Abstract
Background/Aims. Low serum folate levels can alter inflammatory reactions. Both phenomena have been linked to Alzheimer's disease (AD), but the effect of folic acid on AD itself is unclear. We quantified folate supplementation's effect on inflammation and cognitive function in patients with AD over the course of 6 months. Methods. Patients newly diagnosed with AD (age > 60 years; n = 121; mild to severe; international criteria) and being treated with donepezil were randomly assigned into two groups with (intervention group) or without (control group) supplemental treatment with folic acid (1.25 mg/d) for 6 months. The Mini-Mental State Examination (MMSE) was administered to all patients at baseline and follow-up, and blood samples were taken before and after treatment. We quantified serum folate, amyloid beta (Aβ), interleukin-6 (IL-6), tumor necrosis factor α (TNFα), plasma homocysteine (Hcy), S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), and the mRNA levels of presenilin (PS), IL-6, and TNFα in leukocytes. Data were analyzed using a repeated-measures mixed model. Results. The mean MMSE was slightly increased in the intervention group compared to that in the control group (P < 0.05). Posttreatment, plasma SAM and SAM/SAH levels were significantly higher (P < 0.05), while Aβ 40, PS1-mRNA, and TNFα-mRNA levels were lower in the intervention group than in the control group (P < 0.05). The Aβ 42/Aβ 40 ratio was also higher in the intervention group (P < 0.05). Conclusions. Folic acid is beneficial in patients with AD. Inflammation may play an important role in the interaction between folic acid and AD. This trial is registered with clinical trial registration number ChiCTR-TRC-13003246.
1. Introduction
As the world's population ages, it is becoming increasingly difficult to control the growing burden of dementia. This is especially true for Alzheimer's disease (AD), the most common type of dementia in the elderly [1]. In China, it is estimated that the incidence of AD had reached 6.25 cases per 1000 person-years in 2010 [2]. AD is an age-related neurodegenerative disease, characterized by memory loss and cognitive decline, which leads to death within 7–10 years of diagnosis [3]. The mechanisms underlying the degenerative processes remain unclear, although epigenetic and neuroinflammatory disturbances, in addition to the apparent contribution of aging itself, have been implicated in AD [4, 5].
As mentioned, inflammatory processes are being investigated in the pathological mechanisms of AD and mild cognitive impairment (MCI) [5]. Low folate levels impair vitamin B12 absorption, which in turn may lead to an inflammatory state, and thus explain the relationship between both of these compounds and AD risk [6]. Indeed, studies have reported that folate levels are lower in patients with AD than in normal controls [7–9].
Effective treatments for AD are also relatively few and only mitigate the progression of the disease. To improve cognitive function, many clinicians recommend that patients with AD take folate and other B vitamin supplements, but the effectiveness of these remains controversial [10, 11]. We have previously reported that the risk of AD is increased in individuals with low serum folate levels [7, 12]. Here, we further explore the role of inflammation in mediating the beneficial effects of folic acid on newly diagnosed AD patients with no previous folate supplementation.
2. Materials and Methods
2.1. Participants
The Department of Nutrition and Food Science, School of Public Health, Tianjin Medical University, and Department of Neurology, Huanhu Hospital, Tianjin, designed the TFA-AD trial. Participants were recruited between November 2013 and October 2014 by neurologists at the Neurology Central Hospital of Tianjin.
We recruited participants with a new diagnosis of possible or probable AD of mild to moderate severity, defined as a Mini-Mental State Examination (MMSE) total score between 3 and 26 [13], and those who were currently taking donepezil. Individuals who met the following criteria were included in the study: age 40–90 years; Hachinski Ischemic Scale score <4; Hamilton Depression Rating Scale score <7.0; MMSE score 3.0–26.0 points; activities of daily living (ADL) [14] score ≥22.0; cerebral computed tomography or magnetic resonance imaging showing varying degrees of ventricular dilatation; medial temporal lobe and hippocampal atrophy, widening of the cerebral sulci, and other cortical atrophies; and the absence of encephalopathy, which can appear clinically similar to AD. We excluded patients who had taken the prescribed daily intake of vitamins B, C, and E within the month before baseline and those who had regularly taken B vitamin supplements within the 6 months before baseline. All participants had normocytic anemia (mean corpuscular volume: 82–95 FL) and normal renal function (creatinine clearance: 80–120 mL/min). All participants or their surrogates provided written informed consent. Written consent for collection of caregiver data was also obtained from the participants' designated caregivers.
2.2. Interventions
This was a single-center, single blind, randomized controlled trial (RCT) to assess whether folic acid could effectively delay clinical progression in patients with AD. Patients were randomly assigned in a 1 : 1 ratio to either the treatment or the control group, using randomization software. At the baseline visit, each investigator sequentially assigned a randomization number to each patient. No individual patient randomization code was revealed during the trial. Monitors from the Clinical Research Organization and the sponsor visited investigators regularly to conduct quality control checks and ensure the validity and accuracy of recording and overall adherence to the study protocol. Data were entered by double entry, and computerized checks were performed to ensure the consistency of data.
All participants took donepezil as basic routine therapy. The initial dose of donepezil was 5 mg daily and was increased to 10 mg after one month. Participants having difficulty tolerating the higher dose of donepezil were excluded by the investigator. Although the optimal folic acid dose needed to improve cognitive function is still not known, Durga et al. tested the effects of 800 μg/d folic acid supplementation on cognitive function in older adults over a period of 3 years [11]. McMahon et al. reported the effects of 2 years of 1 mg/d folic acid supplementation on healthy older people [10]. Considering previous durations and dosages, this study examined 6 months of 1.25 mg/d folic acid supplementation in patients with AD. Participants randomized into the folic acid supplementation group received 1.25 mg folic acid daily during or immediately after a meal, in tablet form (Beijing Scrianen Pharmaceutical Co. Ltd., China; 5 mg/tablet; state medical permit number H10970079), for the entire six-month period. These folic acid supplements are available by prescription in China. Adherence was encouraged and monitored throughout the trial by telephone. Clinical outcomes were assessed at baseline and at 6-week intervals.
Each patient had a responsible caregiver, and both participants and their caregivers provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and the ICH-GCP as appropriate to nutritional products and approved by the Ethics Committee of the Tianjin Health Service.
2.3. Outcome Measures
Primary outcome measures included the differences between the baseline and 6-month scores on the MMSE and ADL and the levels of amyloid beta Aβ 40, Aβ 42, Aβ 42/Aβ 40, amyloid precursor protein mRNA (APP-mRNA), PS1-mRNA, PS2-mRNA, IL-6-mRNA, and TNFα-mRNA. Secondary outcome measures included the differences between the baseline and 6-month values of SAM, SAH, SAM/SAH, and Hcy.
2.4. Blood Sample Collection
Fasting venous blood was collected into two tubes for processing. A tube containing coagulant was centrifuged at 3,000 g for 10 min, and the serum was drawn off and stored frozenly at −80°C. Serum samples were divided for the subsequent measurement of (1) folate and vitamin B12 levels and (2) Aβ 40, Aβ 42, IL-6, and TNFα levels. Another tube containing ethylenediaminetetraacetic acid (EDTA) was immediately centrifuged at 2,500 g for 10 min at 4°C. Plasma samples were decanted and stored frozenly at −80°C for the subsequent measurement of Hcy, S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH). The white blood cells were obtained and stored frozenly at −80°C for the subsequent measurement of PS1-mRNA, PS2-mRNA, IL-6-mRNA, and TNFα-mRNA.
2.5. Measurement of Biochemical Parameters
Serum folate and vitamin B12 levels were measured using an automated immunoassay analyzer (Abbott AxSYM system, Abbott Laboratories, Abbott Park, IL, USA) [15, 16]. Plasma levels of Hcy, SAM, and SAH were analyzed via high-performance liquid chromatography (HPLC), as described by Poirier et al. [17], and quantified relative to standards obtained from Sigma Chemical Co. (St. Louis, MO, USA). AD gene-related mRNAs were analyzed using real-time polymerase chain reaction (RT-PCR). The interassay coefficients of variation ranged from 3 to 10%, within an appropriate range unlikely to induce statistical errors.
Aβ 40, Aβ 42, IL-6, and TNFα in the patients' blood were quantified with a standard ELISA kit (Biosources, Camarillo, CA). Briefly, frozen samples were sequentially extracted via a two-step extraction process (sonication in 2% SDS and 70% formic acid). After sonication, the samples were centrifuged at 100,000 g for 1 h at 4°C, and the supernatant was decanted. The serum was then sonicated with the formic acid solution. Aβ 40, Aβ 42, IL-6, and TNFα were quantified separately, using an ELISA kit specific for each amyloid fragment in accordance with the manufacturer's instructions.
Real-time PCR was performed to analyze the different AD gene-related mRNA levels. The total RNA in the blood was reverse-transcribed to cDNA using RNase Inhibitor, dNTP, RT buffer, oligo(dT), DEPC H2O, and AMV (Takara). The mixture was incubated at 42°C for 50 min, 99°C for 5 min, and 5°C for 5 min to generate a cDNA library. Real-time PCR was subsequently performed using a LightCycler 480 SYBR Green I Master system (Roche, USA) according to a standardized protocol. The reactions were incubated at 95°C for 5 min, followed by 45 cycles at an interval of 10 s at 95°C, 5–20 s at 72°C, and 5 s at 95°C and an interval of 60 s at 60°C. Data were analyzed by 2−ΔCT. The primer sequences of the internal control gene and the target genes are listed in Table 1.
Table 1.
Primer sequences of the internal control gene and the target genes for RT-PCR.
| Genes | Primer sequence |
|---|---|
| APP | Forward: CCTACAACAGCAGCCAGTACCCCTG |
| Reverse: GACATTCTCTCTCGGTGCTTGGCC | |
|
| |
| PS1 | Forward: GGTCGTGGCTACCATTAAGTC |
| Reverse: GCCCACAGTCTCGGTATCTT | |
|
| |
| PS2 | Forward: ACCGCTATGTCTGTAGTGGG |
| Reverse: CGCTCCGTATTTGAGGGTCAG | |
|
| |
| IL-6 | Forward: TCTCCACAAGCGCCTTCG |
| Reverse: CTCAGGGCTGAGATGCCG | |
|
| |
| TNFα | Forward: TCTTCTCGAACCCCGAGTGA |
| Reverse: CCTCTGATGGCACCACCAG | |
|
| |
| GAPDH | Forward: CCACTCCTCCACCTTTGAC |
| Reverse: ACCCTGTTGCTGTAGCCA | |
2.6. Sample Size
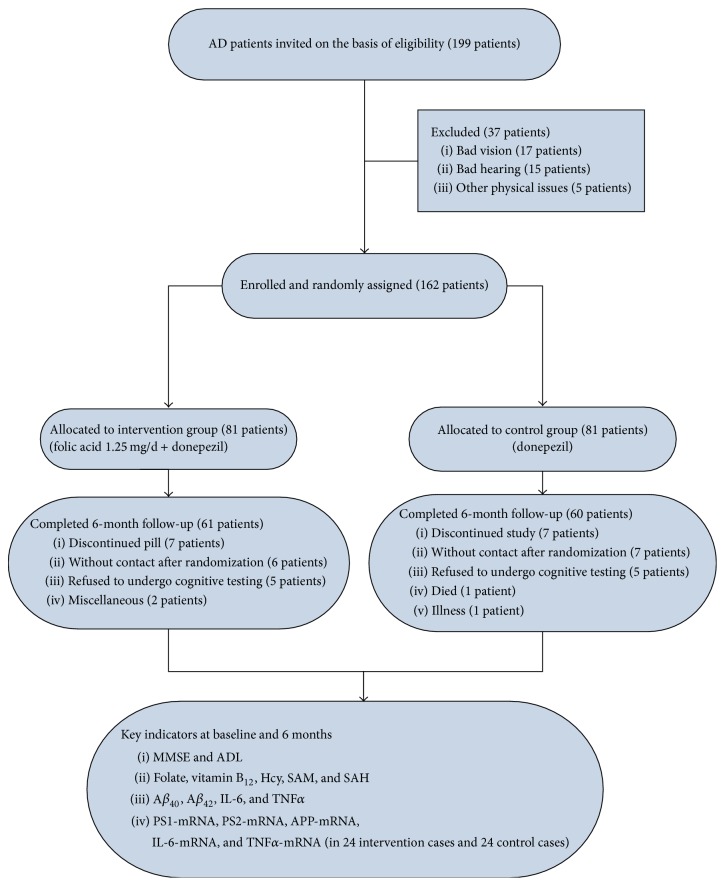
Assuming a hypothetical effect size of 0.6, a sample size in each intervention group provided 90% power at a 2-sided α level of 0.05, based on a 2-sample t-test for change between baseline and 6 months in both MMSE and ADL scores [18]. With allowance for a dropout rate of 25% [19], each intervention group needed at least 75 patients at baseline and 60 patients after 6 months. We started with 81 patients at baseline and concluded with 61/60 patients after 6 months in each intervention group (Figure 1).
Figure 1.
Study participant flow diagram from the initial contact through the completion of the study.
2.7. Statistical Analysis
All statistical tests were performed using SPSS for Windows v.15.0 (SPSS Inc., Chicago, IL, USA). Data are presented as means ± standard deviations (SD), medians (quartiles), or proportions. Statistical significance was set at P < 0.05. Comparisons between categorical variables were conducted using chi-square tests. Quantitative variables were checked for normality using histograms and quartile-quartile plots. Age at diagnosis, education (years), body mass index (BMI), folate, vitamin B12, MMSE, and ADL were normally distributed and thus evaluated using two-tailed Student's t-tests at baseline. Hcy, SAM, SAH, and SAM/SAH were normally distributed after square root transformation. Aβ 40, Aβ 42, Aβ 42/Aβ 40, and AD gene-related mRNA levels were normally distributed after logarithmic transformation.
Repeated-measures ANOVAs were used to evaluate the effects of folic acid and control interventions on biomarker and cognitive performance over 6 months, in which time was treated as a categorical variable and represented by dummies. Data are mean unadjusted scores plus standard deviations, with ηp 2 and P values from repeated-measures ANOVAs that included the time-treatment interaction.
Patients who had one assessment after baseline were included in the intention-to-treat (ITT) efficacy analysis.
3. Results
3.1. Characteristics of the Study Participants
The flow of participants through the study is shown in Figure 1. Of the 199 initial patients with AD, 37 were excluded for bad vision, bad hearing, or other physical issues, leaving 162 patients with AD to be randomly allocated between the groups. Of these, 61 patients in the folate supplementation group were excluded as follows: 7 discontinued the supplement, 6 could not be contacted after randomization, 5 refused to undergo cognitive testing, and 2 were excluded for other reasons. In the control group, 60 patients were excluded as follows: 7 discontinued the study, 7 could not be contacted after randomization, 5 refused to undergo cognitive testing, 1 died, and 1 became ill. No relevant differences among the groups were detected at baseline.
A summary of participants' characteristics at baseline is shown in Table 2. Demographics, lifestyle, family history, comorbidities, and current AD-related medications were not significantly different between the two groups. Furthermore, the intervention (folate supplementation) and control groups had similar levels of folate and vitamin B12 (P > 0.05).
Table 2.
Baseline characteristics of the study population.
| Profile | Intervention group (n = 61) |
Control group (n = 60) |
P valuea |
|---|---|---|---|
| Demography | |||
| Age at diagnosis, years (mean ± SD) | 68.10 ± 8.50 | 67.63 ± 7.92 | 0.756 |
| Males, number (%) | 33 (54.10) | 28 (46.67) | 0.414 |
| Education, years (mean ± SD) | 10.07 ± 4.08 | 9.27 ± 4.39 | 0.302 |
| Lifestyle | |||
| Right-handedness, number (%) | 57 (93.44) | 53 (88.33) | 0.363 |
| Marital status: married, number (%) | 50 (81.97) | 49 (81.67) | 0.966 |
| Living with others, number (%) | 60 (98.36) | 58 (96.67) | 0.619 |
| Smoking, number (%) | 12 (19.67) | 14 (23.33) | 0.624 |
| Consuming alcohol, number (%) | 10 (16.39) | 12 (20.00) | 0.607 |
| BMI (mean ± SD), kg/m2 | 23.25 ± 3.06 | 23.58 ± 4.28 | 0.623 |
| Profession: intellectual work, number (%) | 32 (52.46) | 28 (46.67) | 0.524 |
| Family history, number (%) | 16 (26.23) | 15 (25.00) | 0.877 |
| Comorbid disease, n (%) | 25 (40.98) | 26 (43.33) | 0.794 |
| Biochemical measures | |||
| Folate (nmol/L) [median (Q1, Q3)] | 11.98 (8.04, 17.68) | 10.76 (7.53, 16.37) | 0.484 |
| Vitamin B12 (pmol/L) [median (Q1, Q3)] | 289.96 (233.88, 406.16) | 329.06 (231.86, 455.04) | 0.446 |
| Medicine on AD | |||
| AChEI, number (%) | 61 (100.00) | 60 (100.00) | 1.000 |
| Memantine, number (%) | 10 (16.39) | 13 (15.48) | 0.460 |
| MMSE (mean ± SD) | 18.56 ± 6.23 | 17.63 ± 7.77 | 0.471 |
| ADL (mean ± SD) | 32.87 ± 10.88 | 33.97 ± 13.42 | 0.622 |
| Folate insufficiencyb | 18 (29.51) | 24 (40.00) | 0.225 |
AChEI: acetylcholinesterase inhibitor; AD: Alzheimer's disease; ADL: activities of daily living. Domains of comorbidity include diabetes, hypertension, coronary heart disease, cerebral infarction, cerebral hemorrhage, transient cerebral hemorrhage, cerebral trauma, Parkinson's disease, CO poisoning, tumor, exposure to heavy metals, anxiety, and depression. Results are expressed as proportion, mean ± SD, or median (Q1, Q3). Comparisons between intervention groups and controls were performed by two-tailed Student's t-test (for normally distributed data) and Mann-Whitney test (for skewed distribution). Pearson's chi-square and Fisher's Exact Test were performed for frequencies analysis; n: number of individuals. a P < 0.05, significant difference between groups; bbased on WHO definitions of folate sufficiency [below 9.06 nmol/L (4 ng/mL)].
3.2. Methionine-Related Metabolites in Serum
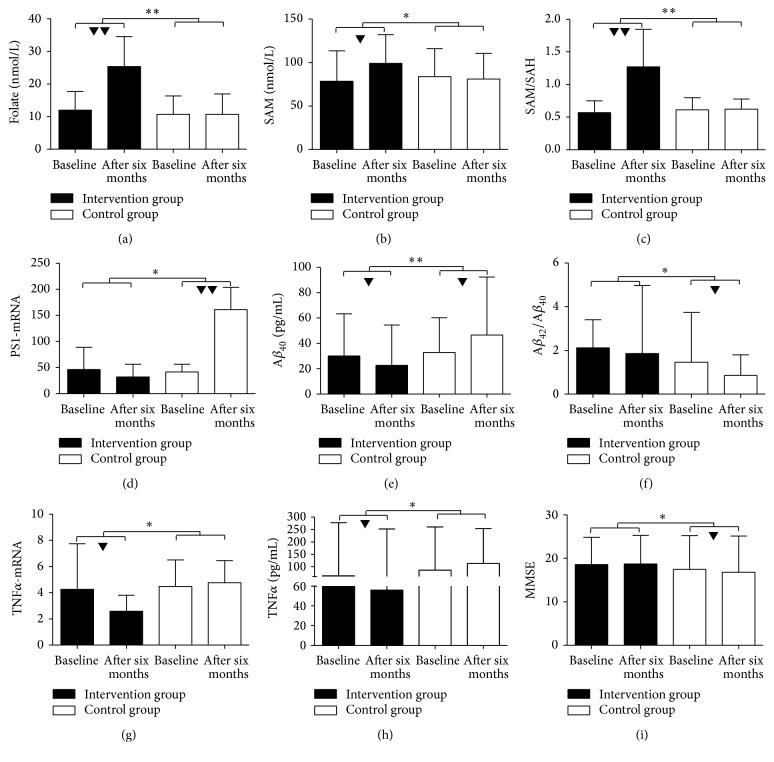
ITT analyses showed that, over 6 months, participants in the intervention group had a significantly greater increase in serum folate than those in the control group (P = 0.001, ηp 2 = 0.937). Plasma SAM (P = 0.012, ηp 2 = 0.712) and SAM/SAH levels (P = 0.000, ηp 2 = 0.999) demonstrated greater increases in the intervention group than in the control group. However, there were no significant differences in levels of vitamin B12, Hcy, and SAH (Table 3, Figure 2).
Table 3.
The levels of serum biomarkers parameters at baseline and 6-month follow-up in folic acid and control groups.
| Item | Groups | Cases (n) | Treatment time | Repeated measures | |||
|---|---|---|---|---|---|---|---|
| Before treatment | At 6 months | Interaction effect, P (ηp 2) |
Time effect, P (ηp 2) |
Group effect, P (ηp 2) |
|||
| Folate (nmol/L) |
Intervention | 61 | 11.98 (8.04, 17.68) | 25.37 (10.87, 34.54) | 0.001 (0.937) | 0.000 (0.972) | 0.001 (0.933) |
| Control | 60 | 10.76 (7.53, 16.37) | 10.76 (7.99, 16.99) | ||||
|
| |||||||
| Vitamin B12
(pmol/L) |
Intervention | 61 | 289.96 (233.88, 406.16) | 289.22 (240.89, 397.68) | 0.627 (0.077) | 0.854 (0.054) | 0.488 (0.106) |
| Control | 60 | 329.06 (231.86, 455.04) | 328.33 (225.58, 445.82) | ||||
|
| |||||||
| Hcy (µmol/L) | Intervention | 61 | 17.55 ± 7.25 | 12.25 ± 6.93 | 0.096 (0.385) | 0.000 (0.994) | 0.001 (0.903) |
| Control | 60 | 19.16 ± 7.34 | 16.73 ± 6.13 | ||||
|
| |||||||
| SAM (nmol/L) | Intervention | 61 | 78.53 ± 35.02 | 99.04 ± 33.14 | 0.012 (0.712) | 0.055 (0.485) | 0.092 (0.391) |
| Control | 60 | 83.81 ± 32.11 | 81.06 ± 29.55 | ||||
|
| |||||||
| SAH (nmol/L) | Intervention | 61 | 127.20 ± 45.48 | 97.24 ± 52.19 | 0.051 (0.499) | 0.004 (0.826) | 0.078 (0.422) |
| Control | 60 | 126.19 ± 54.33 | 120.39 ± 38.40 | ||||
|
| |||||||
| SAM/SAH | Intervention | 61 | 0.57 (0.49, 0.75) | 1.27 (0.69, 1.85) | 0.000 (0.999) | 0.000 (0.991) | 0.004 (0.882) |
| Control | 60 | 0.61 (0.51, 0.80) | 0.62 (0.53, 0.78) | ||||
|
| |||||||
| Aβ 40 (pg/mL) | Intervention | 61 | 30.11 (11.98, 63.39) | 22.72 (8.83, 54.48) | 0.003 (0.843) | 0.827 (0.048) | 0.029 (0.592) |
| Control | 60 | 32.94 (12.39, 60.23) | 46.47 (16.33, 92.43) | ||||
|
| |||||||
| Aβ 42 (pg/mL) | Intervention | 61 | 53.88 (35.72, 115.56) | 45.17 (31.11, 68.22) | 0.306 (0.175) | 0.009 (0.754) | 0.021 (0.642) |
| Control | 60 | 42.32 (19.62, 76.66) | 35.67 (23.66, 56.01) | ||||
|
| |||||||
| Aβ 42/Aβ 40 | Intervention | 61 | 2.12 (1.07, 3.40) | 1.86 (0.81, 4.98) | 0.029 (0.594) | 0.136 (0.319) | 0.001 (0.940) |
| Control | 60 | 1.46 (0.56, 3.75) | 0.85 (0.29, 1.80) | ||||
|
| |||||||
| IL-6 (pg/mL) | Intervention | 61 | 8.12 (5.93, 12.61) | 9.94 (6.87, 13.30) | 0.334 (0.161) | 0.398 (0.134) | 0.004 (0.834) |
| Control | 60 | 10.23 (6.12, 16.98) | 10.88 (6.77, 18.71) | ||||
|
| |||||||
| TNFα (pg/mL) | Intervention | 61 | 62.88 (26.53, 277.39) | 56.08 (21.06, 252.61) | 0.021 (0.638) | 0.504 (0.102) | 0.308 (0.174) |
| Control | 60 | 85.89 (18.81, 261.05) | 112.42 (26.37, 254.75) | ||||
|
| |||||||
| MMSE | Intervention | 61 | 18.56 ± 6.23 | 18.72 ± 6.56 | 0.041 (0.538) | 0.167 (0.281) | 0.274 (0.193) |
| Control | 60 | 17.63 ± 7.77 | 16.80 ± 8.26 | ||||
|
| |||||||
| ADL | Intervention | 61 | 32.87 ± 10.88 | 32.93 ± 10.93 | 0.895 (0.052) | 0.698 (0.067) | 0.615 (0.079) |
| Control | 60 | 33.97 ± 13.42 | 34.10 ± 14.15 | ||||
Biochemical variables are presented as mean ± SD or median (quartiles). Results are expressed as proportion, mean ± SD, or median (Q1, Q3). Hcy, SAM, SAH, MMSE, and ADL, which were normally distributed, and folate, vitamin B12, SAM/SAH, Aβ 40, Aβ 42, Aβ 42/Aβ 40, IL-6, and TNFα, which were normally distributed after logarithmic transformation, were analyzed by repeated-measures ANOVA. ηp 2 describes the percentage of variance explained in the dependent variable by a predictor variable.
Figure 2.
Changes in peripheral biochemical factors (folate, SAM, SAM/SAH, TNFα-mRNA, TNFα, PS1-mRNA, Aβ 40, and Aβ 42/Aβ 40) and MMSE at baseline and 6 months, in the intervention and control groups. Results are given as mean ± SD or median with interquartile range. Figures indicate a significant difference from the baseline to six months, between intervention group and control group by analysis of repeated-measures ANOVA (▼, ∗ P < 0.05; ▼▼, ∗∗ P < 0.01).
3.3. Aβ, IL-6, and TNFα ELISAs
ELISAs of serum amyloid load and ITT analyses demonstrated that Aβ 40 was significantly lower in the intervention group than in the control group (P = 0.003, ηp 2 = 0.843). The Aβ 42/Aβ 40 ratio was significantly higher in the intervention group (P = 0.029, ηp 2 = 0.594). TNFα was significantly lower in the intervention group versus the control group (P = 0.021, ηp 2 = 0.638) (Table 3, Figure 2).
3.4. RT-PCR of Alzheimer's Disease-Related mRNA
Of the Alzheimer's disease-related mRNA species in the blood, PS1-mRNA was significantly lower in the intervention group compared to the control group (P = 0.017, ηp 2 = 0.676). TNFα-mRNA was also significantly lower in the intervention group versus the control group (P = 0.044, ηp 2 = 0.527). However, there were no significant differences in PS2-mRNA or IL-6-mRNA (Table 4, Figure 2).
Table 4.
The mRNA levels of genes related to serum biomarker parameters at baseline and 6-month follow-up in folic acid and control groups.
| Item | Groups | Cases (n) | Treatment time | Repeated measures | |||
|---|---|---|---|---|---|---|---|
| Before treatment | At 6 months | Interaction effect, P (ηp 2) |
Time effect, P (ηp 2) |
Group effect, P (ηp 2) |
|||
| PS1-mRNA | Intervention | 24 | 50.71 (28.66, 128.88) | 43.44 (21.81, 85.86) | 0.017 (0.676) | 0.008 (0.781) | 0.000 (0.988) |
| Control | 24 | 37.16 (30.98, 66.76) | 143.10 (107.14, 241.16) | ||||
|
| |||||||
| PS2-mRNA | Intervention | 24 | 21.34 (2.87, 70.20) | 4.68 (1.58, 22.78) | 0.055 (0.488) | 0.823 (0.056) | 0.775 (0.059) |
| Control | 24 | 5.86 (2.09, 0.29) | 9.50 (5.76, 13.77) | ||||
|
| |||||||
| APP-mRNA | Intervention | 24 | 14.56 (6.99, 30.98) | 20.68 (8.79, 24.40) | 0.358 (0.149) | 0.970 (0.050) | 0.064 (0.458) |
| Control | 24 | 17.79 (8.12, 73.90) | 19.88 (9.82, 86.05) | ||||
|
| |||||||
| IL-6-mRNA | Intervention | 24 | 0.43 (0.21, 0.89) | 0.28 (0.17, 0.47) | 0.101 (0.373) | 0.195 (0.251) | 0.080 (0.417) |
| Control | 24 | 0.26 (0.12, 0.51) | 0.22 (0.11, 0.53) | ||||
|
| |||||||
| TNFα-mRNA | Intervention | 24 | 6.20 (3.00, 11.12) | 2.86 (1.44, 5.73) | 0.044 (0.527) | 0.091 (0.393) | 0.520 (0.097) |
| Control | 24 | 5.43 (2.19, 8.51) | 5.50 (3.35, 8.48) | ||||
Variables are presented as median (Q1, Q3). Serum biomarkers, which were normally distributed after logarithmic transformation, are analyzed by repeated-measures ANOVA. ηp 2 describes the percentage of variance explained in the dependent variable by a predictor variable.
3.5. Cognitive Function
Repeated-measures analyses revealed that, over 6 months, the MMSE scores were significantly higher in the intervention group than in the control group (P = 0.041, ηp 2 = 0.538). However, there was no significant difference in ADL scores (Table 3, Figure 2).
4. Discussion
In this study, the patients with AD were not exposed to folic acid derived from fortification of food items. In western countries, folic acid fortification programs may mask the relationship between folate status and cognitive decline, as suggested by the limited and inconclusive findings of previous studies. The level of folate intake in China is usually 30–40% lower than the recommended dietary allowance [20] because of the lack of a governmental folic acid fortification program and traditional cooking methods that may cause folate to be lost from cooked vegetables. We treated patients with AD with or without folic acid (1.25 mg/d) for 6 months. All patients were to maintain stable donepezil therapy at the entry dose prescribed by the patient's physician for the duration of the study, and if donepezil therapy was discontinued, patients were excluded from the study.
4.1. Effect of Folic Acid Supplementation on Levels of Inflammatory Cytokines and Cognitive Impairment/Dementia
Many clinicians recommend that patients with AD take B vitamin supplements, in hopes of maintaining cognition. Peripheral inflammation is an important and potentially modifiable condition for patients who complain of changes in cognitive performance. Several population-based studies have shown that peripheral inflammatory cytokines, especially IL-6, TNFα, and IL-1β, are correlated with the risk of dementia [21, 22]. Increased peripheral cytokine levels have been associated with disrupted hippocampal stem cell function, reduced hippocampal volume, and reduced memory performance [23–25]. The present study also supports the role of inflammation in increasing the risk of developing dementia. Our data indicated that TNFα protein (interaction effect: P = 0.021, ηp 2 = 0.638) and TNFα-mRNA (interaction effect: P = 0.044, ηp 2 = 0.527) were significantly lower in the intervention group versus the control group.
The neuropathological features of AD include extracellular senile plaques composed primarily of Aβ [26–28]. The two major forms of Aβ are Aβ 40 and Aβ 42. In a normal individual, the majority of Aβ produced is Aβ 40, whereas mutations causing familial AD lead to either increased Aβ 42 production or reduced Aβ 40 without a concomitant increase in Aβ 42 production (i.e., an increased Aβ 42/Aβ 40 ratio) [29, 30]. This differs from our results, which showed a decreased Aβ 42/Aβ 40 ratio (interaction effect: P = 0.029, ηp 2 = 0.594), although, in our patients, those with a family history of AD accounted for just 26.23% of the intervention group and 25% of the control group. We found that Aβ 40 (interaction effect: P = 0.003, ηp 2 = 0.843), but not Aβ 42, was significantly reduced in the serum of the intervention group versus the control group. We also found that PS1-mRNA (interaction effect: P = 0.017, ηp 2 = 0.676) was significantly lower in the intervention group versus the control group. Thus, our study participants were mostly sporadic cases, with potentially different underlying mechanisms of pathology. The results of other studies support our findings. For example, in one study, the Aβ 42/Aβ 40 ratio in cerebrospinal fluid (CSF) samples from patients with AD was significantly (approximately 38%) lower than in age-matched controls, and a significant linear correlation was detected between Aβ 40 concentration and MMSE scores [31]. In patients with MCI, CSF Aβ 42 concentrations and Aβ 42/Aβ 40 ratio at baseline were significantly lower in those patients who subsequently developed AD than in those who were cognitively stable or who developed other forms of dementia [32]. Finally, a lower plasma Aβ 42/Aβ 40 ratio is also associated with greater cognitive decline among nondemented elderly individuals [33].
4.2. Effect of Folic Acid Supplementation on Levels of SAM and SAM/SAH
Folate is an essential vitamin that is involved in various biochemical reactions including the SAM metabolic cycle (i.e., one-carbon metabolism). There is an inverse relationship between folic acid and SAM concentrations throughout the one-carbon cycle. In this trial, daily use of folic acid supplements increased plasma SAM (interaction effect: P = 0.012, ηp 2 = 0.712) and SAM/SAH (interaction effect: P = 0.000, ηp 2 = 0.999) concentrations in elderly individuals with AD. SAM itself appears to be altered in some neurological disorders, including AD [34–36]. For example, Trolin and coworkers treated patients with AD with vitamin B12, SAM, and folate for 6 months, causing a significant decrease in Hcy levels [37], while Bottiglieri et al. [38] noted that oral SAM treatment (1200 mg daily) for 4 to 8 months was associated with a significant increase in CSF SAM, indicating that oral SAM does cross the blood-brain barrier.
4.3. Effect of Folic Acid Supplementation on Levels of Inflammatory Cytokines and Cognitive Impairment/Dementia Mediated by SAM
Inflammation is one of the earliest neuropathological events in AD. TNFα plays a critical role in the development of inflammation-induced AD. SAM has been associated with the proinflammatory response in vitro and in animal models and in humans. Declining or already low concentrations of folate may exert detrimental effects on cognitive function through systemic inflammation.
SAM has been shown to lower lipopolysaccharide- (LPS-) induced expression of the proinflammatory cytokine TNFα and increase the expression of the anti-inflammatory cytokine IL-10 in macrophages. Relative to controls, treatment with 500 μmol/L SAM caused a significant decrease in TNFα expression and increase in IL-10 expression in human monocytic THP1 cells [39]. Inhibition of LPS-induced PDE4B2 upregulation and increased cAMP-dependent protein kinase A activation are significant mechanisms contributing to the anti-TNF effect of SAM [40]. SAM supplementation can also decrease oxidative stress by upregulating glutathione synthesis, reducing inflammation via downregulating TNFα, upregulating IL-10 synthesis, increasing the ratio of SAM to SAH, inhibiting the apoptosis of normal hepatocytes, and stimulating the apoptosis of liver cancer cells. Folate deficiency may accelerate or promote alcoholic liver disease (ALD) by increasing hepatic homocysteine and SAH concentrations, decreasing hepatic SAM and glutathione concentrations and the SAM-SAH ratio, and inhibiting apoptosis. In addition, folate deficiency has been found to increase Hcy and SAH concentrations [41].
4.4. The Combination of Folic Acid and Donepezil as an Improved Therapy for AD
After 6 months, significant improvement in the MMSE scores (i.e., cognitive function) (interaction effect: P = 0.041, ηp 2 = 0.538) was noted in the intervention group versus the control group (P = 0.041, ηp 2 = 0.538). There is evidence suggesting that donepezil/folate combination therapy could be beneficial. Sharma and Singh found that sodium diethyldithiocarbamate trihydrate, folacin, and donepezil significantly improved hyperhomocysteinemia/cholesterol-induced impairment of learning, memory, endothelial dysfunction, and changes in various biochemical parameters in patients with vascular dementia [42]. Connelly et al. also found significant improvement when an acetylcholinesterase inhibitor (AChEI) was combined with folate [43]. Though the mechanism is unclear, the benefits may be related to coaction on methylation-controlled Aβ production and modification of brain gene expression [44]. Folic acid deficiency may compromise antioxidative activity at multiple levels [44] and may elevate Hcy. Hcy stimulates NMDA receptors, leading to increased reactive oxygen species, which in turn stimulate hyperphosphorylation of tau [45]. It is particularly intriguing that folic acid could compensate for the probable toxicity of cholinesterase [46] as well. Therefore, we hypothesize that there is a possible synergistic effect of folic acid with donepezil, a combination therapy that is increasingly used in clinical practice [47, 48].
Our study also had some limitations. One is its short duration, which may have caused underestimation of the effectiveness of folate. An additional limitation of the study may be the dosing. The optimal dose of folic acid needed to improve cognitive function is currently unknown. Although the present study provides some insight, larger dosages may produce different effects than those observed in the current investigation. Lastly, because all participants received donepezil, the effects on AD patients who are not prescribed donepezil must be identified in future studies.
5. Conclusions
This small pilot study examined the effect of folic acid supplementation on newly diagnosed patients with AD. Folic acid improved cognition and markers of inflammation. The full effects of folate may lie in its ability to work in concert with donepezil and may lead to improved clinical practices in the treatment of AD.
Acknowledgments
This research was supported by a grant from the National Natural Science Foundation of China (no. 81130053).
Abbreviations
- AChEI:
Acetylcholinesterase inhibitor
- AD:
Alzheimer's disease
- ADL:
Activities of daily living
- APP:
Amyloid precursor protein
- Aβ:
Amyloid beta
- BMI:
Body mass index
- CSF:
Cerebrospinal fluid
- EDTA:
Ethylenediaminetetraacetic acid
- Hcy:
Homocysteine
- HPLC:
High-performance liquid chromatography
- ITT:
Intention-to-treat
- IL-6:
Interleukin-6
- LPS:
Lipopolysaccharide
- MCI:
Mild cognitive impairment
- MMSE:
Mini-Mental State Examination
- PS:
Presenilin
- RCT:
Randomized controlled trial
- RT-PCR:
Real-time polymerase chain reaction
- SAM:
S-Adenosylmethionine
- SAH:
S-Adenosylhomocysteine
- SD:
Standard deviation
- TNFα:
Tumor necrosis factor alpha.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Hui Chen and Shuai Liu contributed equally to this study.
References
- 1.Evans D. A., Funkenstein H. H., Albert M. S., et al. Prevalence of Alzheimer's disease in a community population of older persons, higher than previously reported. The Journal of the American Medical Association. 1989;262(18):2551–2556. doi: 10.1001/jama.1989.03430180093036. [DOI] [PubMed] [Google Scholar]
- 2.Chan K. Y., Wu J. J., Liu L., et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. The Lancet. 2013;381(9882):2016–2023. doi: 10.1016/s0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 3.Masters C. L., Cappai R., Barnham K. J., Villemagne V. L. Molecular mechanisms for Alzheimer's disease: implications for neuroimaging and therapeutics. Journal of Neurochemistry. 2006;97(6):1700–1725. doi: 10.1111/j.1471-4159.2006.03989.x. [DOI] [PubMed] [Google Scholar]
- 4.Chouliaras L., Mastroeni D., Delvaux E., et al. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer's disease patients. Neurobiology of Aging. 2013;34(9):2091–2099. doi: 10.1016/j.neurobiolaging.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gezen-Ak D., Dursun E., Hanağası H., et al. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer's disease or mild cognitive impairment. Journal of Alzheimer's Disease. 2013;37(1):185–195. doi: 10.3233/jad-130497. [DOI] [PubMed] [Google Scholar]
- 6.Das U. N. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer's disease—but how and why? Prostaglandins Leukotrienes and Essential Fatty Acids. 2008;78(1):11–19. doi: 10.1016/j.plefa.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Chen H., Liu S., Ji L., et al. Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: a case-control study. Current Alzheimer Research. 2015;12(1):88–94. doi: 10.2174/1567205012666141218144035. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R., Smith A. D., Jobst K. A., Refsum H., Sutton L., Ueland P. M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Archives of Neurology. 1998;55(11):1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 9.Postiglione A., Milan G., Ruocco A., Gallotta G., Guiotto G., Di Minno G. Plasma folate, vitamin B12, and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer's dementia. A case-control study. Gerontology. 2001;47(6):324–329. doi: 10.1159/000052822. [DOI] [PubMed] [Google Scholar]
- 10.McMahon J. A., Green T. J., Skeaff C. M., Knight R. G., Mann J. I., Williams S. M. A controlled trial of homocysteine lowering and cognitive performance. The New England Journal of Medicine. 2006;354(26):2764–2772. doi: 10.1056/nejmoa054025. [DOI] [PubMed] [Google Scholar]
- 11.Durga J., van Boxtel M. P., Schouten E. G., et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. The Lancet. 2007;369(9557):208–216. doi: 10.1016/s0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 12.Zheng M., Zhang M., Yang J., et al. Relationship between blood levels of methyl donor and folate and mild cognitive impairment in Chinese patients with type 2 diabetes: a case-control study. Journal of Clinical Biochemistry and Nutrition. 2014;54(2):122–128. doi: 10.3164/jcbn.13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack C. R., Jr., Albert M. S., Knopman D. S., et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng F., Han X. Q., Chen J., Shang L., Li J. Application of the activities of daily living rating scale in screening dementia. J Clin Psychol Med. 2004;14:193–194. [Google Scholar]
- 15.Papandreou D., Mavromichalis I., Makedou A., Rousso I., Arvanitidou M. Total serum homocysteine, folate and vitamin B12 in a Greek school age population. Clinical Nutrition. 2006;25(5):797–802. doi: 10.1016/j.clnu.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Quinlivan E. P. In vitamin B12 deficiency, higher serum folate is associated with increased homocysteine and methylmalonic acid concentrations. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(5, article E7) doi: 10.1073/pnas.0711541105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirier L. A., Wise C. K., Delongchamp R. R., Sinha R. Blood determinations of S-adenosylmethionine, S-adenosylhomocysteine, and homocysteine: correlations with diet. Cancer Epidemiology Biomarkers and Prevention. 2001;10(6):649–655. [PubMed] [Google Scholar]
- 18.Sun Z. Q. Medical Statistics. 2nd. Beijing, China: People's Medical Publishing House (PMPH); 2013. [Google Scholar]
- 19.Ballard C., Margallo-Lana M., Juszczak E., et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial. The British Medical Journal. 2005;330(7496):874–877. doi: 10.1136/bmj.38369.459988.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry R. J., Li Z., Erickson J. D., et al. Prevention of neural-tube defects with folic acid in China. The New England Journal of Medicine. 1999;341(20):1485–1490. doi: 10.1056/nejm199911113412001. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt R., Schmidt H., Curb J. D., Masaki K., White L. R., Launer L. J. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Annals of Neurology. 2002;52(2):168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 22.Engelhart M. J., Geerlings M. I., Meijer J., et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of Neurology. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 23.Das S., Basu A. Inflammation: a new candidate in modulating adult neurogenesis. Journal of Neuroscience Research. 2008;86(6):1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 24.Marsland A. L., Gianaros P. J., Abramowitch S. M., Manuck S. B., Hariri A. R. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biological Psychiatry. 2008;64(6):484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAfoose J., Baune B. T. Evidence for a cytokine model of cognitive function. Neuroscience and Biobehavioral Reviews. 2009;33(3):355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Chaney M. O., Baudry J., Esh C., et al. Aβ, aging, and Alzheimer's disease: a tale, models, and hypotheses. Neurological Research. 2003;25(6):581–589. doi: 10.1179/016164103101202011. [DOI] [PubMed] [Google Scholar]
- 27.De Strooper B., König G. Alzheimer's disease: a firm base for drug development. Nature. 1999;402(6761):471–472. doi: 10.1038/44973. [DOI] [PubMed] [Google Scholar]
- 28.Selkoe D. J. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid β-protein. Annals of the New York Academy of Sciences. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 29.Findeis M. A. The role of amyloid β peptide 42 in Alzheimer's disease. Pharmacology and Therapeutics. 2007;116(2):266–286. doi: 10.1016/j.pharmthera.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Bentahir M., Nyabi O., Verhamme J., et al. Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. Journal of Neurochemistry. 2006;96(3):732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- 31.Fukuyama R., Mizuno T., Mizuno T., et al. Age-dependent change in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to Aβ40 level in cerebrospinal fluid from Alzheimer's disease patients. European Neurology. 2000;43(3):155–160. doi: 10.1159/000008156. [DOI] [PubMed] [Google Scholar]
- 32.Hansson O., Zetterberg H., Buchhave P., et al. Prediction of Alzheimer's disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2007;23(5):316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K., Weston A., Graff-Radford N. R., et al. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. The Journal of the American Medical Association. 2011;305(3):261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bottiglieri T., Hyland K. S-adenosylmethionine levels in psychiatric and neurological disorders: a review. Acta Neurologica Scandinavica. 1994;89(supplement 154):19–26. doi: 10.1111/j.1600-0404.1994.tb05405.x. [DOI] [PubMed] [Google Scholar]
- 35.Morrison L. D., Smith D. D., Kish S. J. Brain S-adenosylmethionine levels are severely decreased in Alzheimer's disease. Journal of Neurochemistry. 1996;67(3):1328–1331. doi: 10.1046/j.1471-4159.1996.67031328.x. [DOI] [PubMed] [Google Scholar]
- 36.Tchantchou F., Graves M., Ortiz D., Chan A., Rogers E., Shea T. B. S-adenosyl methionine: a connection between nutritional and genetic risk factors for neurodegeneration in Alzheimer's disease. Journal of Nutrition, Health and Aging. 2006;10(6):541–544. [PubMed] [Google Scholar]
- 37.Trolin C. G., Regland B., Oreland L. Decreased methionine adenosyltransferase activity in erythrocytes of patients with dementia disorders. European Neuropsychopharmacology. 1995;5(2):107–114. doi: 10.1016/0924-977X(95)00007-C. [DOI] [PubMed] [Google Scholar]
- 38.Bottiglieri T., Godfrey P., Flynn T., Carney M. W. P., Toone B. K., Reynolds E. H. Cerebrospinal fluid S-adenosylmethionine-in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. Journal of Neurology, Neurosurgery and Psychiatry. 1990;53(12):1096–1098. doi: 10.1136/jnnp.53.12.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfalzer A. C., Choi S.-W., Tammen S. A., et al. S-adenosylmethionine mediates inhibition of inflammatory response and changes in DNA methylation in human macrophages. Physiological Genomics. 2014;46(17):617–623. doi: 10.1152/physiolgenomics.00056.2014. [DOI] [PubMed] [Google Scholar]
- 40.Gobejishvili L., Avila D. V., Barker D. F., et al. S-adenosylmethionine decreases lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates tumor necrosis factor expression via cAMP/protein kinase A pathway. Journal of Pharmacology and Experimental Therapeutics. 2011;337(2):433–443. doi: 10.1124/jpet.110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purohit V., Abdelmalek M. F., Barve S., et al. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. American Journal of Clinical Nutrition. 2007;86(1):14–24. doi: 10.1093/ajcn/86.1.14. [DOI] [PubMed] [Google Scholar]
- 42.Sharma B., Singh N. Salutary effect of NFκB inhibitor and folacin in hyperhomocysteinemia-hyperlipidemia induced vascular dementia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;38(2):207–215. doi: 10.1016/j.pnpbp.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Connelly P. J., Prentice N. P., Cousland G., Bonham J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer's disease. International Journal of Geriatric Psychiatry. 2008;23(2):155–160. doi: 10.1002/gps.1856. [DOI] [PubMed] [Google Scholar]
- 44.Chen T.-F., Huang R.-F. S., Lin S.-E., Lu J.-F., Tang M.-C., Chiu M.-J. Folic acid potentiates the effect of memantine on spatial learning and neuronal protection in an Alzheimer's disease transgenic model. Journal of Alzheimer's Disease. 2010;20(2):607–615. doi: 10.3233/jad-2010-1396. [DOI] [PubMed] [Google Scholar]
- 45.Shea T. B., Lyons-Weiler J., Rogers E. Homocysteine, folate deprivation and Alzheimer neuropathology. Journal of Alzheimer's Disease. 2002;4(4):261–267. doi: 10.3233/jad-2002-4401. [DOI] [PubMed] [Google Scholar]
- 46.Creeley C. E., Wozniak D. F., Nardi A., Farber N. B., Olney J. W. Donepezil markedly potentiates memantine neurotoxicity in the adult rat brain. Neurobiology of Aging. 2008;29(2):153–167. doi: 10.1016/j.neurobiolaging.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atri A., Shaughnessy L. W., Locascio J. J., Growdon J. H. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2008;22(3):209–221. doi: 10.1097/wad.0b013e31816653bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez O. L., Becker J. T., Wahed A. S., et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(6):600–607. doi: 10.1136/jnnp.2008.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]