Abstract
Background: In the elderly patients, optimal surgical treatment can be difficult to achieve, because of comorbidity. Therefore, we aimed to clarify the preferred surgical management in this patient group.
Methods: A retrospective study was conducted between April 2008 and March 2015 that included patients with non-small cell lung cancer (NSCLC) aged ≥75 years.
Results: We included 44 patients who underwent partial resection (n = 20) or lobectomy (n = 24). There were no significant differences between the two groups on most variables, except for some character. Survival analysis revealed a significant difference in overall survival (OS) between the two groups; however, no significant differences existed in the disease-free survival or in the OS for stage I disease. Postoperative complications led to poor prognoses. Cox regression analysis revealed statistical significance for the Brinkman Index, the ratio of the pulmonary artery diameter to the ascending aorta diameter (PA:A), and the alveolar–arterial oxygen gradient. Only the PA:A ratio remained significant after multivariate analysis, with a higher ratio associated with better survival.
Conclusion: In elderly patients with NSCLC, surgical resection should not be denied because of age alone. However, partial resection should be favored to lobectomy when possible.
Keywords: elderly patient, lobectomy, NSCLC, partial resection, PA:A ratio
Introduction
The Japanese Thoracic Surgery Society (JTSS) reported that the number of patients aged 80 years or older with lung cancer was 8.7% in 2007 and that this had increased to 11.6% by 2012.1) In addition, comorbidity rates of 42.9%–84.5% have been identified in 33%–66% of patients aged 75 years or older who developed postoperative complications.2) This leads to difficulties when selecting the most appropriate surgical treatment in this population.
Elderly patients with lung cancer should not be denied resection because of their age alone.3–6) Indeed, lung surgery can be both safe and feasible in octogenarians, provided surgical candidates are appropriately selected. However, because elderly patients have a higher incidence of morbidity and mortality than that of younger patients,7) the long-term benefits of surgery remain unclear for this population. Thus, the surgeons face a clinical dilemma as to whether elderly patients should undergo surgery because of the increased potential for postoperative complications in this group.
Recently, the potential for exacerbation of chronic obstructive pulmonary disease (COPD) has become a major clinical problem, potentially resulting in a poor quality of life and increased hospitalization costs.8) To identify patients at risk, Wells et al. described the role of pulmonary arterial enlargement, reporting that a ratio of the diameter of the pulmonary artery to the diameter of the ascending aorta (the PA:A ratio) >1 was associated with an increased risk of severe exacerbation of COPD.8) The Japanese Respiratory Society has supplemented this by proposing the concept of “lung age” to help patients understand the risk posed by COPD.9) Moreover, Haruki et al. reported that lung age predicts postoperative respiratory morbidity.9) COPD clearly has an important relationship with outcomes following lung cancer surgery.
The issue of whether or not to treat elderly patients with non-small cell lung cancer (NSCLC) is increasingly significant not only in Japan but also in other countries because of the growth in the proportion of elderly patients in the population. Therefore, we aimed to clarify the preferred surgical option for elderly patients with NSCLC based on a retrospective review of the data at our institution. We hypothesized that these results could help decide whether lobectomy or partial resection is preferable in this context.
Patients and Methods
We included patients with NSCLC aged ≥75 years who underwent surgical resection at the Nippon Medical School, Musashikosugi Hospital between April 2008 and March 2015. All patient data were collected retrospectively. For comparison, we divided the cohort into those who underwent partial resection (P group) and those who underwent lobectomy (L group). Basically, Video-Assisted Thoracoscopic Surgery (VATS) was performed; with one or two ports with one small window (from 5 cm to 15 cm). The operation was conducted by one chief surgeon and patients were followed up at our outpatient department. Although lymph node dissection was performed in patients undergoing lobectomy, it was not performed in most patients undergoing partial resection. Thus, lymph node metastasis was determined by clinical stage using computed tomography (CT) for those who underwent partial resection.
Informed consent was obtained for all patients preoperatively, including for later access to their clinical records. In addition, the Nippon Medical School ethics committee permitted access to and use of patient data for the purpose of this study.
Two-category comparisons were performed using chi-square tests, t-tests, and Mann–Whitney U tests for quantitative data. To estimate the prognosis, we used the Kaplan–Meier method with Log-rank or Wilcoxon tests. To investigate the effect on overall survival (OS), Cox’s hazard regression model was used in both univariate and multivariate analyses. All analyses were considered statistically significant when the p-value was <0.05. Data analyses were conducted using StatView version 5.0 (SAS Institute, Inc, Cary, NC).
Results
Patients’ characteristics
We included 44 patients (28 men and 16 women) aged 79.0 ± 0.6 years (range 75–90); 20 patients were included in the P group, and 24 patients were included in the L group. The patients’ characteristics are shown in Tables 1a–c, as previously reported.3–16) There were no significant differences between the two groups on most variables, except for preoperative diabetes mellitus (DM) (p = 0.0271), tumor size on CT (p = 0.0002), operation time (p <0.0001), length of postoperative hospitalization (p = 0.0003), or pathological tumor size (p <0.0001).
Table 1.
Patient characteristics
| (a) Preoperative patient characteristics | ||||||
|---|---|---|---|---|---|---|
| Characteristics | P-group | L-group | p-value | |||
| (n = 20) | (%) | (n = 24) | (%) | |||
| Gender | Male | 10 | 50.0 | 18 | 75.0 | p = 0.1267 |
| Female | 10 | 50.0 | 6 | 25.0 | ||
| Age (y/o) | Average | 80.0 ± 1.1 | 78.3 ± 0.5 | p = 0.1196 | ||
| Range | 75–90 | 75–84 | ||||
| Body mass index (BMI) | Average | 22.6 ± 0.6 | 23.6 ± 0.6 | p = 0.2324 | ||
| Range | 16.0–27.0 | 17.9–30.9 | ||||
| Smoking status | Non-smoker | 7 | 35.0 | 5 | 20.8 | p = 0.3293 |
| Smoker | 13 | 65.0 | 19 | 79.2 | ||
| Brinkman index | Average | 724.3 ± 160.1 | 1052.9 ± 198.9 | p = 0.2176 | ||
| Range | 0–2440 | 0–4000 | ||||
| Smoking cessation (y/o) | Average | 2.8 ± 1.1 | 8.0 ± 2.2 | p = 0.0757 | ||
| Range | 0–10 | 0–31 | ||||
| PA:A ratio | Average | 0.74 ± 0.03 | 0.75 ± 0.03 | p = 0.7301 | ||
| Range | 0.58–1.04 | 0.46–1.03 | ||||
| Lung age (y/o) | Average | 85.8 ± 3.7 | 91.4 ± 4.2 | p = 0.3323 | ||
| Range | 53–111 | 58–160 | ||||
| Pulmonary comorbidity | Yes | 14 | 70.0 | 19 | 79.2 | p = 0.5093 |
| No | 5 | 25.0 | 5 | 20.8 | ||
| N/A | 1 | 5.0 | 0 | 0.0 | ||
| Cardiac comorbidity | Yes | 9 | 45.0 | 7 | 29.2 | p = 0.3520 |
| No | 11 | 55.0 | 17 | 70.8 | ||
| Diabetes mellitus (DM) | Yes | 1 | 5.0 | 8 | 33.3 | p = 0.0271* |
| No | 19 | 95.0 | 16 | 66.7 | ||
| Hypertension | Yes | 14 | 70.0 | 14 | 58.3 | p = 0.5344 |
| No | 6 | 30.0 | 10 | 41.7 | ||
| Tumor size (cm) | Average | 2.1 ± 0.2 | 3.3 ± 0.2 | p = 0.0002* | ||
| Range | 1.0–4.5 | 1.7–5.3 | ||||
| eGFR* grade | G1 | 1 | 5.0 | 3 | 12.5 | p = 0.5362 |
| G2 | 7 | 35.0 | 7 | 29.2 | ||
| G3a | 8 | 40.0 | 10 | 54.0 | ||
| G3b | 3 | 15.0 | 2 | 8.4 | ||
| G5 | 1 | 5.0 | 0 | 0.0 | ||
| N/A | 0 | 0.0 | 2 | 8.4 | ||
| Performance status (PS) | 0 | 15 | 75.0 | 19 | 79.1 | p = 0.7453 |
| 1 | 4 | 20.0 | 3 | 12.5 | ||
| N/A | 1 | 5.0 | 2 | 8.4 | ||
| c-stage | IA | 16 | 80.0 | 9 | 37.5 | p = 0.0287* |
| IB | 2 | 10.0 | 10 | 41.7 | ||
| IIA | 1 | 5.0 | 4 | 16.7 | ||
| IIIA | 0 | 0.0 | 1 | 4.1 | ||
| N/A | 1 | 5.0 | 0 | 0.0 | ||
*statistical significant; P group: partial resection; L group: lobectomy; PA:A: the pulmonary artery diameter to the ascending aorta diameter; N/A: not available; eGFR: estimated glomerular filtration rate
| (b) Preoperative pulmonary function test | ||||||
|---|---|---|---|---|---|---|
| Characteristics | P-group | L-group | p-value | |||
| (n = 20) | (n = 24) | |||||
| VC (L) | Average | 2.73 ± 0.15 | 2.90 ± 0.11 | p = 0.3535 | ||
| Range | 1.82–3.99 | 1.59–3.73 | ||||
| %VC (%) | Average | 106.6 ± 3.26 | 103.2 ± 2.64 | p = 0.4177 | ||
| Range | 87.9–130.2 | 78.8–129.2 | ||||
| FEV1.0 (L) | Average | 1.83 ± 0.11 | 1.98 ± 0.08 | p = 0.2549 | ||
| Range | 0.86–2.87 | 1.07–2.67 | ||||
| FEV1.0% (%) | Average | 67.5 ± 2.4 | 69.6 ± 1.6 | p = 0.4751 | ||
| Range | 47.1–86.5 | 53.9–86.2 | ||||
| %FEV1.0 (%) | Average | 113.7 ± 6.5 | 106.7 ± 4.5 | p = 0.3693 | ||
| Range | 70.1–161.3 | 68.3–146.9 | ||||
| %TLC (%) | Average | 102.4 ± 3.8 | 97.3 ± 3.3 | p = 0.3272 | ||
| Range | 84.8–120.0 | 72.6–121.3 | ||||
| %DLCO (%) | Average | 87.9 ± 8.4 | 85.9 ± 7.4 | p = 0.8642 | ||
| Range | 50.6–131.2 | 45.9–141.4 | ||||
| pO2 (Torr) | Average | 83.5 ± 2.3 | 82.5 ± 2.0 | p = 0.7539 | ||
| Range | 67.0–103.0 | 66.0–103.0 | ||||
| pCO2 (Torr) | Average | 41.7 ± 0.7 | 40.2 ± 0.9 | p = 0.2140 | ||
| Range | 37.0–47.0 | 31.7–47.0 | ||||
| A–aDO2 (Torr) | Average | 14.1 ± 2.1 | 16.9 ± 2.0 | p = 0.3419 | ||
| Range | 2.0–29.6 | 0.8–32.7 | ||||
| KL-6 (U/ml) | Average | 488.2 ± 81.7 | 435.6 ± 76.7 | p = 0.6457 | ||
| Range | 248–1340 | 35–1370 | ||||
| SP-D (ng/ml) | Average | 64.3 ± 11.3 | 124.6 ± 23.9 | p = 0.0528 | ||
| Range | 17.2–133.0 | 27.2–366.0 | ||||
P group: partial resection; L group: lobectomy; VC: vital capacity; FEV1.0: forced expiratory volume in 1 s; FEV1.0%: a forced expiratory volume for 1 s expressed as a percentage of the forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity for carbon monoxide; A–aDO2: alveolar–arterial oxygen gradient
| (c) Perioperative and postoperative characteristics | ||||||
|---|---|---|---|---|---|---|
| Characteristics | P-group | L-group | p-value | |||
| (n = 20) | (%) | (n = 24) | (%) | |||
| Operation time | Average (min) | 85.8 ± 8.2 | 212.3 ± 12.2 | p <0.0001 | ||
| Range (min) | 39–188 | 148–428 | ||||
| Postoperative hospital days | Average (days) | 4.8 ± 1.6 | 6.7 ± 0.7 | p = 0.0003 | ||
| Range (days) | 2–34 | 3–15 | ||||
| p-stage | IA | 15 | 75.0 | 10 | 41.5 | p = 0.0663 |
| IB | 5 | 25.0 | 6 | 25.0 | ||
| IIA | 0 | 0.0 | 2 | 8.4 | ||
| IIB | 0 | 0.0 | 4 | 16.7 | ||
| IIIA | 0 | 0.0 | 2 | 8.4 | ||
| Pathological tumor size | Average (cm) | 1.9 ± 0.2 | 3.1 ± 0.2 | p <0.0001 | ||
| Range (cm) | 0.7–3.5 | 1.6–5.5 | ||||
| Histology | Adenocarcinoma | 12 | 60.0 | 15 | 62.4 | p = 0.9768 |
| Squamous cell carcinoma | 6 | 30.0 | 7 | 29.2 | ||
| Others | 2 | 10.0 | 2 | 8.4 | ||
| Pathological COPD | Yes | 9 | 45.0 | 7 | 29.2 | p = 0.0663 |
| No | 1 | 5.0 | 8 | 33.3 | ||
| N/A | 10 | 50.0 | 9 | 37.5 | ||
| Pathological interstitial pneumonia | Yes | 5 | 25.0 | 8 | 33.3 | p = 0.5058 |
| No | 4 | 20.0 | 7 | 29.2 | ||
| N/A | 11 | 55.0 | 9 | 37.5 | ||
P group: partial resection; L group: lobectomy; COPD: chronic obstructive pulmonary disease; N/A: not available
Survival analysis
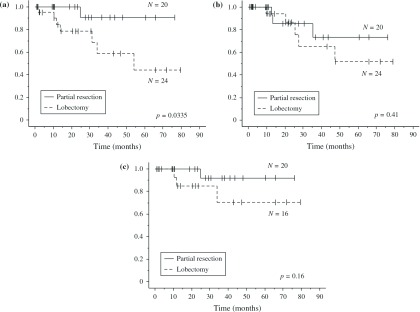
Kaplan–Meier analyses revealed a significant difference in OS between the P group and L group (p = 0.0335) (Fig. 1a). However, no significant differences were found in the disease-free survival (DFS) rate between the two categories (p = 0.41) (Fig. 1b) or in the OS among patients with stage I disease (p = 0.16) (Fig. 1c).
Fig. 1.
(a) Postoperative overall survival (OS) curves by operation for NSCLC (P group versus L group; p = 0.0335 by Log-rank test). (b) Postoperative disease-free survival (DFS) curves by operation (P group versus L group; p = 0.41 by Log-rank test). (c) Postoperative overall survival (OS) curves by operation for stage I NSCLC (P group versus L group; p = 0.16 by Log-rank test). NSCLC: non-small cell lung cancer; P group: partial resection; L group: lobectomy
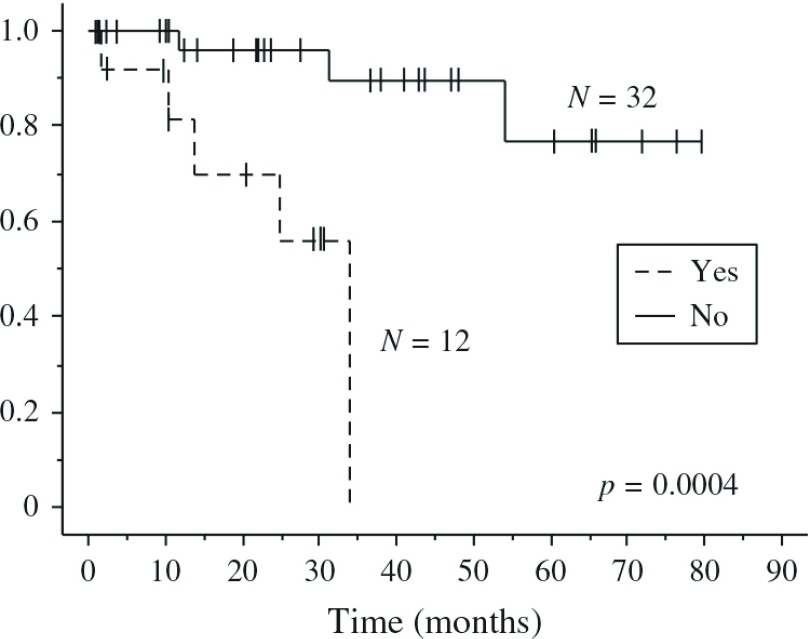
To understand the differences in DFS and OS, we investigated the postoperative complication rates. Figure 2 shows that OS between those with (Yes) postoperative complications were significantly worse survival than those without (No) postoperative complications. However, both groups had statistically similar postoperative morbidities (p = 0.36) (Table 1c). Thus, postoperative morbidity was associated with poor postoperative mortality, but the operation procedure itself did not correlate with postoperative morbidity.
Fig. 2.
Postoperative overall survival (OS) curves according to the presence (Yes) and absence (No) of morbidity among patients with NSCLC (p = 0.0004 by Log-rank test). NSCLC: non-small cell lung cancer
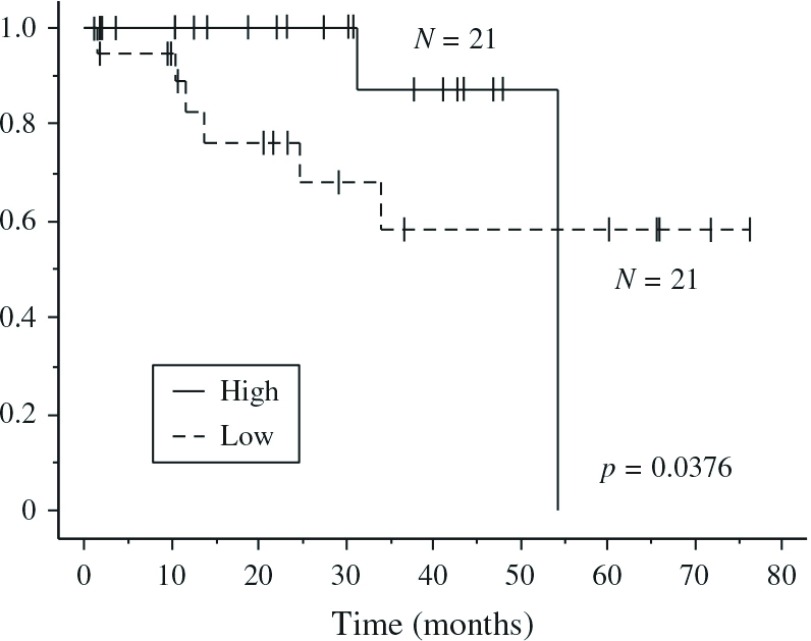
To evaluate the comorbidities associated with a poor prognosis, we reviewed the preoperative risk factors (Table 2). In univariate analysis, the Brinkman Index (BI) (p = 0.0318), the PA:A ratio (p = 0.0182), and the alveolar–arterial oxygen gradient (A–aDO2) (p = 0.0300) (Table 2a) were statistically significant, but only the PA:A ratio (p = 0.0462) (Table 2b) remained significant in the multivariate analysis. Figure 3 shows that a high PA:A ratio was associated with a better survival than a low ratio.
Table 2.
Preoperative predictive factors
| (a) Cox proportional hazards univariate analysis | |||
|---|---|---|---|
| Predictive factor | Odds ratio | 95% Confidence interval | p-value |
| Preoperative comorbidity | |||
| Cardiac | 0.805 | 0.144–4.501 | 0.8046 |
| Lung | 0.244 | 0.029–2.042 | 0.1930 |
| Kidney | 0.756 | 0.149–3.837 | 0.7353 |
| Diabetes mellitus | 0.504 | 0.097–2.624 | 0.4159 |
| Hypertension | 4.932 | 0.987–24.637 | 0.0518 |
| Smoking status | |||
| Brinkman index | 1.001 | 1–1.001 | 0.0318* |
| Smoking cessation period | 1.012 | 0.947–1.081 | 0.7239 |
| Preoperative CT image | |||
| Tumor size | 0.477 | 0.887–2.926 | 0.1171 |
| PA:A ratio | 4.35E-05 | 1.043E-8–0.181 | 0.0182* |
| Preoperative condition | |||
| BMI | 1.078 | 0.850–1.367 | 0.5368 |
| eGFR | 1.021 | 0.982–1.061 | 0.3016 |
| Preoperative pulmonary function test | |||
| %VC | 0.948 | 0.896–1.004 | 0.0668 |
| %FEV1.0 | 0.976 | 0.933–1.021 | 0.2853 |
| %DLCO | 0.984 | 0.937–1.033 | 0.5136 |
| %TLC | 1.004 | 0.917–1.100 | 0.9262 |
| Lung age | 1.004 | 0.976–1.034 | 0.7680 |
| A–aDO2 | 1.118 | 1.011–1.236 | 0.0300* |
| Operative procedure | |||
| Partial resection | 7.055 | 0.8654–57.51 | 0.0680 |
*statistically significant; CT: computed tomography; PA:A: the pulmonary artery diameter to the ascending aorta diameter; BMI: body mass index; eGFR: estimated glomerular filtration rate; VC: vital capacity; FEV1.0: forced expiratory volume in 1 s; DLCO: diffusing capacity for carbon monoxide; TLC: total lung capacity; A–aDO2: alveolar– arterial oxygen gradient
| (b) Cox proportional hazards multivariate analysis | |||
|---|---|---|---|
| Predictive factor | Odds ratio | 95% Confidence interval | p-value |
| PA:A ratio | 1.48E-04 | 2.523E-8–0.864 | 0.0462* |
| A-aDO2 | 1.072 | 0.968–1.187 | 0.1799 |
| Brinkman index | 1.001 | 1.000–1.001 | 0.1906 |
*statistically significant; PA:A: the pulmonary artery diameter to the ascending aorta diameter; A–aDO2: alveolar–arterial oxygen gradient
Fig. 3.
Postoperative overall survival (OS) curves according to the PA:A ratio for patients with NSCLC. Patients with higher (High) and the lower (Low) PA:A ratios are compared by the Wilcoxon test (p = 0.0376). PA:A: the pulmonary artery diameter to the ascending aorta diameter; NSCLC: non-small cell lung cancer
Discussion
Elderly patients with NSCLC have lower tolerance to the stress of operations because of their comorbidities. Regardless of age and the individual cancer character, the treatment of NSCLC forced to be selected according to the TNM classification. Consequently, elderly patients often suffer worse outcomes with higher morbidity and mortality, poorer quality of life, and the high cost of the procedure. However, previous studies have produced conflicting results, with different operations considered superior depending on the perspective taken.1–7) For example, the efficacy of radical systemic lymphadenectomy byVATS has been shown to increase the risk of morbidity without prolonging survival.10) In the current study, our aim was to identify the option that avoided excessive treatment to help select the most appropriate surgical option for elderly patients with NSCLC.
First, we divided our cohort into the P group and the L group. As shown in Fig. 1, the P group had better OS than the L group (p = 0.0335); however, there were no significant differences between the two groups in the DFS or in the OS of patients with stage I NSCLC. Furthermore, Table 1 shows very similar characteristics between the two patient groups. However, tumor size warrants special mention as an important factor because increased tumor size is clearly correlated with a poor prognosis.11) We cannot deny that the P group was selected by the surgeon according to tumor size and position as well as their performance status, thereby introducing bias. Despite this, analysis by the Cox hazard model identified that tumor size did not contribute to patient prognosis (p = 0.1171) (Table 2). Although these results suggest that the operative procedure was an important predictive factor, the procedure choice did not contribute to recurrence in elderly patients with NSCLC. Furthermore, our results are consistent with those of several papers that have demonstrated sub-lobar resection to be associated with reduced surgical risk and better surgical outcomes in elderly patients with lung cancer.12) On the other hand, recently, Razi et al. reported that OS and DFS were significantly lower in the wedge resection group as compared with those in lobectomy, but sublobar resection was not inferior to lobectomy for only T1aN0M0 NSCLC in the elderly and should be considered a viable alternative in this high-risk population.13) Our study showed that in not only stage I patients (Fig. 1c) but also T1a patients, whose number was 13; eight patients in P group and four patients in L group, there was no significant difference in P group and L group about OS (data not shown). It might be also that these results were related to smaller number of stage I patients than the former study or selection bias of operation procedure.
Next, we studied how postoperative morbidity influenced OS. In Fig. 2, it is clear that postoperative morbidity affected prognosis (p = 0.0004), with poor OS associated with the presence of postoperative morbidity. Hino et al. also reported that octogenarians with postoperative morbidity had significantly poorer prognoses than those without.12) Our results are consistent with those of Hino et al., suggesting the importance of selecting patients with a low risk of postoperative morbidity.
Some investigators have identified perioperative comorbidities associated with poor prognoses in elderly patients. For example, Cerforio et al. reported that neoadjuvant therapy was a major risk factor for postoperative morbidity.3) In addition, Dominguez-Ventura et al. pointed out that congestive heart failure and myocardial infarction both correlated with postoperative mortality and that male gender, operative procedure, a forced expiratory volume for 1 s expressed as a percentage of the forced vital capacity (i.e., FEV1%) of 40% or less, smoking status, and stroke increased the risk of postoperative morbidity.5,6) Hino et al. also reported that male gender and non-adenocarcinoma histology were significant risk factors,14) while Okami et al. reported that comorbidity and mediastinal lymph node dissection were associated with poor prognosis and operative risk.15) Finally, Ogawa et al. reported that the age gap, as previously described, was associated with postoperative morbidity.16)
In the current report, although the Brinkman Index, PA:A ratio, and A–aDO2 correlated significantly with OS in univariate analysis, only the PA:A ratio correlated with OS after multivariate analysis (Fig. 3 and Table 2). However, it was unclear why those with a higher PA:A ratio had better survival than those with a lower ratio because COPD patients with a PA:A ratio >1 tend to suffer from severe exacerbations more frequently.8) It is also known that a PA:A ratio >1 is useful for diagnosing resting pulmonary hypertension in patients with severe COPD.17) We found that patients with a high PA:A ratio had a statistically improved survival than those with lower ratios by the Wilcoxon test (p = 0.0376) but not by the Log-rank test (p = 0.17). Thus, we suggest that the PA:A ratio is not correlated with OS in the long term. Furthermore, few patients in this study had a PA:A ratio of ≥1 (data not shown). We considered that this was due to the small sample size.
Conclusion
Our results have demonstrated sub-lobar resection to be associated with reduced surgical risk and better surgical outcomes in elderly patients with lung cancer. In addition, the operation choice was not associated with recurrence in elderly patients with NSCLC. Although age is an insufficient justification to deny surgical resection, it appears that partial resection is preferable to lobectomy in this age group based on higher PA:A ratios. However, our report was limited by the sample size, retrospective design, and few patients with a PA:A ratio >1. Further study in a randomized prospective study is necessary to confirm our results and to clarify the factors associated with improved postoperative outcomes.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1).Masuda M, Kuwano H, Okumura M, et al. Thoracic and cardiovascular surgery in Japan during 2012: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014; 62: 734-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Nakagawa T, Chiba N, Saito M, et al. Surgical treatment for elderly patients with lung cancer. Nihon Rinsho 2013; 71(Suppl 6): 488–92. (in Japanese) [Google Scholar]
- 3).Cerfolio RJ, Bryant AS. Survival and outcomes of pulmonary resection for non-small cell lung cancer in the elderly: a nested case-control study. Ann Thorac Surg 2006; 82: 424-9; discussion 429-30. [DOI] [PubMed] [Google Scholar]
- 4).Port JL, Kent M, Korst RJ, et al. Surgical resection for lung cancer in the octogenarian. Chest 2004; 126: 733-8. [DOI] [PubMed] [Google Scholar]
- 5).Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg 2006; 82: 1175-9. [DOI] [PubMed] [Google Scholar]
- 6).Dominguez-Ventura A, Cassivi SD, Allen MS, et al. Lung cancer in octogenarians: factors affecting long-term survival following resection. Eur J Cardiothorac Surg 2007; 32: 370-4. [DOI] [PubMed] [Google Scholar]
- 7).Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg 2014; 98: 1953-60. [DOI] [PubMed] [Google Scholar]
- 8).Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012; 367: 913-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Haruki T, Nakamura H, Taniguchi Y, et al. ‘Lung age’ predicts post-operative complications and survival in lung cancer patients. Respirology 2010; 15: 495-500. [DOI] [PubMed] [Google Scholar]
- 10).Aoki T, Tsuchida M, Watanabe T, et al. Surgical strategy for clinical stage I non-small cell lung cancer in octogenarians. Eur J Cardiothorac Surg 2003; 23: 446-50. [DOI] [PubMed] [Google Scholar]
- 11).Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007; 2: 593-602. [DOI] [PubMed] [Google Scholar]
- 12).Dell'Amore A, Monteverde M, Martucci N, et al. Lobar and sub-lobar lung resection in octogenarians with early stage non-small cell lung cancer: factors affecting surgical outcomes and long-term results. Gen Thorac Cardiovasc Surg 2015; 63: 222-30. [DOI] [PubMed] [Google Scholar]
- 13).Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016; 200: 683-9. [DOI] [PubMed] [Google Scholar]
- 14).Hino H, Murakawa T, Ichinose J, et al. Results of lung cancer surgery for octogenarians. Ann Thorac Cardiovasc Surg 2015; 21: 209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009; 4: 1247-53. [DOI] [PubMed] [Google Scholar]
- 16).Ogawa F, Miyata S, Nakashima H, et al. Clinical impact of lung age on postoperative complications in non-small cell lung cancer patients aged >70 y. J Surg Res 2014; 188: 373-80. [DOI] [PubMed] [Google Scholar]
- 17).Iyer AS, Wells JM, Vishin S, et al. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest 2014; 145: 824-32. [DOI] [PMC free article] [PubMed] [Google Scholar]