Abstract
Introduction: Deteriorated alveolar structure at the base of blebs and bullae is known as the reticulated trabecula-like structure. Its clinical significance in primary spontaneous pneumothorax (PSP) remains unclear. This study aimed to investigate the impact of the structure on recurrence of PSP after video-assisted thoracoscopic surgery (VATS) bullectomy.
Methods: Between April 2010 and March 2014, 80 cases of PSP in 76 patients who underwent VATS bullectomy using endoscopic staplers were included. The staple line was covered with polyglycolic acid sheets and fibrin glue. Cases were assigned to a normal alveolar structure (NAS) group (n = 54) and a reticulated trabecula-like structure (RT) group (n = 26) based on the histological analysis. Factors associated with recurrence were analysed using logistic regression.
Results: The reticulated trabecula-like structure was significantly related to apical lung blebs. The recurrence rate of PSP was significantly higher in the RT group than in the NAS group (38.5% vs. 3.7%; P <0.001). On multivariate analysis, the reticulated trabecula-like structure was an independent factor for recurrence of PSP after VATS bullectomy.
Conclusion: The change of alveolar structure at the base of apical lung blebs would increase the risk for recurrence of PSP after VATS bullectomy.
Keywords: spontaneous pneumothorax, video-assisted thoracoscopic surgery, histological analysis, recurrence risk, blebs
Introduction
Primary spontaneous pneumothorax (PSP) usually occurs following rupture of blebs and bullae at apices of the lung.1,2) Video-assisted thoracoscopic surgery (VATS) bullectomy has been widely accepted for the treatment of PSP; however, the recurrence rate is higher with VATS bullectomy than with open bullectomy.3–5) The most common cause of recurrence is air leakage from new growing bullae around the staple line.6) The mechanisms involved remain speculative, and blebs and bullae may be missed with inappropriate surgical techniques.7) Therefore, various surgical techniques are used, and no consensus has been reached on a standard strategy for preventing new growing bullae.2,4)
Structural changes in the lung parenchyma are involved in the pathogenesis of PSP.1,8) However, their relationship with the recurrence of PSP after VATS bullectomy has not been evaluated because PSP occurs without any apparent underlying lung disease.8) After blebs and bullae occur from the degradation of elastic fibres, an increase in alveolar pressure due to small airway obstructions ruptures them, resulting in spontaneous pneumothorax.2) This mechanism often deteriorates the alveolar structure at the base of blebs and bullae,1) and this structure, known as a reticulated trabecula-like structure,1) is well recognized as emphysema in secondary spontaneous pneumothorax (SSP);9) however, it has not been investigated in PSP. Emphysema has been reported to be associated with the recurrence of SSP after surgery.9) We assumed that the reticulated trabecula-like structure in PSP would be involved in postoperative air leakage after VATS bullectomy.
The present study aimed to investigate the mechanisms of new growing bullae in order to improve the outcome of VATS bullectomy for PSP and to investigate the impact of the reticulated trabecula-like structure on the recurrence of PSP after VATS bullectomy.
Materials and Methods
This retrospective study considered 80 cases of PSP in 76 patients who underwent VATS bullectomy at the Department of Thoracic Surgery, Akita Red Cross Hospital, Japan between April 2010 and March 2014, for inclusion. The institutional review board of Akita Red Cross Hospital approved this study, and the requirement for informed consent was waived. Patients with a history of lung diseases or thoracic surgery, those with severe comorbidities, and those aged over 50 years were excluded. For each patient, the following perioperative data were recorded: sex, age, body mass index (BMI), smoking history, pneumothorax clinical history, chest radiographs, computed tomography (CT) images, intraoperative findings, operation time, pathological reports, postoperative complications, and recurrence. Patients who underwent surgery for PSP and developed contralateral PSP were considered as new cases. All patients received clinical follow-up for at least 12 months. Recurrence was defined as further ipsilateral pneumothorax requiring intervention.
The clinical pathway was indicated for the study patients. All patients were initially managed with placement of a 20Fr chest tube. Chest CT scans were performed before surgery. Patients who showed emphysematous lung on CT scans were excluded. Surgery was indicated in patients with recurrence after conservative management, bilateral problems, prolonged air leak, or tension pneumothorax at the first episode. The VATS procedure involved the use of three intercostal ports (12 mm). The complete VATS procedure was performed with the patients in the lateral decubitus position under general anaesthesia, with double-lumen endotracheal intubation. A 10-mm, 30-degree telescope (Olympus, Tokyo, Japan) was first inserted through the hole made for chest tube insertion in order to examine the pleural cavity. After identifying the leakage point, VATS bullectomy was performed using endoscopic staplers (Ethicon ECHELON flex™ 45 mm gold; Ethicon Endo-Surgery, Inc., Cincinnati, OH or Endo GIA™ 45 mm purple; Covidien, Mansfield, MA). A sealing test was performed at 20 cm H2O to check for air leakage from the staple line. The staple line was then covered with polyglycolic acid (PGA) sheets (NEOVEIL® sheet, Gunze, Ayabe, Japan) and fibrin glue (Bolheal®, The Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan). The operation was routinely video-recorded with a DVD/Blu-ray recorder through the video-assisted thoracoscopic device in order to confirm the achievement of complete resection of blebs and bullae. The 20Fr chest tube placed to obtain a water seal during surgery was removed on the first postoperative day. All patients were discharged on the second postoperative day.
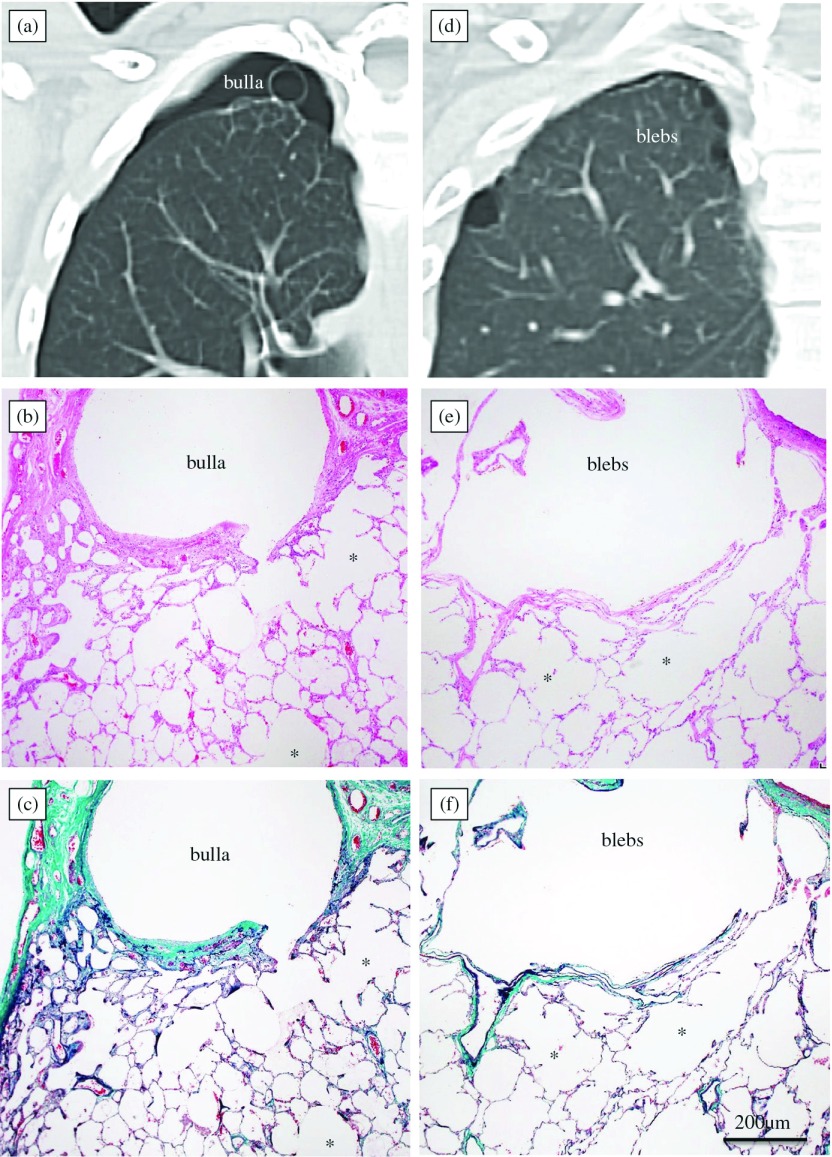
Blebs and bullae were evaluated in axial high-resolution CT (HRCT) and coronal and sagittal multi-planar reconstruction (MPR) images by two radiologists. These CT images were analysed using a commercial workstation (Synapse Vincent®, Fujifilm Medical, Minato-ku, Tokyo, Japan). Blebs were defined as small subpleural thin-walled spaces containing air, with maximum diameters of less than 1 cm on CT images or intraoperative findings, whereas bullae were defined as air-filled emphysematous spaces located in the lung periphery, with maximum diameters of 1 cm or more. A mixture of blebs and bullae was considered bullae. Distributions of blebs and bullae responsible for air leakage based on CT images, pleurographic images, and intraoperative findings were classified into apex or other sites of the lung (Figs. 1a, 1d). The area of residual apical space (RAS)10) on chest radiography at two weeks after VATS bullectomy was measured using Image J software (NIH Image J 1.48; National Institutes of Health, Bethesda, MD).
Fig. 1.
Chest computed tomography (CT) images and histological characteristics of the alveolar structure at the base of lung blebs and bullae in patients with right-sided primary spontaneous pneumothorax. (a–c) An apical lung bulla with a normal alveolar structure in a 20-year-old non-smoker. (d–f) Apical lung blebs with a reticulated trabecula-like structure in a 25-year-old non-smoker. (a, d, Chest CT images; b, e, hematoxylin-eosin staining; c, f, elastic-Masson staining). *alveolar ducts.
Histopathological analysis of the resected specimens was performed with hematoxylin-eosin staining (Figs. 1b, 1e) and elastic-Masson staining (Figs. 1c, 1f). The lung tissue specimens were inflated and fixed with 10% buffered formalin using a 5-mL syringe with a 23-gauge needle for 24–48 h and routinely embedded in paraffin. In the present study, the reticulated trabecula-like structure was defined as a reticulated over dilation of alveolar ducts and alveoli distal to respiratory bronchiole at the base of blebs and bullae, accompanied with the enlarged orifices of alveoli along the alveolar ducts and without the destruction of alveolar walls and obvious fibrosis (Figs. 1e, 1f). An abnormal air space of the reticulated trabecula-like structure is not enlarged to the entire secondary pulmonary lobule. The focal and non-destructive abnormal enlargement of air spaces in the peripheral lung parenchyma displayed the distinguishable pathological features in the reticulated trabecula-like structure, which differ from the histological characteristics of the normal structure of the lung or panacinar emphysema. The histopathological findings of the alveolar tissue at the base of the lung blebs and bullae were classified into normal alveolar structure (Figs. 1b, 1c) or reticulated trabecula-like structure (Figs. 1e, 1f). Elastic-Masson staining was performed to evaluate the connective tissues in the walls of the blebs and bullae. The main component of the walls at the base of the bullae was collagen fibres, whereas that of the blebs was internal elastic layers (Figs. 1c, 1f). All cases were divided into the following two groups based on histopathological examination performed by pathologists: a normal alveolar structure (NAS) group and a reticulated trabecula-like structure (RT) group.
Clinical data are expressed as number (%), median (interquartile range [IQR]), or mean (standard deviation [SD]). The Kolmogorov-Smirnov test was performed to determine if variables were normally distributed. The nonparametric Mann-Whitney U test was used to analyse age, BMI, the area of RAS, and operation time. Fisher’s exact test was used to compare sex, age, smoking history, incidence of bilateral PSP, side of pneumothorax, apical lung blebs, and recurrence between the NAS and RT groups. Variables with P-values <0.10 that were significantly associated with recurrence in univariate analysis were entered into multivariate logistic regression models. Backward stepwise method with P-values <0.05 was used for selection of variables associated with recurrence. Statistical analyses were made using IBM SPSS ver. 21.0 (IBM Co., Armonk, NY). A P-value of <0.05 was considered statistically significant.
Results
The background characteristics of 80 cases of PSP in 76 patients according to the histological groups are summarized in Table 1. The median follow-up period was 43.1 months (range, 12.1–62.6 months). Of the 80 cases, 54 (67.5%) were assigned to the NAS group and 26 (32.5%) were assigned to the RT group. There were no significant differences between the two groups with respect to sex, age, BMI, smoking status, side of PSP, the area of RAS, and operation time (P >0.05). Among the study patients, four patients developed contralateral PSP during the study period. The number of patients with bilateral PSP was significantly higher in the RT group than in the NAS group (P = 0.017). Regarding the distribution of bullae and blebs, in the NAS group, 33 cases showed a single apical bulla, 13 showed apical bullae, four showed a single bulla at other sites, two showed apical blebs, and two showed blebs at other sites, and in the RT group, 24 cases showed apical blebs and two showed apical bullae. The reticulated trabecula-like structure was significantly associated with apical lung blebs (P <0.001). There was no significant association between bilateral PSP and apical lung blebs.
Table 1.
Patient characteristics
| Characteristic | NAS group (n = 54) | RT group (n = 26) | P-value |
|---|---|---|---|
| Sex, male/female (n) | 47/7 | 25/1 | 0.26 |
| Age, median (IQR), years | 20 (17, 28) | 22 (17, 28) | 0.74 |
| Age under 25 years (n) | 35 | 17 | >0.99 |
| BMI, mean (SD), m/kg2 | 19.2 (2.5) | 19.9 (2.4) | 0.26 |
| Smokers/non-smokers (n) | 22/32 | 10/16 | >0.99 |
| Unilateral/bilateral PSP (n) | 47/7 | 16/10 | 0.017 |
| Side, right/left (n) | 33/21 | 11/15 | 0.15 |
| Apical lung blebs (n) | 2 | 24 | <0.001 |
| Area of RAS, median (IQR), cm2 | 7.3 (3.8, 13.6) | 10.3 (6.6, 14.9) | 0.18 |
| Operation time, median (IQR), minutes | 41 (35, 67) | 56 (40, 73) | 0.14 |
| Recurrence (n) | 2 | 10 | <0.001 |
| Treatment for recurrence (n) | |||
| VATS bullectomy | 2 | 6 | |
| VATS suturing | 0 | 2 | |
| Conservative management | 0 | 2 | |
NAS: normal alveolar structure; RT: reticulated trabecula-like structure; IQR: interquartile range; BMI: body mass index; SD: standard deviation; PSP: primary spontaneous pneumothorax; RAS: residual apical space; VATS: video-assisted thoracoscopic surgery
Postoperative complications included bronchitis in one case from the NAS group and wound infection in one case from the RT group. The recurrence rate was significantly higher in the RT group than in the NAS group (38.5% vs. 3.7%; P <0.001). The overall recurrence rate was 15.0% (12/80 cases), and the median time to recurrence was 1.9 months (range, 0.2–7.0 months). Of the 12 recurrence cases, two from the NAS group and eight from the RT group underwent surgical treatment, and two from the RT group were treated conservatively. Of the 10 recurrence cases that underwent surgical treatment, VATS suturing was performed in two cases from the RT group and VATS bullectomy was performed in the remaining eight cases.
The results of univariate and multivariate logistic regression analyses of predictors of recurrence are shown in Table 2. Variables achieving statistical significance on univariate analysis were female sex, young age, non-smoker, presence of bilateral PSP, apical lung blebs, and reticulated trabecula-like structure. There was no significant difference in the area of RAS between patients with and those without recurrence (median, 9.4 cm2 vs. 8.1 cm2; P >0.99). The results of the multivariate logistic regression analysis indicated that the presence of the reticulated trabecula-like structure was an independent factor for recurrence.
Table 2.
Summary of the results of Cox proportional hazards regression analysis
| Covariates | HR (95% CI) | P-value |
|---|---|---|
| Univariate analysis | ||
| Female sex | 2.30 (0.41–13.0) | <0.001 |
| Young age (under 25 years) | 3.93 (0.81–19.2) | 0.09 |
| Non-smoker | 4.37 (0.90–21.3) | <0.001 |
| Bilateral PSP | 5.18 (1.41–19.1) | <0.001 |
| Apical lung blebs | 9.00 (2.18–37.1) | <0.001 |
| Reticulated trabecula-like structure | 16.2 (3.22–82.0) | <0.001 |
| Multivariate analysis | ||
| Reticulated trabecula-like structure | 16.2 (3.22–82.0) | <0.001 |
HR: hazard ratio; CI: confidence interval; PSP: primary spontaneous pneumothorax
Histopathological analysis of eight resected specimens from recurrence cases obtained during VATS bullectomy revealed that the cause of recurrence was the rupture of new growing bullae around the staple line. Among these eight cases, six cases from the RT group showed the reticulated trabecula-like structure at the base of new growing bullae and two cases from the NAS group showed a normal alveolar structure.
Discussion
In the present study, we found that the presence of the reticulated trabecula-like structure was an independent factor for recurrence of PSP after VATS bullectomy. The structure was mainly present at the base of apical lung blebs. The main cause of recurrence was the rupture of new growing bullae around the staple line. Among recurrence cases, this structure was observed at the base of new growing bullae in the RT group. These findings suggested that the reticulated trabecula-like structure would be associated with the development of new growing bullae around the staple line, resulting in recurrence of PSP after VATS bullectomy.
The presence of a change in the alveolar structure at the base of apical lung blebs would be associated with the development of new growing bullae around the staple line after VATS bullectomy. Based on these findings, we propose the following mechanism for the development of new growing bullae. The reticulated trabecula-like structure has a weak structural framework owing their deteriorated elastic fibres. Elastic lamina close to the structure would be vulnerable to increased alveolar pressure at apices of the lungs, resulting in the development of blebs. Because the ability to manipulate thoracoscopic forceps and endoscopic staplers tends to be lower with VATS than with thoracotomy, thoracoscopic procedure would be more easily to cause mechanical injury of the weak structural framework. In this situation, an injured area in the pulmonary parenchyma can remain even after complete surgical bullectomy. Elastic lamina above the injured area would be also vulnerable to increased alveolar pressure. Therefore, new growing bullae can develop around the staple line by excessive surface tension on the visceral pleura above the injured area when the lung is inflated.
The results of the present study can be helpful for standardizing thoracoscopic procedure for PSP. Because of the high recurrence rates with VATS bullectomy using endoscopic staplers alone, the guidelines for the treatment of PSP recommend additional procedures during surgery.4) However, as the pathogenesis associated with the formation of new growing bullae has not been elucidated, the decision to apply additional procedures, such as visceral pleural coverage, and chemical and mechanical pleurodesis, remains controversial.3,11–13) No single surgical procedure has been achieved complete satisfactory results.4) From the results of the present study, VATS bullectomy following staple line coverage with PGA sheets may be less effective for preventing recurrence in PSP patients with apical lung blebs.
The reticulated trabecula-like structure would be one of the histological characteristics of apical lung blebs in patients with PSP. Elastic fibres form the structural framework of the lungs, and they allow the lungs to stretch during inhalation.14) Progressive dilation of air spaces due to degradation of elastic fibres occurs after the age of 30–40 years, and alveolar ducts enlarge while adjacent alveoli appear flattened.15) Cigarette smoke has been shown to decrease the elasticity of the fibres and cause emphysema.16) These age- and smoking-related changes have been reported to be factors that increase the risk of recurrence of PSP after VATS bullectomy.9) However, our results revealed that the reticulated trabecula-like structure was not related to these structural changes, indicating that it would be a unique characteristic of apical lung blebs. We suspected that this histological characteristic may be related to difference of originating nature between bullae and blebs. Bullae are located within the substance of the lung and often accompanied with emphysematous lung, whereas blebs are located within the visceral pleura in the most of patients without underlying lung disease. The reticulated trabecula-like structure was undetectable on HRCT and MRP images because of their limited utility for depicting normal pulmonary acini.16,17)
The histological analysis for the lung parenchyma at the base of bullae and blebs would be useful for predicting the risk of recurrence of PSP after VATS bullectomy. Previous studies have reported that female sex, young age, smoking habit, presence of bilateral PSP, the area of RAS, the size and distribution of bullae, and incomplete bullectomy are risk factors for postoperative recurrence of PSP.8,10,12,18,19) However, few previous studies have reported the impact of a change in the alveolar structure at the base of blebs and bullae on the recurrence of PSP after VATS bullectomy. Histological analysis of resected blebs and bullae is usually performed to identify unexpected lung diseases.20) Therefore, the outcome of VATS bullectomy for PSP in the previous studies may have been influenced or biased by the presence of the reticulated trabecula-like structure. From another aspect, our result would provide histological evidence that PSP patients with apical blebs more likely to be recur after surgery than those with bullae.
The present study had several limitations. First, with regard to retrospective nature and small-sized study many confounding factors attributing to recurrence of pneumothorax cannot be completely ruled out. Second, the presence or absence of the reticulated trabecula-like structure at the staple line could not be actually proved because of crush artifacts from the endoscopic staplers. Third, postoperative CT scans were not performed routinely because non-ruptured new growing bullae around the staple line are of low clinical significance. Therefore, the exact incidence of new growing bullae was not evaluated. Despite these limitations, we believe that this study provides important new insights into the pathogenesis of recurrence of PSP after VATS bullectomy. Further studies would be required to confirm our findings.
Conclusion
The structural change in the lung parenchyma at the base of apical lung blebs would be associated with recurrence of PSP after VATS bullectomy and staple line coverage with PGA sheets. Investigating the change of alveolar structure at the base of bullae and bleb would be useful for improving the outcome of VATS bullectomy for PSP.
Disclosure Statement
None declared.
Acknowledgment
The authors thank K. Enomoto from Department of Diagnostic Pathology, Akita Red Cross Hospital for his valuable comments.
References
- 1).Ohata M, Suzuki H. Pathogenesis of spontaneous pneumothorax. With special reference to the ultrastructure of emphysematous bullae. Chest 1980; 77: 771-6. [DOI] [PubMed] [Google Scholar]
- 2).Alberto H, Willard AF. Pneumothorax In: Thomas WS, Joseph L, Carolyn ER, et al. (eds). General Thoracic Surgery. 7th Philadelphia: Lippincott Williams & Wilkins, 2009. 739-1097. [Google Scholar]
- 3).Vohra HA, Adamson L, Weeden DF. Does video-assisted thoracoscopic pleurectomy result in better outcomes than open pleurectomy for primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2008; 7: 673-7. [DOI] [PubMed] [Google Scholar]
- 4).Goto T, Kadota Y, Mori T, et al. Video-assisted thoracic surgery for pneumothorax: republication of a systematic review and a proposal by the guideline committee of the Japanese association for chest surgery 2014. Gen Thorac Cardiovasc Surg 2015; 63: 8-13. [DOI] [PubMed] [Google Scholar]
- 5).Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet 2007; 370: 329-35. [DOI] [PubMed] [Google Scholar]
- 6).Sawada S, Watanabe Y, Moriyama S. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 2005; 127: 2226-30. [DOI] [PubMed] [Google Scholar]
- 7).Ng CS, Lee TW, Wan S, et al. Video assisted thoracic surgery in the management of spontaneous pneumothorax: the current status. Postgrad Med J 2006; 82: 179-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Imperatori A, Rotolo N, Spagnoletti M, et al. Risk factors for postoperative recurrence of spontaneous pneumothorax treated by video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2015; 20: 647-51; discussion 651-2. [DOI] [PubMed] [Google Scholar]
- 9).Isaka M, Asai K, Urabe N. Surgery for secondary spontaneous pneumothorax: risk factors for recurrence and morbidity. Interact Cardiovasc Thorac Surg 2013; 17: 247-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Gaunt A, Martin-Ucar AE, Beggs L, et al. Residual apical space following surgery for pneumothorax increases the risk of recurrence. Eur J Cardiothorac Surg 2008; 34: 169-73. [DOI] [PubMed] [Google Scholar]
- 11).Shaikhrezai K, Thompson AI, Parkin C, et al. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax-long-term results. Eur J Cardiothorac Surg 2011; 40: 120-3. [DOI] [PubMed] [Google Scholar]
- 12).Jiang L, Jiang G, Zhu Y, et al. Risk factors predisposing to prolonged air leak after video-assisted thoracoscopic surgery for spontaneous pneumothorax. Ann Thorac Surg 2014; 97: 1008-13. [DOI] [PubMed] [Google Scholar]
- 13).Min X, Huang Y, Yang Y, et al. Mechanical pleurodesis does not reduce recurrence of spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014; 98: 1790-6; discussion 1796. [DOI] [PubMed] [Google Scholar]
- 14).Suki B, Stamenović D, Hubmayr R. Lung parenchymal mechanics. Compr Physiol 2011; 1: 1317-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Leong F, Leong A. Anatomy and Histology of the Human Lung. In: Franz JL, Veronique D, Thomas D. (eds). A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis. CRC Press, 2010. 31-51. [Google Scholar]
- 16).Takahashi M, Fukuoka J, Nitta N, et al. Imaging of pulmonary emphysema: a pictorial review. Int J Chron Obstruct Pulmon Dis 2008; 3: 193-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Kim do H. The feasibility of axial and coronal combined imaging using multi-detector row computed tomography for the diagnosis and treatment of a primary spontaneous pneumothorax. J Cardiothorac Surg 2011; 14: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Cardillo G, Carleo F, Giunti R, et al. Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: a single-institution experience in 861 cases. J Thorac Cardiovasc Surg 2006; 131: 322-8. [DOI] [PubMed] [Google Scholar]
- 19).Ingolfsson I, Gyllstedt E, Lillo-Gil R, et al. Reoperations are common following VATS for spontaneous pneumothorax: study of risk factors. Interact Cardiovasc Thorac Surg 2006; 5: 602-7. [DOI] [PubMed] [Google Scholar]
- 20).Sauter JL, Butnor KJ. Pathological findings in spontaneous pneumothorax specimens: does the incidence of unexpected clinically significant findings justify routine histological examination? Histopathology 2015; 66: 675-84. [DOI] [PubMed] [Google Scholar]