SUMMARY
Spinal cord injuries are amongst the most dangerous injuries, leading to high mortality and morbidity. Injured patients are occasionally faced with life-threatening complications and quality-of-life changing neurological deficits. Thoracic and cervical spinal segments are the most effected sites of injury and a wide range of complications including paraplegia, respiratory and cardiovascular compromise secondary to autonomic dysfunction or tetraplegia may ensue. We aim to draw attention to the progressive nature of the neurological deficits in a patient admitted asymptomatically. Also, we would like to discuss the importance of swift diagnosis and management in such patients. In asymptomatic patients in whom no fractures are diagnosed with CT scans, a neurological examination should be repeated several times to exclude any neurological injuries that were missed. MRI should be ordered in an emergency setting even though it is not frequently used as a diagnostic modality. This should be done especially in patients without any fractures on CT but with neurological signs.
Key words: Motor vehicle accident, MRI myelography, spinal injury, spinal radiology, tetraplegia
ÖZET
Spinal kord yaralanmaları yüksek mortalite ve sakatlanma oranlarına neden olan en tehlikeli yaralanmalar arasında sayılmaktadır. Etkilenen hastalarda sıklıkla yaşamı tehdit edici komplikasyonlar ve hastanın hayat kalitesini etkileyen nörolojik bozukluklar gelişebilmektedir. Torakal ve servikal segmentler en sık etkilenen yaralanma yerleri olup, hastalarda otonom disfonksiyona ikincil parapleji, solunumsal ve kardiyovasküler bozukluklar gelişebilir ya da tetrapleji görülebilir. Bu olgu sunumuyla, semptomsuz olarak başvuran bir hastanın nörolojik bozukluklarının ilerleyici doğasına dikkat çekmek istiyoruz. Ayrıca, bu tip hastalarda hızlı tanı ve yönetimin önemini tartışmak istemekteyiz. Semptomsuz olarak başvuran ve bilgisayarlı tomografilerinde kırık saptanmayan hastalarda nörolojik muayene sık aralıklarla tekrarlanarak herhangi bir nörolojik hasarın gelişip gelişmediği izlenmelidir. Acil servislerde manyetik rezonans görüntüleme sık kullanılan tanı testlerinden biri olmamasına rağmen özellikle bilgisayarlı tomografisinde herhangi bir patoloji tespit edilmeyen ancak nörolojik bulguları mevcut olan hastalarda mutlaka istenmelidir.
Anahtar sözcükler: Motorlu taşıt kazası, MR miyelografi, spinal yaralanma, spinal görüntüleme, tetrapleji
Introduction
Spinal cord injury (SCI) is an injury causing temporary or permanent damage to the motor, sensory and autonomic function of the spinal cord. Generally, permanent and progressive neurological disorders are seen.[1] Life threatening complications and neurological disorders affecting quality of life can develop in these patients.
Thoracic and cervical segments are affected most and paraplegia secondary to autonomic dysfunction, respiratory or cardiovascular disorders or tetraplegia can be seen.
While SCI patients can rarely present asymptomatically, progressive neurological disorders and death can be seen due to edema and secondary injury. For this reason, all SCI cases should be thoroughly examined and accompanying pathologies must be excluded.
In this case report, the progressive nature of the neurological disorders in an asymptomatic SCI case, its diagnostic processes and treatment are discussed, with an attempt to emphasize the importance of the approach to injuries with dangerous mechanisms.
Case Report
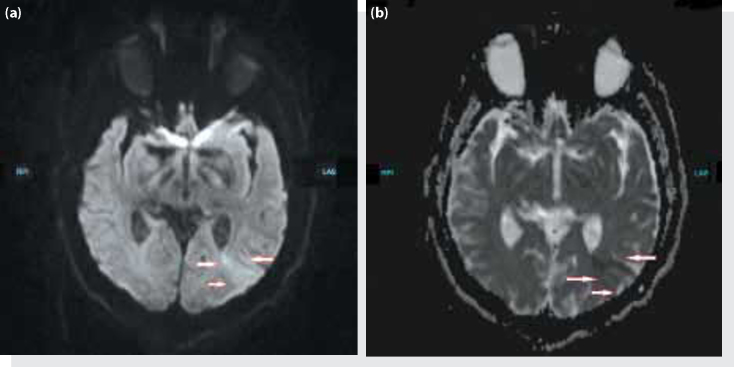
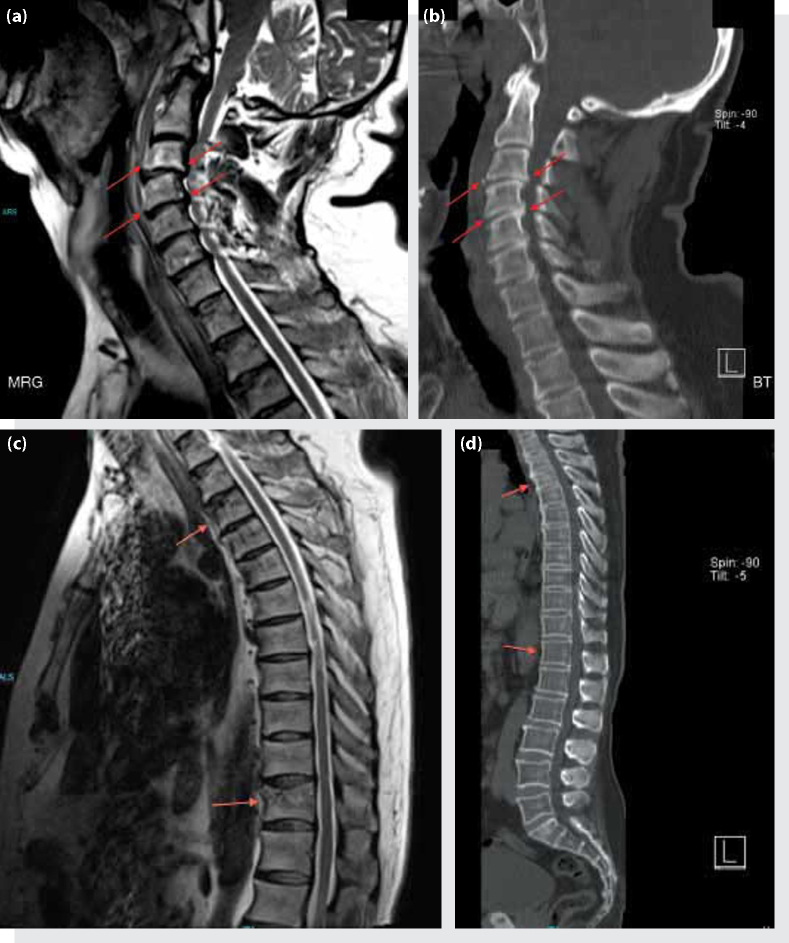
A 59-year-old male patient with known cervical stenosis was brought to the emergency department by provincial ambulance after being involved in a traffic accident as a pedestrian. The patient was on a trauma board with a cervical collar upon arrival. His general condition was good; he was conscious, cooperative and oriented. His Glasgow Coma Scale score (GCS) was calculated as 15 (E4, V5, M6). The patient's vital signs were unremarkable except borderline hypotension (blood pressure 90/65 mmHg, pulse 74/min, PSO2 90%, temperature 36.2°C). According to the information obtained from the patient himself, the vehicle struck him on the diagonal directly in the back area and he experienced a temporary loss of consciousness and vision for three to four minutes afterwards. Upon physical examination, other than a 3 cm cutaneous-subcutaneous laceration on the left parietal area, there were no visible injuries. The neurological examination displayed that motor strength was full on all extremities; however, there was decreased rectal tone upon digital rectal examination. The patient was given 1000 cc of saline through a 16 gauge intravenous catheter in both antecubital areas, and tetanus prophylaxis was given. Laboratory findings were unremarkable (white blood cell: 12.700/mm3, hemoglobin: 14.3 g/dL, MCV: 81 fL, platelets: 235000/dl, glucose: 115 mg/dL, urea: 45 mg/dL, creatinine: 1.2, AST: 15 U/L, ALT: 23 U/L, Na: 143 meq/L, K: 3.4 meq/L, INR: 0.98). Transverse, sagittal and axial slice computed tomography (CT) scans (cranial, spinal, thoracic and IV contrast abdominal and pelvic) examinations were evaluated by the on duty radiologist (1st Radiologist), and a verbal and written report was given stating there were no pathological features. However, evaluation of the CT images by Emergency Medicine physicians revealed a stable fracture of the left lamina on C1 vertebrae. Vital sign evaluation repeated approximately one hour later during the follow up of the patient was as follows: blood pressure: 105/70 mmHg, Pulse: 80 pm, PSO2: 98%, temperature: 36.5°C. Repeat physical examination revealed a motor weakness in the lower extremities, followed by loss of touch and motor weakness in the upper extremities. With the patient rapidly progressing to tetraplegia, a full spinal magnetic resonance imaging (MRI) scan, along with a thoraco-abdominal CT angiography to rule out vertebral artery dissection due to the suspected C1 fracture and an aortic dissection if the progressive tetraplegia was caused by a vascular pathology was carried out. In the diffusion MRI of the patient, whose CT had been unremarkable and vascular pathologies were ruled out according to radiology reports (1st and 2nd Radiologists), an acute cerebral infarct (Figure 1) in the parieto-tempero-occipital region was prominent. In the spinal MRI (2nd Radiologist) central protrusions of the intervertebral discs along C2-C7 were exerting pressure on the spinal cord and a narrowing of the antero-posterior diameter of the spinal canal was present; there was edema secondary to contusions on the C2-C3, C3-C4 levels (Figure 2a, b); there were compression fractures of the T1-T2-T3-T5-T11 vertebral corpus, along with left paracentral protrusions causing compression of the anterior subarachnoid space of the intervertebral discs at the T7-T8 level (Figure 2c, d). The patient was consulted to the Neurology and Neurosurgery Departments, and was admitted by the Neurosurgery Department. Upon no pathology being detected in the Digital Subtraction Angiography (DSA), the patient was operated and C3-to-C6 laminectomy surgery was performed on the same day. The patient, who developed respiratory failure two to three hours following the surgery, was intubated and admitted to the intensive care unit. However, the patient died due to cardiac arrest on the same day.
Figure 1.
(a) Acute infarction in the left parietal-tempero-occipital region in diffusion magnetic resonance imaging. (b) Its ADC diffusion magnetic resonance imaging.
Figure 2.
Central protrusions of the intervertebral discs at the C2-C7 level exerting pressure on the spinal cord and narrowing the spinal canal in the anterior-posterior diameter. (a) Lysthesis at C2 and C3 on the cervical magnetic resonance imaging, intensity changes due to flexion-distraction type opened and closed fracture of the C3 and a teardrop fracture, (b) CT images of the same levels. (c) Compression fractures of the vertebral corpus of T3 and T11, and possible degenerative changes on T1, T2 and T5 on MRI, (d) CT image of the same level.
Discussion
The average incidence of SCI in developing countries is 25.5 million/year (2.1 and 130.7 million/year). 82.8% of all SCI cases are male with an average age of 32.4 years. The leading causes of SCI are motor vehicle accidents (41.4%) and falls (34.9%). Complete SCI is more common than incomplete SCI (56.5% to 43%), and paraplegia is more common than tetraplegia (58.7% to 40.6%).[2] Firearm injuries, sports injuries, and diving accidents can also be included among other etiological factors.[3]
The cervical level (C5) is the most commonly affected area.[4] The pathophysiology of spinal injury generally consists of direct damage to the medulla spinalis by trauma, compression due to bone fragments, hematomas and disc material, ischemia as a result of spinal artery injuries and the accompanying tissue edema.[5]
In studies carried out to evaluate the blood flow in the dorsolateral cord in severe spinal trauma, it has been shown that for 60 to 90 minutes following cord damage autoregulation mechanisms remain intact; however, simultaneously with the onset of ischemia this continuity begins to be disrupted. As a response to SCI, both disruption of autoregulation and vasoconstriction of resistance blood vessels develops. In the early post-traumatic period, intervention aimed at ensuring perfusion can be valuable in terms of reverting or limiting loss of function due to secondary damage caused by ischemia.[6]
In a study by Morais DF et al. it was shown that MRI was distinctly superior to CT in terms of evaluating bone structure, in posterior ligament injuries, spinal cord compression and disc herniation.[7] In our case, the 1st Radiologist reported no osseous pathologies from the CT scan, however the 2nd and 3rd Radiologists reported a fracture of the left C1 lamina. The two Radiologists evaluating the MRI (2nd and 3rd Radiologists) identified cervical and thoracic fractures which were unapparent on the CT. The 2nd Radiologist was an experienced faculty member tasked with routine reporting at the hospital where the case presented, and the 3rd Radiologist was a physician with 10 years of academic experience working in a different city (TA), who was invited to review images blindly upon the preparation of this article. Both radiologists had the same opinion that central protrusions of the intervertebral discs exerting pressure on the spinal cord along C2-C7, edema/bleeding was prominent at C2-C3, C3-C4 levels secondary to contusion, and apparent compression fractures of T3 and T11 along with the degeneration of T1, T2 and T5 (Figure 2a-d). Additionally, in the evaluation carried out by the 3rd Radiologist, minimally displaced flexion-distraction type fracture from the frontal section of the lower plateau of the C3 vertebra corpus towards the upper-mid section, with a tear drop fracture, a minimal retrolisthesis of the C3 according to C4, along with Modic type 2 bone marrow signal intensity changes consistent with degeneration of the T1, T3 and T5 vertebra corpuses and Schmorl nodule indentations were noted. During surgery, loss of the complete integrity of the C3 vertebra was determined and laminectomy surgery was performed on adjacent vertebrae. Thus, the C3 vertebra extension-distraction and tear-drop fractures were responsible for the spinal cord bleeding, which could not be determined by three separate radiologists and the emergency medicine physicians on the CT, and which was only determined by one of two radiologists reporting the MRI, was clinically diagnosed.
The bilateral sensory-motor loss is accepted as an indication of complete SCI. The lack of neuromotor loss upon admission in our case, followed by motor and sensation loss starting from the shoulder level leading to tetraplegia shows that the edema/bleeding due to contusions at the C2-C3-C4 levels were increased and led to a complete SCI. Since updated SCI treatment guidelines (2013) do not recommend high-dose methylprednisolone anymore, immediate surgical treatment of the patient was planned.[8]
Vertebral artery injuries (VAI) may accompany cervical injuries, their mortality is high and they can lead to ischemic stroke.[9] Upper level vertebral fractures (such as C1-C3) – including transverse foramen fractures – are particular risk factors for vertebral artery injury.[10] Today, CT angiography is the diagnostic method of choice for VAI. DSA is also one of the commonly used imaging methods. It has been reported that despite all anticoagulant therapy, fatal complications such as cerebrovascular insufficiency or embolus may develop in 5.8% of patients, while 2.9% of patients die due to cerebrovascular ischemia.[11] In VAI, treatment options are anticoagulants, antiplatelets, thrombolysis, endovascular or surgical treatment.12, 13, 14 In our case, VAI was excluded by the lack of thrombus or dissection of the vertebral artery from CT angiography, which was ordered upon the presence of the stable C1 lamina fracture (one of the risk factors) and worsening findings upon neurological examination. This data suggests that the acute ischemic lesion in the left parieto-temporo-occipital region detected in the diffusion MRI of our patient may have not been developed due to VAI, but may have developed due to hypoperfusion leading to loss of consciousness.
Neurogenic shock may develop in a portion of patients with spinal trauma[15] and can cause neurological disorders to progress to levels which may threaten the patient's life. Spinal stabilization of the patients, vasopressor treatment with fluid support and early surgical decompression of the spinal cord are accepted treatments.[16] In a study by Tuli et al. delayed surgical treatment has been shown to be associated with the development of neurogenic shock.[17] Our patient had a blood pressure of 90/65 upon presentation, and the absence of a source of bleeding to explain the hypotension suggests that the patient may actually have been developing neurogenic shock.
The acute cerebral infarct which appeared in the diffusion MRI of the patient may have developed secondary to hypotension due to neurogenic shock. Additionally, the loss of consciousness for a period of three to four minutes upon the accident and the SPO2 being 90% upon presentation may have contributed to the development of the cerebral infarct. In this context, we believe that our patient, where cerebral infarct and complete high spinal injury developed due to progressive edema, highlights the importance of a skeptical approach to patients presenting with asymptomatic spinal trauma, and not delaying advanced examination by putting forward the indications on radiological imaging.
Respiratory failure, multiple organ failure and gastrointestinal bleeding are the leading causes of death in these patients.[18] In the light of this information, it is of vital importance that emergency physicians predict the possible complications that can develop and take the necessary precautions.
Conclusion
Spinal trauma is likely to cause neurological disorders. In asymptomatic patients in whom no fractures were diagnosed in CTs, neurological examination should be repeated several times to exclude if any neurological injuries were ensued. Even though it is not a frequent diagnostic modality, especially in patients without any fractures on CT but who have neurological signs, MRI should be ordered in emergency department.
Conflict of Interest
The authors declare that there is no potential conflicts of interest.
Footnotes
Published online: January 07, 2014
References
- 1.Dincer F, Oflazer A, Beyazova M, Celiker R, Basgöze O, Altioklar K. Traumatic spinal cord injuries in Turkey. Paraplegia. 1992;30:641–646. doi: 10.1038/sc.1992.127. CrossRef. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi-Movaghar V, Sayyah MK, Akbari H, Khorramirouz R, Rasouli MR, Moradi-Lakeh M. Epidemiology of traumatic spinal cord injury in developing countries: a systematic review. Neuroepidemiology. 2013;41:65–85. doi: 10.1159/000350710. CrossRef. [DOI] [PubMed] [Google Scholar]
- 3.Bellon K, Kolakowsky-Hayner SA, Chen D, McDowell S, Bitterman B, Klaas SJ. Evidence-based practice in primary prevention of spinal cord injury. Top Spinal Cord Inj Rehabil. 2013;19:25–30. doi: 10.1310/sci1901-25. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James G. Adams MD FACEP. Clinical Essentials of Emergency Medicine, September 19, 2012 | ISBN-10: 1437735487.
- 5.Wilson JR, Fehlings MG. Emerging approaches to the surgical management of acute traumatic spinal cord injury. Neurotherapeutics. 2011;8:187–194. doi: 10.1007/s13311-011-0027-3. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senter HJ, Venes JL. Loss of autoregulation and posttraumatic ischemia following experimental spinal cord trauma. J Neurosurg. 1979;50:198–206. doi: 10.3171/jns.1979.50.2.0198. CrossRef. [DOI] [PubMed] [Google Scholar]
- 7.Morais DF, de Melo Neto JS, Meguins LC, Mussi SE, Filho JR, Tognola WA. Clinical applicability of magnetic resonance imaging in acute spinal cord trauma. Eur Spine J. 2014;23:1457–1463. doi: 10.1007/s00586-013-3047-3. [DOI] [PubMed] [Google Scholar]
- 8.Hadley MN, Walters BC. Introduction to the Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries. Neurosurgery. 2013;72(Suppl 2):5–16. doi: 10.1227/NEU.0b013e3182773549. CrossRef. [DOI] [PubMed] [Google Scholar]
- 9.DeVivo MJ, Chen Y, Mennemeyer ST, Deutsch A. Costs of care following spinal cord injury. Top Spinal Cord Inj Rehabil. 2011;16:1–9. CrossRef. [Google Scholar]
- 10.Savitz SI, Caplan LR. Vertebrobasilar disease. N Engl J Med. 2005;352:2618–2626. doi: 10.1056/NEJMra041544. CrossRef. [DOI] [PubMed] [Google Scholar]
- 11.Mueller CA, Peters I, Podlogar M, Kovacs A, Urbach H, Schaller K. Vertebral artery injuries following cervical spine trauma: a prospective observational study. Eur Spine J. 2011;20:2202–2209. doi: 10.1007/s00586-011-1887-2. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaccaro AR, Klein GR, Flanders AE, Albert TJ, Balderston RA, Cotler JM. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine. 1998;23:789–795. doi: 10.1097/00007632-199804010-00009. CrossRef. [DOI] [PubMed] [Google Scholar]
- 13.Dziewas R, Konrad C, Dräger B, Evers S, Besselmann M, Lüdemann P. Cervical artery dissection-clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179–1184. doi: 10.1007/s00415-003-0174-5. CrossRef. [DOI] [PubMed] [Google Scholar]
- 14.Keilani ZM, Berne JD, Agko M. Bilateral internal carotid and vertebral artery dissection after a horse-riding injury. J Vasc Surg. 2010;52:1052–1057. doi: 10.1016/j.jvs.2010.05.065. CrossRef. [DOI] [PubMed] [Google Scholar]
- 15.Popa C, Popa F, Grigorean VT, Onose G, Sandu AM, Popescu M. Vascular dysfunctions following spinal cord injury. J Med Life. 2010;3:275–285. [PMC free article] [PubMed] [Google Scholar]
- 16.Maurin O, de Régloix S, Caballé D, Arvis AM, Perrochon JC, Tourtier JP. Traumatic neurogenic shock. [Article in French] Ann Fr Anesth Reanim. 2013;32:361–363. doi: 10.1016/j.annfar.2013.02.015. [Abstract] CrossRef. [DOI] [PubMed] [Google Scholar]
- 17.Tuli S, Tuli J, Coleman WP, Geisler FH, Krassioukov A. Hemodynamic parameters and timing of surgical decompression in acute cervical spinal cord injury. J Spinal Cord Med. 2007;30:482–490. doi: 10.1080/10790268.2007.11754582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng YX, Nie CY, Yao ZY, Zhu X. Analysis of the risk factors for early death in acute severe traumatic cervical spinal cord injury. [Article in Chinese] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:294–297. doi: 10.3760/cma.j.issn.2095-4352.2013.05.014. [Abstract] [DOI] [PubMed] [Google Scholar]