SUMMARY
Objectives
Traumatic brain injury is a common cause of death and disability worldwide. Early recognition of patients with brain cellular damage allows for early rehabilitation and patient outcome improvement.
Methods
In this prospective study, the clinical conditions of patients with mild to moderate traumatic brain injury (TBI) were assessed, and patient serum S100B levels were measured. Patients were followed up one month later and evaluated for level of consciousness, presence or absence of post-traumatic headache, and daily activity performance (using the Barthel scale). Student's t-test and the chi-square test were used for data analysis, which was performed using SPSS software.
Results
The mean serum S100B value was significantly lower for patients with minor TBI than for patients with moderate TBI (23.1±14.2 ng/dl and 134.0±245.0 ng/dl, respectively). Patients with normal CT scans also had statistically significantly lower serum S100B levels than patients with abnormal CT findings. The mean S100B value was statistically significantly higher for patients with suspected diffused axonal injury (632.18±516.1 ng/dl) than for patients with other abnormal CT findings (p=0.000): 24.97±22.9 ng/dl in patients with normal CT results; 41.56±25.7 ng/dl in patients with skull bone fracture; 57.38 ±28.9 ng/dl in patients with intracranial hemorrhage; and 76.23±38.3 ng/dl in patients with fracture plus intracranial hemorrhage).
Conclusions
Serum S100B levels increase in patients with minor to moderate TBIs, especially in those with diffused axonal injury. However, serum S100B values cannot accurately predict one-month neuropsychological outcomes and performance.
Key words: Biomarker, head trauma, S100B protein, traumatic brain injury
ÖZET
Amaç
Travmatik beyin travması dünya ölçeğinde olağan bir ölüm ve özürlülük nedenidir. Beyin hücre hasarı olan hastaların erkenden tanınması erkendsen rehabilitasyon ve hasta sonuçlarında iyileşmeye olanak tanır.
Gereç ve Yöntem
Bu prospektif çalışmada hafif-orta derecede travmatik beyin hasarı (TBH) olan hastaların klinik durumları değerlendirildi ve hastaların serum S100B düzeyleri ölçüldü. Hastalar bir ay sonra takip edildi, bilinç düzeyleri, travma sonrası baş ağrısı olup olmaması ve günlük aktivite performansı (Barthel ölçeğini kullanarak) açısından değerlendirildi. Veri analizinde SPSS yazılımı ile Student t-testi ve ki-kare testi kullanıldı.
Bulgular
Orta derecede TBH geçirmiş olanlara göre hafif derecede TBH geçirmiş hastalarda ortalama serum S100B değeri anlamlı derecede daha düşüktü (sırasıyla, 134,0±245,0 ng/dl ve 23,1±14,2 ng/dl). BT taramaları normal olmayan hastalara göre normal olanlarda serum S100B düzeyleri istatistiksel açıdan anlamlı derece daha düşüktü. Ortalama S100B değeri yaygın akson hasarından kuşkulanılan hastalarda (632,18±516,1 ng/dl) başka anormal BT bulguları olan hastalardan anlamlı derecede daha düşük idi (p=0.000). Normal BT sonuçları olan hastalarda, 24.97±22.9 ng/dl; kafatası kemiği kırıkları olanlarda 41.56±25.7 ng/dl; intrakraniyal kanaması olanlarda 57.38±28.9 ng/dl, kırıkla birlikte intrakraniyal kanaması olanlarda 76.23±38.3 ng/dl.
Sonuç
Hafif ve orta derecede TBH özellikle yaygın akson travması olanlarda serum S100B düzeyleri yükselmektedir. Ancak serum S100B değerleri 1 ay sonrasının nöropsikolojik sonuçları ve performansını doğru biçimde öngörememektedir.
Anahtar sözcükler: Biyobelirteç, kafa travması, S100B proteini, travmatik beyin hasarı
Introduction
Traumatic brain injury (TBI) is a common cause of death and disability worldwide. TBI is a public health priority because it is associated with extensive physical, psychological and social impacts and a high economic burden.[1] Some studies have demonstrated that more than 10–40% of patients with TBI are still disabled 6–12 months after trauma, including those with mild TBI and unremarkable neuroimaging findings. Although early recognition and proper management of patients with TBI may result in better rehabilitation and substantial outcome improvement, assessing different cellular and clinical aspects and effects of TBI is still less than optimal.2, 3, 4
S100B, a calcium binding protein highly expressed in astroglial cells of the brain and released in cerebrospinal fluid (CSF) and blood, can be measured by available immunoassay kits. Different studies have evaluated S100B as a biomarker for different brain injuries, such as stroke5, 6, bacterial meningitis[7], carbon monoxide poisoning[8] and TBI9, 10, 11, 12. Some recent studies have also highlighted the complex release pattern of S100B and its potential role in brain tissue repair processes13, 14, 15, 16, 17
This prospective study evaluates the diagnostic and prognostic roles of serum S100B protein in emergency department (ED) patients with minor to moderate TBI.
Materials and Methods
Patients were enrolled conveniently between March and May 2012 at two teaching hospitals with a total annual census of 80,000 adult patients. The institutional ethics committee (Faculty of Medicine, Iran University of Medical Sciences) approved this prospective study, and informed consent was obtained from all patients.
Participants
Patients at least 18 years old with a clinical diagnosis of acute mild to moderate TBI were enrolled. Patients with a history of isolated head trauma and Glasgow Coma Scale (GCS) score between 9 and 15 who presented in the ED within the first six hours of their head injury were considered to have mild to moderate TBI. All clinical assessments, including GCS calculations, were performed by a research assistant who was a physician. The research assistant was blinded to other assessments results.
Patients with the following were excluded: severe TBI (GCS≤8); hemodynamic instability; body temperature greater than 38.5°C; concurrent trauma to any other organs; concurrent primary and secondary brain injury, including refractory severe hypoxia (arterial oxygen saturation ≤92% while receiving 100% oxygen), post-traumatic seizure, and skull bone fracture; and any other identified or suspected differential diagnosis for the patient's decreased level of consciousness, including alcohol abuse, drug abuse, substance abuse, drug toxicity, hypo/hyperglycemia, hypo/hypernatremia, endocrine disorder, or infection. Patients who did not undergo a head CT scan were also excluded.
Intervention
S100B assay: A blood sample was drawn from the peripheral veins within the first six hours of ED admission. The time of blood sample collection was recorded. Samples were centrifuged, and the serum was refrigerated at −20°C until analyzed.
Neuroimaging: Ten millimeter thick slices obtained using a GE VCT Lightspeed 64 multi-slice detector were interpreted by a board certified radiologist and confirmed by another consultant radiologist who was blinded to the first interpretation. Both radiologists were blinded to the clinical conditions and S100B results of the patients. All pathologic findings, including skull bone fracture and any type of intracranial hemorrhage (e.g. brain contusion, subdural/epidural intracranial hematoma), were reported as positive computed tomography findings.
Follow up: The patients were called by two blinded research assistant one month later. During follow-up, patients were evaluated for level of consciousness, presence or absence of post-traumatic headaches, and daily activity performance (using the Barthel scale) to determine if any significant intracranial complications had occurred (.i.e. complications requiring further neuroimaging).
Figure 1.
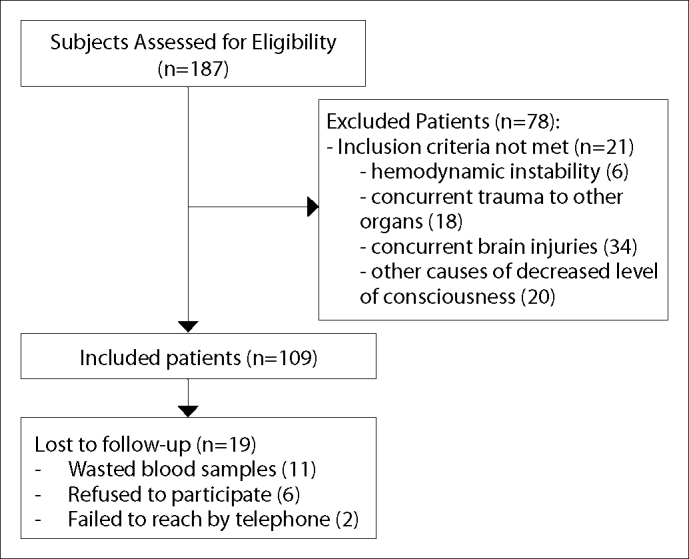
Participant flow over the course of the study.
Measurements
Initial TBI severity was assessed using the GCS. Patients with GCS scores between 9 and 15 were considered to have mild to moderate TBI. To measure S100B serum levels, the human S100 ELISA kit (BioVendor – laboratorni medicina a.s., Brno, Czech Republic) was used. The lowest detection limit of the test is about 15 pg/ml. Serum S100B levels were measured in ng/dl.
The Barthel scale is an ordinal 10-variable scale used to measure patient performance on daily activities and to predict the likelihood a patient will be able to live at home independently. The Barthel scale has high inter-rater and test re-test reliability, as well as, high correlations with other measures of physical disability. The ten Barthel scale variables are: presence/absence of fecal incontinence; presence/absence of urinary incontinence; and help needed with grooming, toilet use, feeding, transfers, walking, dressing, climbing stairs, and bathing. Each variable is given a score (between 0 and 3). These scores are summed to determine the total score (out of 20). The higher the Barthel score, the less assistance the patient is likely to need with daily activities after discharge from the hospital. For example, when a person can perform about 50% of their daily tasks and activities independently, then their Barthel score will be 10 out of 20.18, 19, 20 Patient outcome measures were level of consciousness, residual headache, and Barthel score one month after trauma.
Data Analysis
The Student's t-test was used to compare the mean values of quantitative variables, and the Chi square test was used to compare qualitative variables. All data analyses were performed with SPSS version 13.5 (SPSS, Inc., Chicago, IL).
Results
One hundred eighty-seven patients were assessed for eligibility, and 78 patients were excluded from the study: six patients had hemodynamic instability; 18 patients had concurrent trauma to other organs; 34 patients had concurrent brain injuries; and 20 patients had other causes of decreased level of consciousness. Venous blood samples were obtained from 109 patients with minor to moderate TBI who had undergone CT as a part of their routine diagnostic evaluations. Eleven samples were wasted due to various errors between initial preparation and analysis. A total of 98 patients with mild to moderate TBI and available serum S100B results were followed. During the telephone follow-up one month post-trauma, six patients refused to continue participating in the study, and two additional cases were unreachable by telephone. Follow-up interviews were performed for 90 patients, all of whom completed the study. No patients had died in the month between injury and follow-up, and all patients had GCS scores of 15.
The mean age of the study participants was 33.1±10.3 years (95% CI: 29.99–34.28) and ranged from 18 to 50 years old. Other basic characteristics of the patients are shown in Table 1. In the present study, 38 (80.9%) of the minor TBI patients and 6 (14.0%) of the moderate TBI patients had normal CT results. Suspected diffused axonal injury (DAI) was not seen in the minor TBI patients, but 5 (11.6%) of the moderate TBI patients had suspected DAI. GCS scores were significantly different between the patients with normal CT results and the patients with abnormal CT findings (p=0.000). The mean serum S100B value was 23.1±14.2 ng/dl (95% CI: 17.4–27.3) in patients with minor TBI and 134.0±245.0 ng/dl (95% CI 51.1–179.6) in patients with moderate TBI. Student's t-test demonstrated that the difference was statistically significant (p=0.003). The mean serum S100B value was statistically significantly higher in patients with suspected DAI compared to patients with other abnormal CT findings (p=0.000). Serum S100B results are summarized in Table 2.
Table 1.
Basic characteristics of study participants
| Variable | n | % |
|---|---|---|
| Sex | ||
| Male | 80 | 88.9 |
| Female | 10 | 11.1 |
| Initial GCS | ||
| 15 | 40 | 44.4 |
| 14 | 7 | 7.8 |
| 13 | 13 | 14.4 |
| 12 | 19 | 21.1 |
| 11 | 6 | 6.7 |
| 10 | 5 | 5.6 |
| Mechanism of injury | ||
| Auto-Pedestrian | 36 | 40.0 |
| MVC | 23 | 25.6 |
| Falling | 16 | 17.8 |
| Direct trauma | 9 | 10.0 |
| Others | 6 | 6.7 |
| CT findings | ||
| Normal | 44 | 48.9 |
| DAI | 5 | 5.6 |
| ICH | 10 | 11.1 |
| Fx | 10 | 11.1 |
| Fx+ICH | 21 | 23.3 |
Table 2.
Serum S100B levels in patients with different CT results
| CT Findings | Mean±SD* (ng/dl) | 95% Confidence Interval |
|---|---|---|
| Skull Fracture | 41.56±25.7 | 22.1–58.9 |
| ICH† | 5.38±28.9 | 28.9 |
| Skull Fracture plus ICH | 76.23±38.3 | 57.7–92.7 |
| DAI†† | 632.18±516.1 | −9.7–1272.0 |
| Abnormal | 125.0±238.5 | 53.1–194.8 |
| Normal | 24.9±22.9 | 16.9–30.9 |
: Intracranial hemorrhage;
: Diffused axonal injury;
: Standard deviation.
Initial GCS scores, CT findings, headache, and Barthel scores of patients with Barthel scores ≤18 and with the highest S100B levels are shown in Table 3. At one-month follow-up, 3 (3.3%) patients had Barthel scores less than 18, 12 (13.4%) had Barthel scores of 18 or 19, and 75 (83.3%) had Barthel scores of 20. The mean serum S100B value was 206.43±316.0 ng/dl (95% CI: 49.3–163.4) in patients with Barthel scores less than 18 (range: 68–1047 ng/dl). Patients with Barthel scores of 18 and 19 had a mean serum S100B level of 88.20±46.5 ng/dl (95% CI: 24.8–407.8, range: 48–175 ng/dl). The mean serum S100B level was 59.51±156.9 ng/dl (95% CI: 18.6–99.6) for patients with Barthel scores of 20 (range: 68–1047 ng/dl). Serum S100B levels were higher in patients with lower Barthel scores, but the difference was not statistically significant (p=0.06).
Thirty-eight (42.2%) patients had residual headaches one month after TBI. The mean serum S100B level was 87.03±163.2 ng/dl (95% CI: 26.5–150.6) in patients with residual headaches, and 68.13±188.5 ng/dl (95% CI: 11.3–127.8) in patients without headaches; the difference was not statistically significant (p=0.59).
Discussion
The S100B protein has a half-life of two hours and can be measured both in CSF and in the blood. Although some studies have shown that S100B protein levels increase after extra-cranial injuries in the absence of brain injury,[21] many other studies have introduced S100B protein as a highly sensitive and specific biomarker of CNS injuries.13, 14, 15, 16, 17 S100B has been suggested as a triage tool for identifying patients who need neuroimaging and as a diagnostic tool for early recognition of patients with possible brain tissue injury and timely administration of medication (e.g. benzodiazepines to reduce post-concussion syndrome risk after mild TBI). S100B has also been suggested as a prognostic tool to identify at-risk patients and to begin rehabilitation activities as soon as possible, especially for patients who do not need neurosurgical interventions.22, 23, 24, 25, 26
The present study found that although serum S100B increases in minor to moderate traumatic brain injuries (especially in cases of DAI), it cannot accurately predict one-month outcomes. These results are compatible with some other studies which have emphasized the complicated release pattern of S100B. These past studies have highlighted the role of blood-brain barrier integrity and disruption in S100B release into the serum, the poor correlation between serum and CSF S100B levels, and the possible reparative roles of S100B that may improve outcomes in patients with acute brain injuries. These studies also mention that the relationship between S100B values and likely outcomes in patients with TBI is not necessarily a causative relationship.[27]
A study of a large cohort of patients showed some association between high serum S100B level and poor outcome in patients with brain injury, but not significant enough to support use as an outcome prediction tool.[28] Similarly, a review by Townend showed that, although patients with high serum S100B levels at initial evaluation may be at higher risk for disability after TBI, no association between serum S100B levels and the neuro-psychological performance of injured patients has been established.[2] Metting et al. studied 94 patients with mild TBI and demonstrated that S100B is not related to outcome or imaging results.[29] Some newer studies have proposed that serum S100B level might be used for predicting the probability of brain death in patients with TBI.[30]
Conclusion
The current study showed that serum S100B levels increase with minor to moderate TBIs, especially in patients with suspected DAI. However, serum S100B cannot accurately predict one-month neuropsychological outcomes and performance.
Limitations
The present study has some limitations. The study was conducted at two teaching hospitals, and the human S-100 ELISA kits may not be available at other smaller hospitals. Only patients who had undergone brain CT were enrolled; patients who had not undergone CT or who refused to undergo neuroimaging were not included. The sample size was small, and similar studies with larger sample sizes would be preferable. The study did not focus on any cutoff S100B level to categorize at-risk patients, though it might be helpful to determine a cutoff diagnostic serum S100B value.
Conflict of Interest
The authors declare that there is no potential conflicts of interest.
Footnotes
Published online: November 30, 2014
References
- 1.von Holst H, Cassidy JD. Mandate of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):8–10. doi: 10.1080/16501960410023633. CrossRef. [DOI] [PubMed] [Google Scholar]
- 2.Townend W, Ingebrigtsen T. Head injury outcome prediction: a role for protein S-100B? Injury. 2006;37:1098–1108. doi: 10.1016/j.injury.2006.07.014. CrossRef. [DOI] [PubMed] [Google Scholar]
- 3.Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ. 2000;320:1631–1635. doi: 10.1136/bmj.320.7250.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickering A, Grundy K, Clarke A, Townend W. A cohort study of outcomes following head injury among children and young adults in full-time education. Emerg Med J. 2012;29:451–454. doi: 10.1136/emj.2010.094755. CrossRef. [DOI] [PubMed] [Google Scholar]
- 5.Dassan P, Keir G, Brown MM. Criteria for a clinically informative serum biomarker in acute ischaemic stroke: a review of S100B. Cerebrovasc Dis. 2009;27:295–302. doi: 10.1159/000199468. CrossRef. [DOI] [PubMed] [Google Scholar]
- 6.Brouns R, De Vil B, Cras P, De Surgeloose D, Mariën P, De Deyn PP. Neurobiochemical markers of brain damage in cerebrospinal fluid of acute ischemic stroke patients. Clin Chem. 2010;56:451–458. doi: 10.1373/clinchem.2009.134122. CrossRef. [DOI] [PubMed] [Google Scholar]
- 7.Hamed SA, Hamed EA, Abdella MM. Septic encephalopathy: relationship to serum and cerebrospinal fluid levels of adhesion molecules, lipid peroxides and S-100B protein. Neuropediatrics. 2009;40:66–72. doi: 10.1055/s-0029-1231054. CrossRef. [DOI] [PubMed] [Google Scholar]
- 8.Ide T, Kamijo Y, Ide A, Yoshimura K, Nishikawa T, Soma K, Mochizuki H. Elevated S100B level in cerebrospinal fluid could predict poor outcome of carbon monoxide poisoning. Am J Emerg Med. 2012;30:222–225. doi: 10.1016/j.ajem.2010.11.025. CrossRef. [DOI] [PubMed] [Google Scholar]
- 9.Nylén K, Ost M, Csajbok LZ, Nilsson I, Hall C, Blennow K, Nellgård B. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir (Wien) 2008;150:221–227. doi: 10.1007/s00701-007-1489-2. CrossRef. [DOI] [PubMed] [Google Scholar]
- 10.Savola O, Pyhtinen J, Leino TK, Siitonen S, Niemelä O, Hillbom M. Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J Trauma. 2004;56:1229–1234. doi: 10.1097/01.ta.0000096644.08735.72. [DOI] [PubMed] [Google Scholar]
- 11.Raabe A, Kopetsch O, Woszczyk A, Lang J, Gerlach R, Zimmermann M. Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restor Neurol Neurosci. 2003;21:159–169. [PubMed] [Google Scholar]
- 12.Wiesmann M, Steinmeier E, Magerkurth O, Linn J, Gottmann D, Missler U. Outcome prediction in traumatic brain injury: comparison of neurological status, CT findings, and blood levels of S100B and GFAP. Acta Neurol Scand. 2010;121:178–185. doi: 10.1111/j.1600-0404.2009.01196.x. CrossRef. [DOI] [PubMed] [Google Scholar]
- 13.Kleindienst A, Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–1200. doi: 10.1089/neu.2006.23.1185. CrossRef. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1260. doi: 10.1097/00006123-200106000-00012. CrossRef. [DOI] [PubMed] [Google Scholar]
- 15.Willoughby KA, Kleindienst A, Müller C, Chen T, Muir JK, Ellis EF. S100B protein is released by in vitro trauma and reduces delayed neuronal injury. J Neurochem. 2004;91:1284–1291. doi: 10.1111/j.1471-4159.2004.02812.x. CrossRef. [DOI] [PubMed] [Google Scholar]
- 16.Jackson RG, Samra GS, Radcliffe J, Clark GH, Price CP. The early fall in levels of S-100 beta in traumatic brain injury. Clin Chem Lab Med. 2000;38:1165–1167. doi: 10.1515/CCLM.2000.179. CrossRef. [DOI] [PubMed] [Google Scholar]
- 17.Shinozaki KI, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Abe R. S100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care. 2009;13:R121. doi: 10.1186/cc7973. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahoney Fi, Barthel DW. Functional Evaluation: The barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 19.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. CrossRef. [DOI] [PubMed] [Google Scholar]
- 20.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30:1538–1541. doi: 10.1161/01.str.30.8.1538. CrossRef. [DOI] [PubMed] [Google Scholar]
- 21.Bloomfield SM, McKinney J, Smith L, Brisman J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care. 2007;6:121–138. doi: 10.1007/s12028-007-0008-x. CrossRef. [DOI] [PubMed] [Google Scholar]
- 22.Müller B, Evangelopoulos DS, Bias K, Wildisen A, Zimmermann H, Exadaktylos AK. Can S-100B serum protein help to save cranial CT resources in a peripheral trauma centre? A study and consensus paper. Emerg Med J. 2011;28:938–940. doi: 10.1136/emj.2010.095372. [DOI] [PubMed] [Google Scholar]
- 23.Undén J, Romner B. Can low serum levels of S100B predict normal CT findings after minor head injury in adults?: an evidence-based review and meta-analysis. J Head Trauma Rehabil. 2010;25:228–240. doi: 10.1097/HTR.0b013e3181e57e22. CrossRef. [DOI] [PubMed] [Google Scholar]
- 24.Bazarian JJ, McClung J, Cheng YT, Flesher W, Schneider SM. Emergency department management of mild traumatic brain injury in the USA. Emerg Med J. 2005;22:473–477. doi: 10.1136/emj.2004.019273. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter CD, Clough GF, Pringle AK, Church MK. Outcome following severe traumatic brain injury TBI correlates with serum S100B but not brain extracellular fluid S100B: An intracerebral microdialysis study. World Journal of Neuroscience. 2013;3:93–99. CrossRef. [Google Scholar]
- 26.Goyal A, Failla MD, Niyonkuru C, Amin K, Fabio A, Berger RP. S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J Neurotrauma. 2013;30:946–957. doi: 10.1089/neu.2012.2579. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleindienst A, Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–1200. doi: 10.1089/neu.2006.23.1185. CrossRef. [DOI] [PubMed] [Google Scholar]
- 28.Kleindienst A, Schmidt C, Parsch H, Emtmann I, Xu Y, Buchfelder M. The Passage of S100B from Brain to Blood Is Not Specifically Related to the Blood-Brain Barrier Integrity. Cardiovasc Psychiatry Neurol. 2010;2010:801295. doi: 10.1155/2010/801295. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78:1428–1433. doi: 10.1212/WNL.0b013e318253d5c7. CrossRef. [DOI] [PubMed] [Google Scholar]
- 30.Egea-Guerrero JJ, Murillo-Cabezas F, Gordillo-Escobar E, Rodríguez-Rodríguez A, Enamorado-Enamorado J, Revuelto-Rey J. S100B protein may detect brain death development after severe traumatic brain injury. J Neurotrauma. 2013;30:1762–1769. doi: 10.1089/neu.2012.2606. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]


