Abstract
Purpose: We collected our experience in the use of chimney technique with endovascular aneurysm repair (Ch-EVAR) for juxtarenal aortic aneurysms (JAAs), and reviewed the outcomes.
Methods: The patients who were treated with Ch-EVAR between January 2012 and December 2015 were retrospectively reviewed. All of the patients underwent endovascular aneurysm repair (EVAR) under general anesthesia. Femoral arterial access was obtained to place the main body of the endograft; brachial or axillary access was obtained to perform the placement of the chimney stent.
Results: We treated 12 patients with 15 renal arteries using the Ch-EVAR procedure. Technical success was achieved in 11 of the 12 (91.6%) cases. Within the first 30 days of postoperative period, the target vessel patency rate was 93.3% (14 of 15 renal arteries). After a median follow-up period of 28 months, one patient required Ch-EVAR-related re-intervention due to a type Ia endoleak, and 13 of the 15 renal arteries were patent at the end of the follow-up period.
Conclusion: Our findings demonstrate that Ch-EVAR can be completed with a high rate of success. Although early target vessel occlusion or early postoperative mortality might occur, Ch-EVAR could be an alternative treatment for JAA, especially in high risk patients.
Keywords: endovascular aneurysm repair, chimney technique, juxtarenal aortic aneurysm
Introduction
Endovascular aneurysm repair (EVAR) is widely used in the treatment of infrarenal abdominal aortic aneurysms (AAAs). In up to 20%–30% of AAA patients, the length of normal infrarenal aorta above the aneurysm but below the renal arteries that can be used as a proximal landing zone is inadequate for a conventional aortic endograft.1,2) The management of juxtarenal aortic aneurysms (JAAs) with EVAR remains controversial due to the high level of risk. Several endovascular techniques have therefore been proposed to ensure a secure proximal landing zone. The fenestrated and branched endograft (FBE) has recently been developed and has shown promising early- and medium-term results.3) However, the use of such devices mandates highly precise planning, and involves high costs and long manufacturing delays because the devices are customized to suit each patient’s anatomy. Moreover, in Japan, as in other countries, FBE is not yet commercially available for the treatment of JAA. FBE usage is therefore limited to few investigational centers, and most departments cannot treat JAAs with endovascular procedures. This has led to the development of alternative treatment techniques.
The chimney technique with EVAR (Ch-EVAR) was originally described by Greenberg et al.4) as an adjunctive procedure, which was a bail-out technique to maintain the perfusion of visceral organs due to intentional endograft coverage on the vessel origin. The technique can be used, in the treatment of JAAs, to create an additional proximal fixation zone. The most important point is that the components used to achieve Ch-EVAR are commercially available off-the-shelf stents, even in Japan. Ch-EVAR is a readily available technique that can be completed with endovascular skills that are possessed by most vascular surgeons and which can be applied to elective and emergent settings. Several reports have been published on the use of Ch-EVAR in the treatment of JAAs.5,6) The aim of the present study was to review our experience with Ch-EVAR in the treatment of JAAs and to report the medium-term outcomes.
Materials and Methods
Patient selection
The data for 128 consecutive patients who underwent EVAR in the Division of Vascular and Endovascular Surgery, Department of Surgery, Tokyo Medical and Dental University Hospital, between January 2012 and December 2015 were retrospectively reviewed. All of the patients provided informed consent, and approval for a retrospective review of the patients’ medical records and images was obtained from our institutional review board. Among these patients, 12 patients had undergone Ch-EVAR for JAAs using bifurcated endografts. During the same period, we performed open surgical repair for 57 patients with AAAs, which included 24 patients, who needed suprarenal cross-clamping for vascular reconstructions.
Indications and definitions
Twelve of the patients who underwent Ch-EVAR were considered to be at high risk for open surgery due to their physiological and anatomical characteristics. The selection criteria for endovascular repair included >75 years of age or <75 years of age in patients with severe comorbidities, including cardiovascular disease, chronic obstructive pulmonary disease, and hostile abdomen. The patients’ comorbidities were reviewed as described below. Hypertension was diagnosed as a systolic blood pressure of >140 mmHg, a diastolic blood pressure of >80 mmHg or a history of treatment for hypertension. Coronary artery disease was defined as the presence of angina pectoris, myocardial infarction or both, as documented on coronary angiography or based on a history of any revascularization procedures of the coronary arteries. Cerebrovascular disease was defined as a history of stroke, transient ischemic attack, carotid artery revascularization or cerebral hemorrhage. Chronic obstructive pulmonary disease was identified based on pulmonary function (<70% of forced expiratory volume in 1.0 s) or when the patient was actively medicated for the condition. Diabetes mellitus was identified in patients undergoing active medical treatment or diet modification. Hostile abdomen mainly included visceral surgery patients with a pre-operated abdomen. The present analysis only included patients in whom the coverage of the renal arteries with the main body endograft, and the parallel adjacent placement of a chimney stent were planned. We excluded AAAs with infectious or inflammatory etiologies, and ruptured AAAs, from this analysis. Ch-EVAR might be unfavorable for JAAs with access route problems, including severe iliac arterial stenotic or occlusive diseases, and abundant and/or multiple atherosclerotic plaques or mural thrombi between the ascending aorta to iliac arteries. In our Ch-EVAR series, we had no JAAs with access route problems. We obtained the data about patients’ conditions, surgical procedures and postoperative outcomes from the in-hospital and outpatient clinical records. We evaluated the aneurysm concerning its location, sac diameter, and surgical approach by the meaning of contrast-enhanced computed tomography angiography (CTA).
JAAs include degenerative aneurysms or penetrating atherosclerotic ulcers up to the level of the renal arteries. Target vessel patency and endoleaks (ELs) were defined based on the definitions of the Society for Vascular Surgery.7) Technical success was defined as the successful completion of Ch-EVAR, in which both the endograft and involved target vessels were patent and in which there was no evidence of type I or III ELs. Ch-EVAR-related secondary procedures due to chimney stent occlusions or angiography-confirmed high-grade (>70%) stenosis, or type Ia ELs were defined as re-interventions.
Technical aspects
We have previously reported our Ch-EVAR techniques.8) In brief, bilateral femoral arterial access was obtained through a bilateral femoral cut-down, which was performed in the usual manner, and percutaneous left brachial access was obtained for the single chimney technique. If more than one vessel required chimney stent placement, bilateral percutaneous brachial arterial access or open left axillary access was employed. A 4.5-6 Fr guiding sheath (Parent Plus™, Medikit Co., LTD, Tokyo, Japan) was inserted via upper limb access, and the target renal artery was cannulated. The main body of the endograft was deployed first, followed by a 4–6-mm chimney stent (Express SD™, Boston Scientific, Cork, Ireland). After deployment, simultaneous balloon molding was performed using a compliant aortic balloon in the endograft and the re-inflation of the renal stent balloon, which was a bare stent (due to insurance coverage in Japan). After the completion of procedure, we evaluate the aneurysmorrhaphy, the patency of the treated vessels, and ELs by angiography.
Postoperative management
After the procedure, all of the patients underwent at least 24 h of surveillance in an intensive care unit. In the absence of specific contraindications (renal failure, iodine contrast allergy), we performed and evaluate the treated aneurysms by a contrast-enhanced CTA scan before discharge. During the follow-up period, CTA was performed at 3, 6 and 12 months after surgery; thereafter, CTA was performed biannually. We investigated the diameter of the aneurysm, the patency of the endograft and chimney stent, and the presence of ELs and stent graft migration using CTA. If contrast-enhanced CTA was contraindicated, we surveyed the chimney stent patency using duplex ultrasonography and non-contrast computed tomography (CT).
Outcomes and statistical analysis
The main outcomes of the present study included target vessel patency, the performance of Ch-EVAR-related secondary procedures, and a >25% decrease in the estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) in comparison to the preoperative value. The eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration formula.9) The aneurysm sac diameter was considered to be stable if <5 mm of growth was measured on postoperative CT scans. More than 5 mm of growth was defined as growth; <5 mm of growth was defined as shrinkage.
The time-to-event was analyzed using Kaplan-Meier curves for target vessel patency, freedom from Ch-EVAR-related secondary procedures, and overall survival. The statistical analyses were performed using the Stat View software program (version 5, Abacus Concept Inc., Berkley, CA, USA).
Results
Patient demographics
The demographics of the patients in the present study are shown in Table 1. During the study period, 12 consecutive patients underwent JAA treatment with the Ch-EVAR procedure. The median age at intervention was 77 years (range, 63–85 years); nine of the patients (75%) were men. The comorbidities included hypertension (n = 11), diabetes mellitus (n = 4), coronary arterial disease (n = 3), chronic obstructive pulmonary disease (n = 3), cerebrovascular disease (n = 3), chronic heart failure (n = 2), and hostile abdomen (n = 2). The preoperative median eGFR was 58.1 mL/min/1.73 m2 (range, 36.4–97.1 mL/min/1.73 m2).
Table 1.
Patient demographics
| Patient characteristics | Number (%) |
|---|---|
| Age, years, median (range) | 77 (63–85) |
| Gender, male:female | 9:3 |
| Patient comorbidities | Number (%) |
| Hypertension | 11 |
| Diabetes mellitus | 4 |
| Coronary artery disease | 3 |
| Chronic obstructive pulmonary disease | 3 |
| Cerebrovascular disease | 3 |
| Chronic heart failure | 2 |
| Hostile abdomen | 2 |
| eGFR, ml/min/1.73 m2, median (range) | 58.1 (36.4–97.1) |
| Aneurysmal features | |
| Maximum diameter, mm, median (range) | 52.0 (33–85) |
| Infrarenal neck length, mm, median (range) | 6 (3–14) |
| Neck with thrombus | 7 (58.3) |
*eGFR: estimated glomerular filtration rate
Regarding the preoperative aneurysmal features, the median maximum diameter of the aneurysm was 52.0 mm (range, 33.0–85.0 mm), and the median length of the infrarenal neck was 6 mm (3–14 mm). Two of the 12 aneurysms had penetrating atherosclerotic ulcers and seven had mural thrombi at the level of the renal arteries.
Intraoperative management
The intraoperative data are listed in Table 2. Emergent Ch-EVAR was performed in 2 of the 12 cases (16.7%) due to symptomatic aneurysms. We used 8 Excluder™ bifurcated endograft (W. L. Gore and Associates, Flagstaff, AZ, USA), and 4 Endologic Powerlink™ bifurcated graft (Endologix, Inc. Irvine, CA, USA) for the EVAR procedures. Nine of the 12 patients underwent Ch-EVAR with a single chimney stent through the unilateral renal artery; three had double chimney stents through the bilateral renal arteries. Among 15 renal arteries, six renal arteries (40%) had a stenotic lesion >50% in diameter. The median operation time was 216 min (range, 136–511 min), and the median volume of intraoperative blood loss was 405 ml (range, 96–2204 ml). The median volume of contrast agent used was 100 ml (range, 50–200 ml).
Table 2.
Intraoperative variables
| Variables | Number (%) |
|---|---|
| Type of operation | |
| Elective | 10 (83.3%) |
| Emergency | 2 (16.7%) |
| Main device | |
| Excluder | 8 (66.7%) |
| Powerlink | 4 (33.3%) |
| Target vessels | |
| Unilateral renal artery | 9 (75%) |
| Bilateral renal arteries | 3 (25%) |
| Chimney stent; Express SD™ | 15 |
| Diameter; 4 mm:5 mm:6 mm | 3:4:8 |
| Length; 14 mm:15 mm:18 mm:19 mm | 1:3:7:4 |
| Operative time, min, median (range) | 216 (136–511) |
| Intraoperative blood loss, ml, median (range) | 405 (96–2204) |
| Contrast volume, ml, median (range) | 100 (50–200) |
| New neck length, mm, median (range) | 13.5 (10.2–19.7) |
In each renal artery, we used one chimney stent with a 4–6 mm sized (4 mm; three renal arteries, 5 mm; four renal arteries, 6 mm; eight renal arteries) and a 14–19 mm length (14 mm; 1 renal artery, 15 mm; three renal arteries; 18 mm; seven renal arteries, 19 mm; four renal arteries) (Express SD™, Boston Scientific, Cork, Ireland). In 14 of the 15 renal arteries that were treated by chimney stent placement, the chimney stents were successfully positioned through the target renal arteries via peri-endografts. In one case, a chimney stent migrated distally, and a further approach failed due to a loss of access to the wire. The target renal artery with the migrated stent was found to be patent by intraoperative angiography. After the completion of Ch-EVAR, intraoperative angiography showed no type Ia ELs in any of the 12 patients. Thus, the technical success rate was 91.6% (=11/12 cases). With the stent placement, we achieved new neck length with the median of 13.5 mm (range, 10.2–19.7 mm).
Postoperative outcomes (Table 3)
Table 3.
The outcomes of postoperative and follow-period
| Variables | Number (%) |
|---|---|
| Early postoperative period | |
| 30-day death | 0 (0%) |
| In-hospital death | 1 (8.3%) |
| Major complications | 3 (25%) |
| Pneumonia | 1 (8.3%) |
| Colonic ischemia | 1 (8.3%) |
| Occlusion of renal stent | 1 (8.3%) |
| Endoleak | 2 (16.7%) |
| Type I | 0 |
| Type II | 2 (16.7%) |
| Follow-up period | |
| Death | 1 (In-hospital death) |
| Patency of targeted renal artery | 13 (86.7%) |
| eGFR decrease >25% | 2 (18.2%) |
| Change of aneurysmal size | |
| Increase | 1 (9.1%) |
| Stable | 7 (64.6%) |
| Shrinkage | 3 (27.3%) |
| Endoleak | 5 (45.5%) |
| Type I | 1 (9.1%) |
| Type II | 4 (36.4%) |
*eGFR: estimated glomerular filtration rate
During the 30-day postoperative period, major complications developed in three patients (25%). One patient developed pneumonia but recovered with conservative treatment. One chimney stent in one patient was found to be occluded on CTA at discharge; however the patient’s postoperative eGFR was within normal limit. The patient was treated conservatively because he did not wish to undergo another operation. One patient developed colonic ischemia and subsequent multi organ failure that resulted in death on postoperative day 74. Two patients showed a type II EL on CTA at discharge; no type I ELs were documented. The 30-day and in-hospital mortality rates were 0% and 8% (1 of 12), respectively. The target vessel patency rate in the 30-day postoperative period was 93.3% (14 of 15 renal arteries).
Follow-up outcomes (Table 3)
One death occurred (the case of in-hospital mortality) during the median follow-up period of 28 months (range, 2–45 months). During the follow-up period, five patients were surveyed by CTA, and six patients by non-contrast CT scanning and duplex ultrasonography. Regarding aneurysm morphology, four patients had a type II EL, which was detected on CTA; however, all type II ELs were considered minor, and re-intervention was not required. CTA in the 2-year follow-up of one patient revealed a type Ia EL; it was successfully treated by coil embolization. With regard to target vessel patency, 13 renal arteries were patent during the follow-up period; the exceptions include one case of in-hospital mortality and one case of early occlusion. The aneurysm size appeared to be stable in seven patients, while shrinkage occurred in three patients. The case with a treated type Ia EL showed shrinkage in comparison to the preoperative diameter. No aneurysms became enlarged or ruptured during the follow-up period. Two of 11 cases showed postoperative renal dysfunction with a >25% decrease in eGFR.
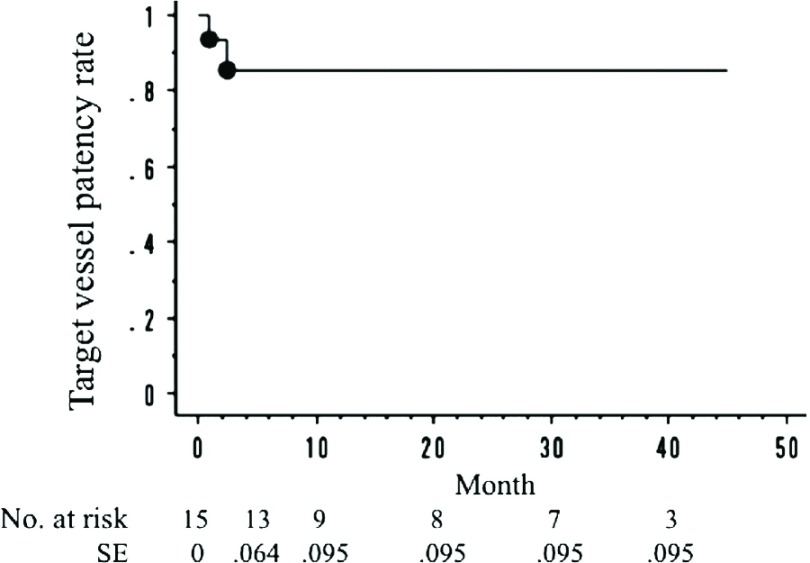
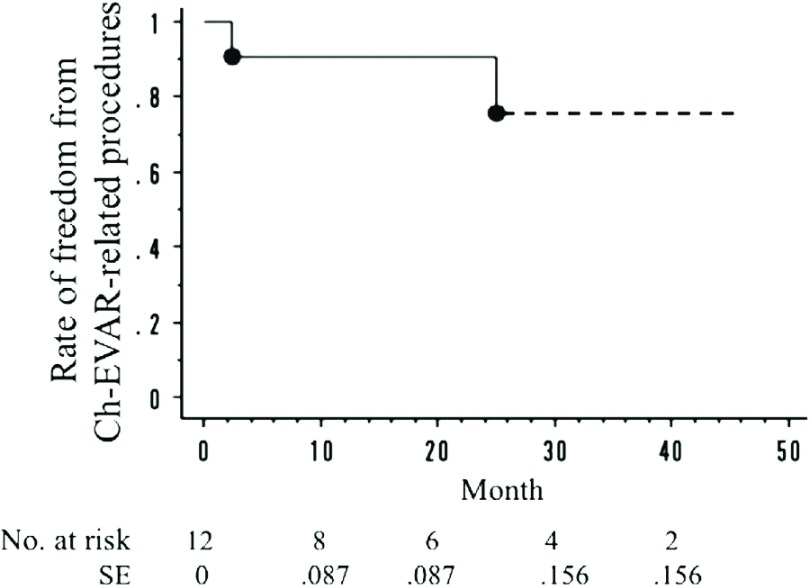
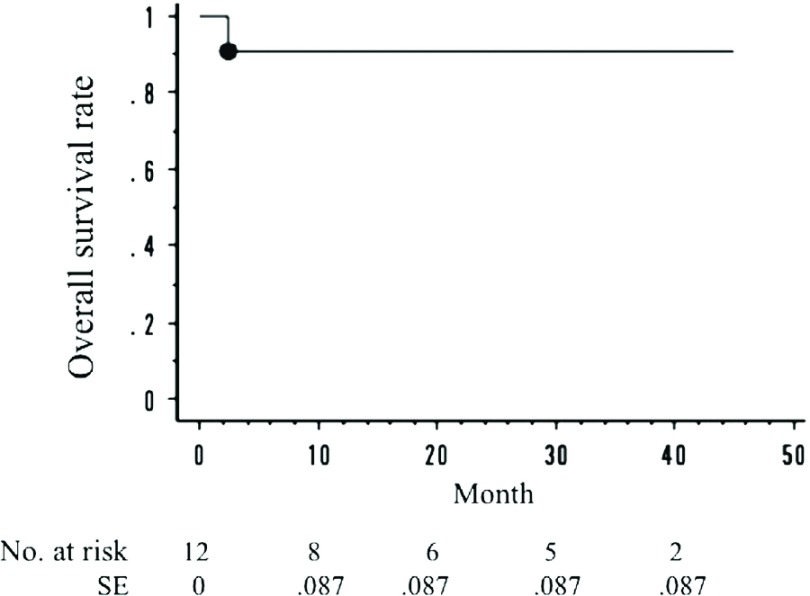
The cumulative target vessel patency rate was 86.7% (13 of 15 renal arteries). The cumulative rate of freedom from Ch-EVAR-related secondary procedures was 91.3% (11 of 12 cases). The cumulative mortality rate was 8% (1 of 12 cases). The target vessel patency rates at 1 year and 3 years, as determined by the Kaplan-Meier method, were 85.6% and 85.6%, respectively (Fig. 1). The rates of freedom from Ch-EVAR-related procedures at 1 year and 3 years were 90.9% and 75.8%, respectively (Fig. 2). The overall survival rates at 1 year and 3 years were 90.9% and 90.9%, respectively (Fig. 3).
Fig. 1.
The primary patency rate of the chimney stents. One-year: 85.6%; 3-year: 85.6%. *SE: standard error
Fig. 2.
The rate of freedom from Ch-EVAR-related procedures. One-year: 90.9%; 3-year: 75.8%. *Ch-EVAR: chimney technique with endovascular aneurysm repair; SE: standard error
Fig. 3.
The overall survival rate. One-year: 90.9%; 3-year: 90.9%. *SE: standard error
Discussion
Even though the open operative management remains the gold standard in AAAs with anatomically challenging necks, the rates of postoperative complications and mortality are relatively high, especially in high risk patients.10) Less invasive treatments, such as Ch-EVAR might therefore be more appropriate than open surgery. The therapeutic efficacy and safety of endovascular treatments for JAAs have been reported. To date, the outcomes of Ch-EVAR have been overwhelmingly successful, with several reports describing an excellent technical success rate of >95%, and short-term chimney stent patency rate of >90%. Furthermore, aneurysmal sac enlargement has been documented in <10% of cases.11,12) In the present study, the technical success rate and target vessel patency rate were 91.6% and 86.7%, respectively, and the diameter of the aneurysmal sac remained stable or shrank in all cases. These outcomes are comparable with those of previous reports; Ch-EVAR procedures therefore show promising clinical results in terms of the morbidity, mortality, the preservation of target organ perfusion, and aneurysmorrhaphy. Furthermore, the follow-up period in the present study (median; 28 months) was longer than the 19.2-month period of previous studies.1)
In theory, Ch-EVAR appears to work well with good conformability between the main endograft, chimney graft, and the aortic wall. However, Ch-EVAR is associated with a potential risk of gutters, which results in type Ia ELs between the main endograft and the chimney graft.13) Coscas et al.14) reported that 4 of 12 patients (30%) developed type Ia ELs intraoperatively during Ch-EVAR procedures. Several issues need to be clarified, including the suitable endograft, the type of chimney stent (balloon- or self-expandable; covered or bare). Donas et al.15) showed the good results with both balloon-expandable and self-expandable covered stents in Ch-EVAR. On the other hand, some authors have reported that bare stents are not inferior to covered stents with regard to renal patency or protection against type Ia ELs.16) Furthermore, in Japan, are only able to use bare stents for Ch-EVAR due to national insurance coverage. Thus, the existing data have not provided any firm conclusions as to whether these devices were associated with an increased risk of type Ia EL.
Donas et al.5) reported that the primary patency rate was 95.7%, and the majority of occlusions were identified during the first 2 months. This kind of early stent occlusion was seen in our study. However, as was noted in our study, the renal function was not permanently impaired; it probably led to the overfunction of the contralateral kidney. Furthermore, it is interesting that Donas et al.13) reported that some patients (8.9%) experienced a clinically significant improvement in renal function caused by the treatment of coexisting renal artery stenosis with a chimney stent. Postoperative renal dysfunction might be caused by renal artery stenosis or occlusion, or by intraoperative manipulation (injury of renal arteries, embolization, and the repeated use of contrast-enhanced agents). Even though postoperative renal function might be affected by various factors, we should carefully evaluate the morphology of the chimney stents and determine the state of perfusion in angiography at the completion of surgery in order to recognize and correct problems.
This study is associated with several limitations. This study includes only small sample size, which might be difficult to evaluate the factors affecting the technical success for Ch-EVAR. For this reasons, we need much larger sample size to determine the efficacy and feasibility of Ch-EVAR. A larger sample size could clarify several issues, including the most suitable types of main endograft and chimney stent. However, our results may be compatible with those of previous reports.
Conclusion
In this study, we presented our experience with Ch-EVAR. The technical success, target vessel patency, Ch-EVAR-related re-intervention, and survival rates were comparable with previous reports, which could indicate that Ch-EVAR is an attractive and efficient alternative treatment for JAA. We believe that vascular surgeons should consider the use of Ch-EVAR in the treatment of JAAs, because it provides an immediate off-the-shelf solution that is safe, effective, and durable in the midterm. Ch-EVAR is a useful treatment method, which deserves further study and wider usage.
Disclosure Statement
Igari and the other co-authors have no conflicts of interest to declare.
References
- 1).Scali ST, Feezor RJ, Chang CK, et al. Critical analysis of results after chimney endovascular aortic aneurysm repair raises cause for concern. J Vasc Surg 2014; 60: 865-73; discussion 873-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Igari K, Kudo T, Toyofuku T, et al. Outcomes following endovascular abdominal aortic aneurysm repair both within and outside of the instructions for use. Ann Thorac Cardiovasc Surg 2014; 20: 61-6. [DOI] [PubMed] [Google Scholar]
- 3).Haulon S, Amiot S, Magnan PE, et al. An analysis of the French multicentre experience of fenestrated aortic endografts: medium-term outcomes. Ann Surg 2010; 251: 357-62. [DOI] [PubMed] [Google Scholar]
- 4).Greenberg RK, Clair D, Srivastava S, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg 2003; 38: 990-6. [DOI] [PubMed] [Google Scholar]
- 5).Donas KP, Torsello GB, Piccoli G, et al. The PROTAGORAS study to evaluate the performance of the Endurant stent graft for patients with pararenal pathologic processes treated by the chimney/snorkel endovascular technique. J Vasc Surg 2016; 63: 1-7. [DOI] [PubMed] [Google Scholar]
- 6).Patel RP, Katsargyris A, Verhoeven EL, et al. Endovascular aortic aneurysm repair with chimney and snorkel grafts: indications, techniques and results. Cardiovasc Intervent Radiol 2013; 36: 1443-51. [DOI] [PubMed] [Google Scholar]
- 7).Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35: 1048-60. [DOI] [PubMed] [Google Scholar]
- 8).Igari K, Kudo T, Uchiyama H, et al. Early experience with the endowedge technique and snorkel technique for endovascular aneurysm repair with challenging neck anatomy. Ann Vasc Dis 2014; 7: 46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Beck AW, Goodney PP, Nolan BW, et al. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg 2009; 49: 838-43; discussion 843-4. [DOI] [PubMed] [Google Scholar]
- 11).Tolenaar JL, van Keulen JW, Trimarchi S, et al. The chimney graft, a systematic review. Ann Vasc Surg 2012; 26: 1030-8. [DOI] [PubMed] [Google Scholar]
- 12).Moulakakis KG, Mylonas SN, Avgerinos E, et al. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg 2012; 55: 1497-503. [DOI] [PubMed] [Google Scholar]
- 13).Donas KP, Lee JT, Lachat M, et al. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies: the PERICLES registry. Ann Surg 2015; 262: 546-53; discussion 552-3. [DOI] [PubMed] [Google Scholar]
- 14).Coscas R, Becquemin JP, Majewski M, et al. Management of perioperative endoleaks during endovascular treatment of juxta-renal aneurysms. Ann Vasc Surg 2012; 26: 175-84. [DOI] [PubMed] [Google Scholar]
- 15).Donas KP, Pecoraro F, Torsello G, et al. Use of covered chimney stents for pararenal aortic pathologies is safe and feasible with excellent patency and low incidence of endoleaks. J Vasc Surg 2012; 55: 659-65. [DOI] [PubMed] [Google Scholar]
- 16).Bruen KJ, Feezor RJ, Daniels MJ, et al. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J Vasc Surg 2011; 53: 895-904; discussion 904-5. [DOI] [PubMed] [Google Scholar]