assessed using Student's t-test and one-way analysis of variance. Differences among groups were determined using Tukey's multiple range test. A p < 0.05 was considered significant.
3. Results
3.1. Amla Extract Components
The total polyphenol and vitamin C contents of Amla extract were 23.9% and 1.32%, respectively (Table 2), and the extract contained low levels of glucose and fructose (3.5% and 4.3%, resp.). Because Amla extract was applied to cells at a concentration of 200 μg/mL, extract-derived sugars (~0.8 mg/dL each) were regarded as negligible.
Table 2.
Components of Amla extract.
| Content, % | ||
|---|---|---|
| Mean | SD | |
| Total polyphenols | 23.9 | 0.6 |
| Vitamin C | 1.32 | 0.2 |
| Glucose | 3.5 | 0.2 |
| Fructose | 4.3 | 0.4 |
Values are means of three replicate samples.
3.2. Amla Treatment Enhanced Mitochondrial Spare Respiratory Capacity
To evaluate the effects of Amla treatment on mitochondrial function, we analyzed the OCR in C2C12 myotubes with or without Amla pretreatment (100 μg/mL or 200 μg/mL). Nonmitochondrial respiration (Figure 1(a), right panel) and Leak (Figure 1(a), right panel, and Figure 1(b)) were unchanged by Amla treatment; however, Base and Max OCRs increased in a dose-dependent manner following Amla treatment (Figure 1(a), right panel, and Figure 1(b)). Mitochondrial spare respiratory capacity also increased in a dose-dependent manner following Amla treatment, though the effects were not significant at lower Amla doses (Figure 1(c)). The ATP-transratio was unchanged by Amla treatment (Figure 1(d)). In order to clarify the mechanisms associated with Amla treatment, we conducted further experiments using the higher dosage (200 μg/mL).
3.3. Amla Treatment Stimulated Mitochondrial Biogenesis by AMPK Activation
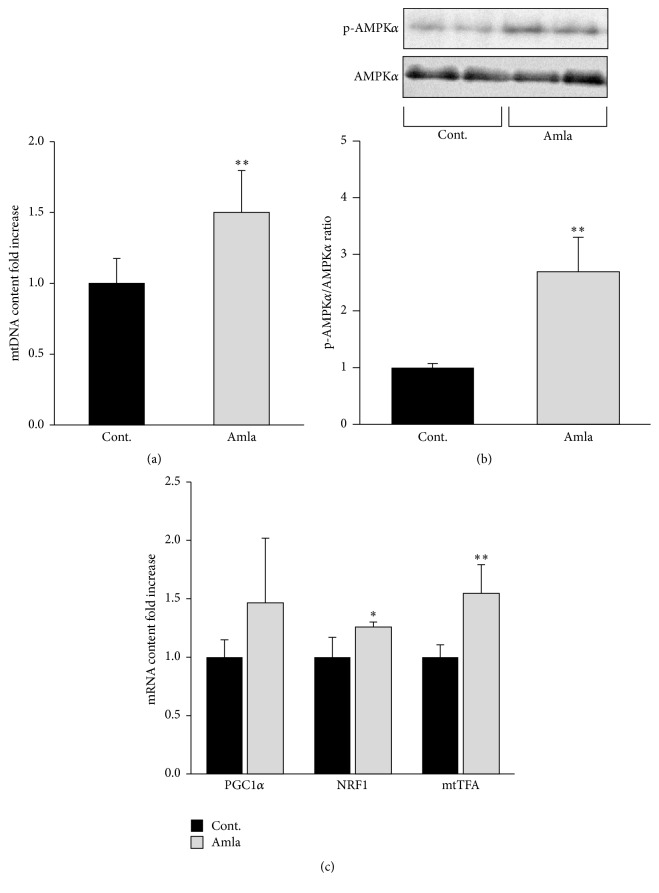
To elucidate how Amla treatment enhanced OCR and mitochondrial spare respiratory capacity, we evaluated its effects on mitochondrial biogenesis in C2C12 myotubes. mtDNA copy number increased 1.5-fold following Amla treatment (Figure 2(a)). To evaluate the molecular effects associated with Amla treatment, we analyzed activation of the AMPKα/PGC1α/NRF1/mtTFA pathway, a key regulatory pathway involved in mitochondrial biogenesis. In cells treated with Amla, AMPKα-phosphorylation levels increased dramatically (Figure 2(b)), NRF1 and mtTFA mRNA expression increased significantly, and PGC1α mRNA exhibited trends indicating increased expression levels (Figure 2(c)).
Figure 2.
Amla treatment stimulated mitochondrial biogenesis by AMPK activation. C2C12 myotubes were incubated with Amla (200 μg/mL) for 48 h. Cell lysates were prepared for western blotting, RT-qPCR, and mtDNA analysis. (a) Relative mtDNA content was determined by qPCR using specific primer sets for the mitochondrial and nuclear genome. ∗∗ p < 0.01; n = 8. (b) Phosphorylated AMPKα to total AMPKα ratios were determined by western blot. ∗∗ p < 0.01; n = 6. (c) Relative contents of PGC1α, NRF1, and mtTFA mRNAs were determined by RT-qPCR. ∗ p < 0.05 and ∗∗ p < 0.01; n = 5. 18S rRNA was used as an internal control for RT-qPCR.
3.4. Amla Treatment Stimulated Antioxidant Systems
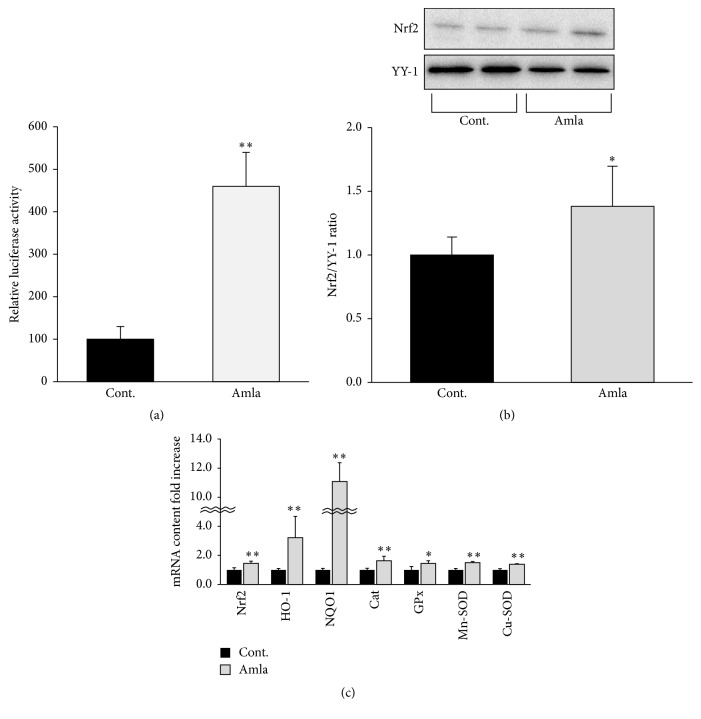
In order to maintain mitochondrial function, ROS reduction and mitochondrial biogenesis are important. Therefore, we assessed the effects of Amla treatment on antioxidant systems in C2C12 myotubes. The activity of Nrf2, a key transcriptional factor involved in cellular antioxidant systems, was analyzed by luciferase reporter assay using a vector containing an ARE. As shown in Figure 3(a), Amla treatment dramatically increased ARE-driven luciferase activity. To further evaluate Nrf2 activation, we analyzed translocation of Nrf2 and observed that Amla treatment significantly increased Nrf2 translocation to the nucleus in C2C12 myotubes (Figure 3(b)). Furthermore, we found that several antioxidant enzymes that are Nrf2 target genes, such as HO-1, NQO-1, Cat, Mn-SOD, and Cu-SOD, were significantly upregulated at the transcriptional level following Amla treatment (Figure 3(c)).
Figure 3.
Amla treatment stimulated antioxidant systems by Nrf2 activation. (a) Activation of Nrf2 was analyzed using an ARE luciferase assay. Data are expressed as relative activities (reporter luciferase activity/control luciferase activity) as compared with data from control cells. ∗∗ p < 0.01; n = 5. (b and c) C2C12 myotubes were incubated with Amla (200 μg/mL) for 48 h. (b) Nuclear lysates were analyzed by western blot, and YY-1 was used an internal control for nuclear protein. ∗ p < 0.05; n = 6. (c) Relative levels of mRNA for antioxidant system related genes were analyzed by RT-qPCR. ∗ p < 0.05; ∗∗ p < 0.01; n = 5. 18S rRNA was used as an internal control for RT-qPCR.
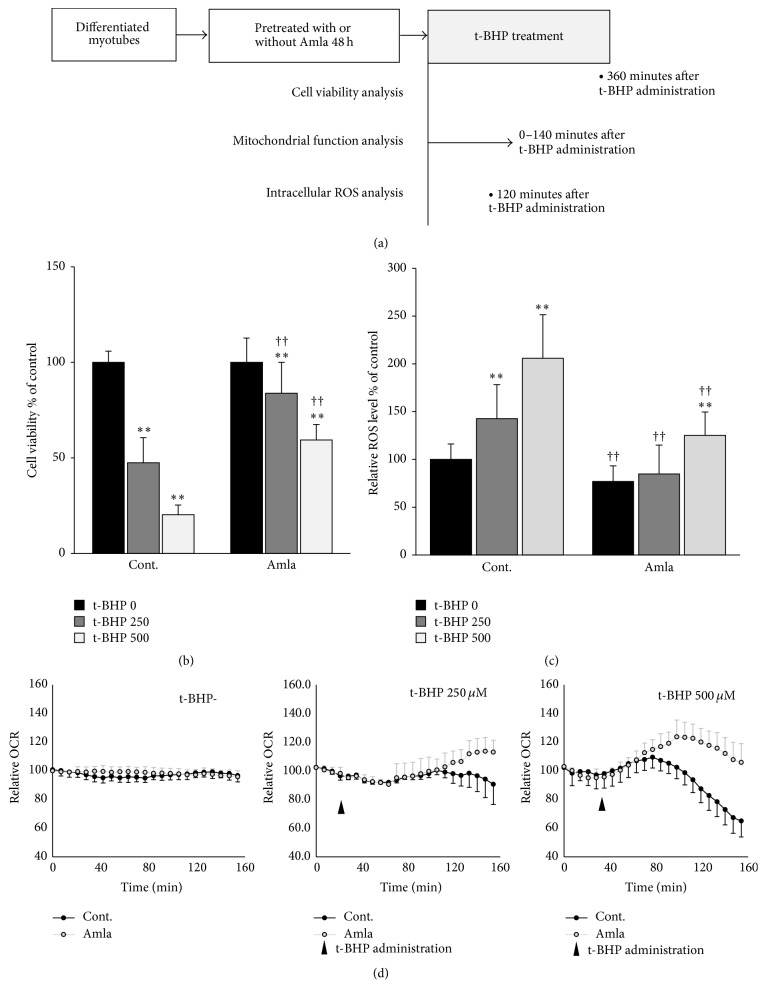
3.5. Amla Treatment Exhibited Cytoprotective Effects on Cells Subjected to Oxidative Stress
To evaluate the cytoprotective effects of Amla treatment against oxidative stress, we first evaluated cell viability using the MTT assay in cells treated with t-BHP for 6 h to induce oxidative stress (Figure 4(a)). As shown in Figure 4(b), t-BHP treatment (250 μM and 500 μM) induced cytotoxicity in a dose-dependent manner, whereas this was significantly attenuated by pretreatment with Amla for 48 h. Next, to evaluate oxidative stress status, we analyzed ROS levels in C2C12 myotubes treated with t-BHP for 2 h (Figure 4(a)). Without t-BHP treatment, Amla pretreatment significantly reduced ROS levels, whereas t-BHP treatment increased ROS levels in a dose-dependent manner, which was significantly suppressed by pretreatment with Amla for 48 h (Figure 4(c)). We then evaluated oxygen consumption under oxidative stress conditions in C2C12 myotubes with or without Amla pretreatment (Figure 4(a)). In control myotubes, t-BHP at high doses caused a time-dependent increase in oxygen consumption, followed by a gradual decline, exhibiting a maximum OCR of 109.5 ± 7.3% that of basal OCR. Amla pretreatment increased maximum OCR in the presence of t-BHP (113.7 ± 9.7% and 120.6 ± 10.0% that of basal OCR at 250 μM and 500 μM t-BHP, resp.). OCRs were unchanged following Amla pretreatment in the absence of t-BHP treatment (Figure 4(d)).
Figure 4.
Amla treatment exhibited a cytoprotective effect against oxidative stress and concomitantly increased oxygen consumption. C2C12 myotubes were pretreated with Amla (200 μg/mL) for 48 h and then treated with t-BHP (250 μM or 500 μM). (a) Schematic showing the time points for three experiments performed to evaluate the cytoprotective effects of Amla treatment. (b) Cell viability was analyzed by MTT assay at 6 h after t-BHP treatment. ∗∗ p < 0.01 versus t-BHP-untreated cells; †† p < 0.01 versus Amla-untreated cells treated with each t-BHP concentration; n = 20. (c) Relative ROS levels in cells were analyzed at 2 h after t-BHP stimulation. ∗∗ p < 0.01 versus t-BHP-untreated cells; †† p < 0.01 versus Amla-untreated cells treated with each t-BHP concentration; n = 12. (d) OCR after t-BHP stimulation was analyzed following t-BHP injection after three basal OCR measurements. OCR was measured every 8 min for a total of 160 min. Data are represented as relative-OCR values divided by the basal OCR values measured prior to t-BHP treatment (n = 10).
4. Discussion
In this study, we made three observations relevant to the beneficial effects of Amla treatment in murine skeletal muscle cells. First, Amla treatment stimulated mitochondrial function, specifically increasing mitochondrial spare respiratory capacity. Second, Amla treatment stimulated mitochondrial biogenesis and antioxidant systems along with activation of the AMPK and Nrf2 pathways. Third, Amla treatment protected cells against oxidative stress accompanied by increased oxygen consumption.
Amla treatment stimulated mitochondrial function by increasing mitochondrial spare respiratory capacity (Figure 1). There have been few studies reporting the ability of food ingredients to enhance mitochondrial spare respiratory capacity. In mouse cortical neuronal cultures, epicatechin and quercetin, both major polyphenols in plant-derived food materials, enhanced mitochondrial spare respiratory capacity and protected cells against oxygen-glucose deprivation stress [19]. Additionally, pharmaceutical approaches, such as administering thyroid hormones, can positively regulate mitochondrial spare respiratory capacity in skeletal muscle [20]. Decreased mitochondrial spare respiratory capacity was also reported in aging [21], and its depletion was implicated in various pathologies in high energy-requiring tissues, such as the heart, brain, and skeletal muscle [22–24]. Our findings suggested that Amla and other food ingredients are potentially novel approaches to improving age-related disorders in skeletal muscle by enhancing mitochondrial spare respiratory capacity. We confirmed that polyphenol rich Amla fractions prepared by adsorption chromatography stimulated mitochondrial respiratory spare capacity (see Supplemental Figures 1(A)–1(C) in Supplementary Material available online at http://dx.doi.org/10.1155/2016/1735841). These results indicated that the polyphenols were the functional components responsible for the observed effects, and given that Amla was reported to contain higher polyphenol content [6], it was expected to be superior to other fruits in mitochondrial maintenance.
Amla treatment stimulated mitochondrial biogenesis and antioxidant systems along with activation of the AMPKα and Nrf2 pathways (Figures 2 and 3). Studies showed that both mitochondrial biogenesis and antioxidant systems could regulate mitochondrial spare respiratory capacity. Increased mitochondrial biogenesis and energy production caused by thyroid hormone treatment leads to stimulation of mitochondrial spare respiratory capacity in skeletal muscle [20]. In striatal neurons, decreased mitochondrial density accompanying mutant huntingtin expression indicated reduced mitochondrial spare respiratory capacity [25]. Furthermore, depletion of an antioxidant enzyme impaired mitochondrial spare respiratory capacity in cortical synaptosomes isolated from SOD2 null mice [26]. These reports were consistent with our findings, and we speculated that the effects of Amla treatment on mitochondrial spare respiratory capacity were related to activation of mitochondrial biogenesis and antioxidant systems. AMPKα and Nrf2 activation reportedly play key roles in mitochondrial biogenesis and antioxidant systems, and there are many studies showing that plant-derived polyphenols activate AMPKα or Nrf2 [27, 28]. In an animal model, dietary gallic acid protected mice from diet-induced obesity by stimulating the AMPKα/Sirt1/PGC1α pathway in liver, muscle, and brown adipose tissues [29]. Furthermore, ellagic acid consumption improved oxidant-induced endothelial dysfunction through Nrf2 activation [30]. These reports supported our findings, because the major polyphenols in Amla include gallic and ellagic acid [6]. Therefore, we proposed that gallic acid and ellagic acid were key factors involved in AMPK and Nrf2 activation following Amla treatment in our experiments.
Amla treatment protected cells against oxidative stress accompanied by increased oxygen consumption (Figure 4). Many reports showed that activation of antioxidant systems could increase cell survival in the presence of oxidative stress [31, 32]. In our study, we observed that Amla treatment protected cells against t-BHP-induced cell death, likely by reducing ROS levels through activation of the Nrf2 pathway (Figures 4(b) and 4(c)). Various oxidative stresses led to increased oxygen consumption, suggesting a critical role for mitochondrial spare respiratory capacity in cell survival and maintenance of biological functions under oxidative stress [15, 33]. We observed that t-BHP treatment increased OCR and that this was likely mediated by the spare respiratory capacity against oxidative stress. Additionally, pretreatment with Amla elevated the maximum OCR against t-BHP treatment (Figure 4(d)). Therefore, we speculated that not only activation of antioxidant systems but also increased mitochondrial function contributed to the cytoprotective effects of Amla treatment against oxidative stress.
In addition to being toxic, ROS are involved in various physiological processes as messenger molecules [34]. Signaling by insulin, several growth factors, and transcriptional factors can be mediated by physiological ROS levels [35, 36]. Because of these multiple roles, ROS homeostasis is regulated by numerous systems [37]. In our experiments, the magnitude by which Amla treatment decreased ROS increased with t-BHP concentration (Figure 4(c)). Therefore, our findings suggested that Amla treatment might remove excessive and damaging levels of ROS without neutralizing the ROS required for physiological function.
One limitation of our study was that it only demonstrated the effects of Amla treatment in vitro. Component analysis and previous research strongly suggested that polyphenols contributed to the effects of Amla treatment [29, 30] and that Amla contained various kinds of polyphenols, with gallic acid as a major component [6]. In one report, gallic acid was absorbed better in humans as compared to other polyphenols [38], and a previous clinical study investigating the bioavailability of gallic acid from red wine showed that glucuronidated and intact forms of gallic acid were detected in plasma [39]. These findings indicated that gallic acid in Amla may partially account for the biological relevance of our findings. Further experiments will be needed to clarify whether the effects of Amla treatment reported here can be reproduced in vivo.
Our results indicated that Amla treatment enhanced mitochondrial spare respiratory capacity, which was supported by its effect on mitochondrial biogenesis and antioxidant systems, through activation of the AMPKα and Nrf2 pathways, respectively. Furthermore, we found that Amla treatment decreased ROS levels and increased cell viability in the presence of t-BHP induced oxidative stress. We attributed the cytoprotective effects of Amla treatment not only to activation of antioxidant systems, but also to enhancement of the mitochondrial spare respiratory capacity (Figure 5).
Figure 5.
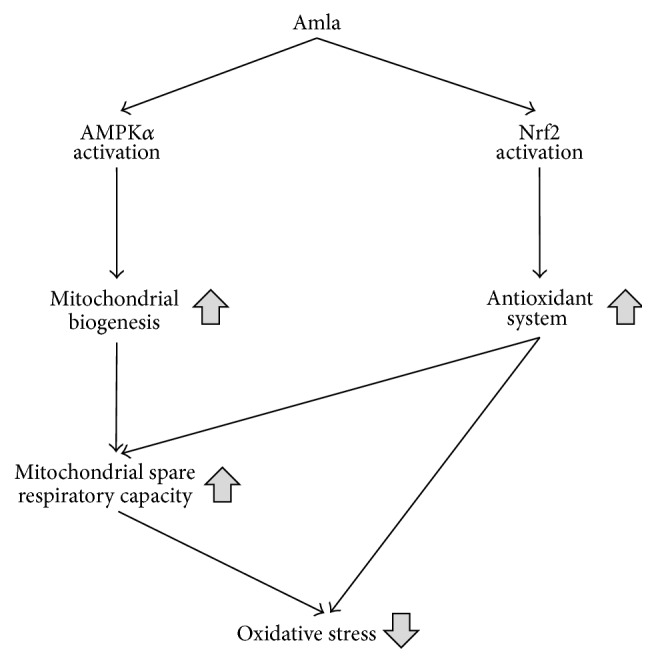
Schematic figure showing the effects of Amla treatment resulting in reduced oxidative stress.
5. Conclusions
In conclusion, our study demonstrated that Amla enhanced mitochondrial spare respiratory capacity through activation of mitochondrial biogenesis and antioxidant systems. Furthermore, we showed that these effects of Amla resulted in decreased ROS levels and increased cell viability under oxidative stress conditions. Therefore, our findings suggested novel potential mechanisms for the beneficial effects associated with Amla intake, including decreased oxidative stress.
Supplementary Material
Amla treatment stimulated mitochondrial bioenergetics function. To explore an active integrant in Amla, Amla extract was separated by reversed-phase chromatography to obtain a polyphenol-rich fraction (a mixture of polyphenols corresponding to what is present in the Amla fruit) and a polyphenol-poor fraction (a mixture of the constituents of Amla extract without polyphenols). We analyzed mitochondrial function and found that the effect of Amla treatment was due to the polyphenol-rich fraction. These results indicated that the polyphenols were the functional components responsible for the observed effects.
Acknowledgments
The authors thank Misako Kitano, Takahiro Okuda, Yuichiro Mori, and Takashi Fujii for their technical assistance. The Department of Medicine, SUMS, received research promotion grants (Shougaku Kifukin) from Astellas, AstraZeneca, Bayer, Boehringer-Mannheim, Bristol-Myers Squibb, Chugai Pharma, Daiichi-Sankyo, Dainippon Sumitomo, Eisai, Eli Lilly, Fuji Yakuhin, Glaxo Smithkline, Kaken Pharmaceutical, Kaneka Medix, Kissei, Kowa Pharmaceuticals, Kyowa-hakko-Kirin, Miki Corporation, Mitsubishi Tanabe, Mochida, MSD, Nihon Medi-Physics, Nipro, Novartis, Novo Nordisk, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, Sunstar, Taisho-Toyama, Takeda, Teijin Pharma, Terumo, and Torii Pharmaceutical. The research topics funded by these grants are unrestricted.
Competing Interests
Hirotaka Yamamoto, Takao Ikami, Kohei Kiriyama, and Taishi Ishibashi are employees of Miki Corporation. Miki Corporation has a food product containing Amla extract. This work was conducted in collaboration with Shiga University of Medical Science (SUMS). Hirotaka Yamamoto is a Visiting Assistant Professor at SUMS.
Authors' Contributions
Hirotaka Yamamoto and Katsutaro Morino conceived and designed the research; Hirotaka Yamamoto, Katsutaro Morino, Taishi Ishibashi, and Kohei Kiriyama performed experiments; Hirotaka Yamamoto and Katsutaro Morino analyzed data; Hirotaka Yamamoto, Katsutaro Morino, and Lemecha Mengistu interpreted the experimental results; Hirotaka Yamamoto and Katsutaro Morino prepared the figures and drafted the paper; Hirotaka Yamamoto, Katsutaro Morino, Takao Ikami, and Hiroshi Maegawa edited, revised, and approved the final version of paper.
References
- 1.Patel S. S., Goyal R. K. Emblica officinalis Geart.: a comprehensive review on phytochemistry, pharmacology and ethnomedicinal uses. Research Journal of Medicinal Plant. 2012;6(1):6–16. doi: 10.3923/rjmp.2012.6.16. [DOI] [Google Scholar]
- 2.Bhandari P., Kamdod M. Emblica officinalis (Amla): a review of potential therapeutic applications. International Journal of Green Pharmacy. 2012;6(4):257–269. doi: 10.4103/0973-8258.108204. [DOI] [Google Scholar]
- 3.Usharani P., Fatima N., Muralidhar N. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2013;6:275–284. doi: 10.2147/dmso.s46341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya A., Chatterjee A., Ghosal S., Bhattacharya S. K. Antioxidant activity of active tannoid principles of Emblica officinalis (amla) Indian Journal of Experimental Biology. 1999;37(7):676–680. [PubMed] [Google Scholar]
- 5.Damodara Reddy V., Padmavathi P., Gopi S., Paramahamsa M., Varadacharyulu N. C. Protective effect of Emblica officinalis against alcohol-induced hepatic injury by ameliorating oxidative stress in rats. Indian Journal of Clinical Biochemistry. 2010;25(4):419–424. doi: 10.1007/s12291-010-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokozawa T., Kim H. Y., Juneja L. R., et al. Amla (Emblica officinalis Gaertn.) attenuates age-related renal dysfunction by oxidative stress. Journal of Agricultural and Food Chemistry. 2007;55(19):7744–7752. doi: 10.1021/jf072105s. [DOI] [PubMed] [Google Scholar]
- 7.Yokozawa T., Kim H. Y., Kim H. J., Okubo T., Chu D.-C., Juneja L. R. Amla (Emblica officinalis Gaertn.)prevents dyslipidaemia and oxidative stress in the ageing process. British Journal of Nutrition. 2007;97(6):1187–1195. doi: 10.1017/s0007114507691971. [DOI] [PubMed] [Google Scholar]
- 8.Kim H. Y., Okubo T., Juneja L. R., Yokozawa T. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. British Journal of Nutrition. 2010;103(4):502–512. doi: 10.1017/s0007114509991978. [DOI] [PubMed] [Google Scholar]
- 9.Ojha S., Golechha M., Kumari S., Arya D. S. Protective effect of Emblica officinalis (amla) on isoproterenol-induced cardiotoxicity in rats. Toxicology and Industrial Health. 2012;28(5):399–411. doi: 10.1177/0748233711413798. [DOI] [PubMed] [Google Scholar]
- 10.Patel S. S., Goyal R. K. Prevention of diabetes-induced myocardial dysfunction in rats using the juice of the Emblica officinalis fruit. Experimental and Clinical Cardiology. 2011;16(3):87–91. [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar M. S., Ramzan A., Ali A., Ahmad M. Effect of amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. International Journal of Food Sciences and Nutrition. 2011;62(6):609–616. doi: 10.3109/09637486.2011.560565. [DOI] [PubMed] [Google Scholar]
- 12.Bratic A., Larsson N.-G. The role of mitochondria in aging. The Journal of Clinical Investigation. 2013;123(3):951–957. doi: 10.1172/jci64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safdar A., Hamadeh M. J., Kaczor J. J., Raha S., deBeer J., Tarnopolsky M. A. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010778.e10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso C., Scapagini G., Currò D., et al. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Frontiers in Bioscience. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- 15.Hill B. G., Dranka B. P., Zou L., Chatham J. C., Darley-Usmar V. M. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochemical Journal. 2009;424(1):99–107. doi: 10.1042/bj20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dennison D. B., Brawley T. G., Hunter G. L. K. Rapid high-performance liquid chromatographic determination of ascorbic acid and combined ascorbic acid-dehydroascorbic acid in beverages. Journal of Agricultural and Food Chemistry. 1981;29(5):927–929. doi: 10.1021/jf00107a009. [DOI] [PubMed] [Google Scholar]
- 17.Kim S., Park J. M., Kim C. H. Ethanol production using whole plant biomass of Jerusalem artichoke by Kluyveromyces marxianus CBS1555. Applied Biochemistry and Biotechnology. 2013;169(5):1531–1545. doi: 10.1007/s12010-013-0094-5. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H., Morino K., Nishio Y., et al. MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. American Journal of Physiology—Endocrinology and Metabolism. 2012;303(12):E1419–E1427. doi: 10.1152/ajpendo.00097.2012. [DOI] [PubMed] [Google Scholar]
- 19.Nichols M., Zhang J., Polster B. M., et al. Synergistic neuroprotection by epicatechin and quercetin: activation of convergent mitochondrial signaling pathways. Neuroscience. 2015;308:75–94. doi: 10.1016/j.neuroscience.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Lesmana R., Sinha R. A., Singh B. K., et al. Thyroid hormone stimulation of autophagy is essential for mitochondrial biogenesis and activity in skeletal muscle. Endocrinology. 2016;157(1):23–38. doi: 10.1210/en.2015-1632. [DOI] [PubMed] [Google Scholar]
- 21.Rohrer B., Bandyopadhyay M., Beeson C. Reduced metabolic capacity in aged primary retinal pigment epithelium (RPE) is correlated with increased susceptibility to oxidative stress. Advances in Experimental Medicine and Biology. 2016;854:793–798. doi: 10.1007/978-3-319-17121-0_106. [DOI] [PubMed] [Google Scholar]
- 22.Desler C., Hansen T. L., Frederiksen J. B., Marcker M. L., Singh K. K., Juel Rasmussen L. Is there a link between mitochondrial reserve respiratory capacity and aging? Journal of Aging Research. 2012;2012:9. doi: 10.1155/2012/192503.192503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sansbury B. E., Jones S. P., Riggs D. W., Darley-Usmar V. M., Hill B. G. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chemico-Biological Interactions. 2011;191(1–3):288–295. doi: 10.1016/j.cbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadava N., Nicholls D. G. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. The Journal of Neuroscience. 2007;27(27):7310–7317. doi: 10.1523/jneurosci.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui A., Sánchez S. R., Castro M. D. R., et al. Mitochondrial DNA damage is associated with reduced mitochondrial bioenergetics in Huntington's disease. Free Radical Biology and Medicine. 2012;53(7):1478–1488. doi: 10.1016/j.freeradbiomed.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn J. M., Choi S. W., Day N. U., Gerencser A. A., Hubbard A., Melov S. Impaired spare respiratory capacity in cortical synaptosomes from Sod2 null mice. Free Radical Biology and Medicine. 2011;50(7):866–873. doi: 10.1016/j.freeradbiomed.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang M., Xu S., Maitland-Toolan K. A., et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55(8):2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 28.Erlank H., Elmann A., Kohen R., Kanner J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radical Biology and Medicine. 2011;51(12):2319–2327. doi: 10.1016/j.freeradbiomed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Doan K. V., Ko C. M., Kinyua A. W., et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology. 2015;156(1):157–168. doi: 10.1210/en.2014-1354. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y., Zhang B., Zhou K., et al. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: role of Nrf2 activation. International Journal of Cardiology. 2014;175(3):508–514. doi: 10.1016/j.ijcard.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 31.Zhong R.-Z., Fang Y., Qin G.-X., Li H.-Y., Zhou D.-W. Tea catechins protect goat skeletal muscle against H2O2-induced oxidative stress by modulating expression of phase 2 antioxidant enzymes. Journal of Agricultural and Food Chemistry. 2015;63(36):7921–7928. doi: 10.1021/acs.jafc.5b00816. [DOI] [PubMed] [Google Scholar]
- 32.Kang J. S., Han M. H., Kim G.-Y., et al. Schisandrae semen essential oil attenuates oxidative stress-induced cell damage in C2C12 murine skeletal muscle cells through Nrf2-mediated upregulation of HO-1. International Journal of Molecular Medicine. 2015;35(2):453–459. doi: 10.3892/ijmm.2014.2028. [DOI] [PubMed] [Google Scholar]
- 33.Dranka B. P., Hill B. G., Darley-Usmar V. M. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radical Biology and Medicine. 2010;48(7):905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. The Journal of Biological Chemistry. 2014;289(13):8735–8741. doi: 10.1074/jbc.r113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heffetz D., Bushkin L., Dror R., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. The Journal of Biological Chemistry. 1990;265(5):2896–2902. [PubMed] [Google Scholar]
- 36.Sundaresan M., Yu Z.-X., Ferrans V. J., Irani K., Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270(5234):296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 37.Rahal A., Kumar A., Singh V., et al. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Research International. 2014;2014:19. doi: 10.1155/2014/761264.761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daglia M., Di Lorenzo A., Nabavi S. F., Talas Z. S., Nabavi S. M. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Current Pharmaceutical Biotechnology. 2014;15(4):362–372. doi: 10.2174/138920101504140825120737. [DOI] [PubMed] [Google Scholar]
- 39.Cartron E., Fouret G., Carbonneau M.-A., et al. Red-wine beneficial long-term effect on lipids but not on antioxidant characteristics in plasma in a study comparing three types of wine—description of two O-methylated derivatives of gallic acid in humans. Free Radical Research. 2003;37(9):1021–1035. doi: 10.1080/10715760310001598097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amla treatment stimulated mitochondrial bioenergetics function. To explore an active integrant in Amla, Amla extract was separated by reversed-phase chromatography to obtain a polyphenol-rich fraction (a mixture of polyphenols corresponding to what is present in the Amla fruit) and a polyphenol-poor fraction (a mixture of the constituents of Amla extract without polyphenols). We analyzed mitochondrial function and found that the effect of Amla treatment was due to the polyphenol-rich fraction. These results indicated that the polyphenols were the functional components responsible for the observed effects.