Abstract
Purpose: The aim of this study was to elucidate the characteristics of chronic hemodialysis (HD) patients requiring surgery during the active phase of infective endocarditis (IE).
Methods: From December 2004 to July 2015, 58 patients underwent surgery in our institute for active IE. Seven patients had been on HD for 1–15 years. Their preoperative profiles and surgical outcomes were compared to those of the other 51 patients (non-HD group).
Results: The predominant causative microorganisms in the HD group were Staphylococcus spp, particularly methicillin-resistant Staphylococcus aureus (MRSA), whereas Streptococcus spp were predominant in the non-HD group. Prosthetic dysfunction (stuck valve after mechanical and structural valve dysfunction following bioprosthetic valve replacement), complete atrioventricular (AV) block, and annular abscess formation were more frequent in the HD group. In-hospital mortality was higher in the HD group (29% vs. 6%, p = 0.044). Actuarial survival in the HD and non-HD groups was 43% vs. 87% at 5 years and 43% vs. 76% at 10 years (p = 0.007).
Conclusions: Early and long term outcomes in patients with chronic HD were poor. Compared to other patients, chronic HD patients undergoing valve surgery during active IE had higher incidences of MRSA infection, annular abscess formation, postoperative valve dysfunction, and postoperative complete AV block.
Keywords: infective endocarditis, hemodialysis
Introduction
Infective endocarditis (IE) occurs 18 times more frequently in chronic hemodialysis (HD) patients than in the general population.1) Several previous studies have demonstrated high postoperative mortality (11%–80%) in HD patients who require surgical intervention for IE,2–4) however, little information is available on the preoperative management of these patients.
Here, to improve preoperative management in HD patients requiring surgical intervention for IE, we reviewed surgical experience at our institution over a 10-year period to elucidate the characteristics of chronic HD patients requiring surgery during the active phase of IE. We also reviewed long-term outcomes in these HD patients.
Materials and Methods
This study was approved by the institutional review board of Showa University, School of Medicine, and informed consent was obtained from all patients. We reviewed the cases of all patients undergoing surgery on native valves for active IE in our institute between December 2004 and July 2015. The surgical strategy in our institute remained consistent over that time period. Redo operations were excluded from the study. Clinical parameters including initial clinical presentation, blood and laboratory data, results of blood cultures, operative variables, and postoperative outcomes were assessed. Preoperative head, chest, and abdomen and pelvic computed tomography (CT) examinations were performed, and the modified Duke criteria5) were applied in all patients.
IE was defined as “active” if surgical intervention was required before completion of a standard course of antibiotics, and “healed” if no antibiotics were being administered at the time of surgical intervention other than for prophylactic purposes.6,7) Operative indications in the active phase were (1) persistent infection despite appropriate antibiotic therapy, (2) recurrent embolic events, (3) refractory heart failure, and (4) large (>1 cm) and mobile vegetation. The surgical strategy for active IE was (1) complete debridement of infected intra-cardiac tissue; (2) in the case of annular abscess formation, annular reconstruction with patching performed before prosthetic implantation; (3) in patients with mitral valve infection, mitral valve repair was the first choice if repair was considered feasible on preoperative echocardiography; and (4) if native valve repair was not feasible, a prosthesis was selected according to the risk of bleeding complications, especially intracranial bleeding, and patient age. In patients on chronic HD, HD was performed 12 to 24 h before surgical intervention and on the second postoperative day, but earlier if volume overload or hyperkalemia was present. Hemofiltration using hemoconcentration incorporated with the circuit was used to avoid excess hemodilution and washed red blood cell treated with autotransfusion system was used during surgery in patients on chronic HD. Postoperative antibiotics were administered intravenously for 4 weeks in all patients, and for an additional 2 weeks if C-reactive protein had not dropped within the normal range during that time.
Patient profile and operative outcomes were compared between patients in the chronic HD (HD group) and other patients who underwent cardiac surgery during the active phase of IE (non-HD group). The diagnostic criterion for peripheral arterial disease was an ankle-brachial index value of 0.9 or less. Follow-up data were obtained from the patient’s medical records, current medical charts and as ascertained by cardiologists. The data are summarized as mean ± standard deviation or number (percentage). Baseline differences in categorical variables were tested using the Pearson χ2 test, while continuous variables were tested using Student’s t-test. Values of p <0.05 were considered statistically significant. Long-term survival curve was calculated using the Kaplan-Meier method, and survival curves in the two groups were compared by the log rank test.
Results
Preoperative characteristics
Fifty-eight patients underwent valve surgery during the active stage of IE during the study period. Of these, 7 patients (12%) were classified into the HD group and 51 into the non-HD group. Preoperative serum creatinine level was 7.3 ± 1.9 mg/dl in the HD group and 0.9 ± 0.5 mg/dl in non-HD group (p <0.0001). Other preoperative characteristics in the HD group are summarized in Table 1. Two patients were previously diagnosed with degenerative valvular disease (mild aortic valve stenosis, 1; and mild mitral valve stenosis, 1) by echocardiography at a dialysis center before the onset of IE.
Table 1.
Preoperative patient profile of the HD group and non-HD group
| HD (n = 7) | Non HD (n = 51) | p-value | |
|---|---|---|---|
| Age | 65 ± 11.6 | 57.3 ± 17.2 | NS |
| Female | 28.5% | 25.4% | NS |
| DM | 28.5% | 12.5% | NS |
| HTN | 57.1% | 20.0% | NS |
| PAD | 28.5% | 1.9% | 0.003 |
| Preop. Afib/PAfib | 28.6% | 11.7% | NS |
| Preop. WBC (/µl) | 10.2 ± 8.0 | 9.2 ± 3.0 | NS |
| Preop.CRP (mg/dl) | 8.1 ± 9.9 | 6.4 ± 6.7 | NS |
| Preop. Albumin (mg/dl) | 2.9 ± 0.4 | 2.9 ± 0.6 | NS |
HD: hemodialysis; DM: diabetes mellitus; HTN: hypertension; PAD: peripheral artery disease; WBC: white blood cell; CRP: C-reactive protein; NS: not significant
The preoperative patient profiles did not otherwise differ significantly between the two groups, except for a significantly higher incidence of peripheral arterial disease in the HD group (Table 1). In the non-HD group, Streptococcus spp. were identified as the predominant causative bacteria (32 patients, 63%), whereas no Streptococcus spp. were identified in the HD group (Table 2). MRSA was detected in three patients in the non-HD group, a significantly lower incidence than in the HD group (6% vs. 57%, p <0.001).
Table 2.
Causative microorganisms and affected valves in HD and non-HD groups
| HD (n = 7) | Non HD (n = 51) | p-value | |
|---|---|---|---|
| Causative microorganisms | |||
| Streptococcus spp. | 0 | 32 (63%) | 0.002 |
| Staphylococcus spp. | 5 (71%) | 8 (16%) | 0.0001 |
| MRSA | 4 (57%) | 3 (6%) | <0.001 |
| Enterococcus faecalis | 0 | 4 (8%) | <0.001 |
| Not specified | 1 | 2 | NS |
| Valve lesion | |||
| Aortic valve | 4 (57%) | 13 (25%) | 0.002 |
| Mitral valve | 3 (43%) | 25 (49%) | NS |
| Aortic + mitral valve | 0 | 13 (25%) | <0.001 |
| Abscess formation | 4 (57%) | 8 (16%) | 0.011 |
HD: hemodialysis; MRSA: methicillin-resistant Staphylococcus aureus; NS: not significant
Valve lesions in both groups are summarized in Table 2. In the HD group, the valve most often involved was the aortic valve. In the non-HD group, involvement was predominantly of the mitral valve, with equal numbers of aortic involvement and involvement of both valves. Annular abscess formation was significantly more frequent in the HD group.
Early postoperative outcomes, morbidity and mortality
Clinical data of HD patients are summarized in Table 3, and early postoperative outcomes are compared in Table 4. In-hospital mortality was higher in the HD group than in the non-HD group (29% vs. 6%, p = 0.044). Prosthetic valve dysfunction, the cause of the two re-operations (postoperative day (POD) 4 and 10) and early deaths (POD 5 and 21, respectively) in the HD group, did not occur in the non-HD group. There was no difference between the two groups in the occurrence of pneumonia or neurological complications, including stroke and intracranial bleeding. There was no recurrence of IE in either group. The incidence of other infective complications was significantly higher in the HD group than in the non-HD group (29% vs. 2%, p = 0.003). The incidence of postoperative complete atrioventricular (AV) block was significantly higher in the HD group.
Table 3.
Preoperative and postoperative patient profiles of the hemodialysis group
| Age/Sex | Indication | Duration of HD | Cause of RF | Pathogen | Preop. NA | Predisposing factors | Operation | Annular abscess | Postop. complication | Remarks | In-hosp death | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 M | Persistent infection | 6Y | ? | MRSA | none | Mild MS (for 2 Y) | MVR (mech.) + Maze | + | GI bleeding (OP), mediastinitis | ||
| 2 | 68 M | Heart failure | 1Y2M | GN | MRSA | none | CV line (not for HD) infection | AVR (mech.) | + | Stuck valve | Redo POD 3 | †POD 5 |
| 3 | 79 M | Persistent infection | 1Y6M | ? | MRSA | none | Ruptured mycotic aneurysm of the AV shunt | AVR (mech.) | + | Candida endophthalmitis, IB, AVB III | ||
| 4 | 55 F | Vegetation | 8Y | ? | E.coli | none | AVR (mech.) + CABG | + | AVB III | |||
| 5 | 55 F | Persistent infection | 15Y | GN | MSSA | IB | MVR (bio.) | – | ||||
| 6 | 59 M | Vegetation | 9Y | ? | Not specified | none | MV repair + Maze | – | Brady Afib | |||
| 7 | 83 M | Persistent infection | 1Y3M | DM | MRSA | IB | Mild AR (for 1Y) | AVR (bio.) | – | Structural valve destruction | †POD 21 | |
HD: hemodialysis; RF: renal failure; NA: neurological abnormality; MRSA: methicillin-resistant Staphylococcus aureus; MS: mitral stenosis; MVR: mitral valve replacement; GI: gastrointestinal bleeding; OP: operation; GN: glomerulonephritis; CV: central venous; AVR: aortic valve replacement; AV: arteriovenous; IB: intracranial bleeding; AVB: atrioventricular block; MSSA: methicillin-susceptible Staphylococcus aureus; CABG: coronary arterial bypass graft; DM: diabetes mellitus; AR: aortic regurgitation; POD: postoperative day; †: death
Table 4.
Operative outcome in HD and non-HD groups
| HD (n = 7) | Non HD (n = 51) | p-value | |
|---|---|---|---|
| In-hospital mortality | 2 (29%) | 3 (6%) | 0.044 |
| Complications | |||
| Valve dysfunction | 2 (29%) | 0 | 0.0001 |
| Pneumonia | 1 | 2 | NS |
| Neurological | 1 | 3 | NS |
| GI bleeding | 1 | 1 | 0.093 |
| AV Block III | 2 (29%) | 2 (4%) | 0.015 |
| Recurrence of IE | 0 | 0 | NS |
| Other infectious complication | 2 (mediastinitis, central line infection) | 1 (Cholecystitis) | 0.003 |
HD: hemodialysis; NS: not significant; GI: gastrointestinal bleeding; AV: atrioventricular; IE: infective endocarditis
Long-term outcomes
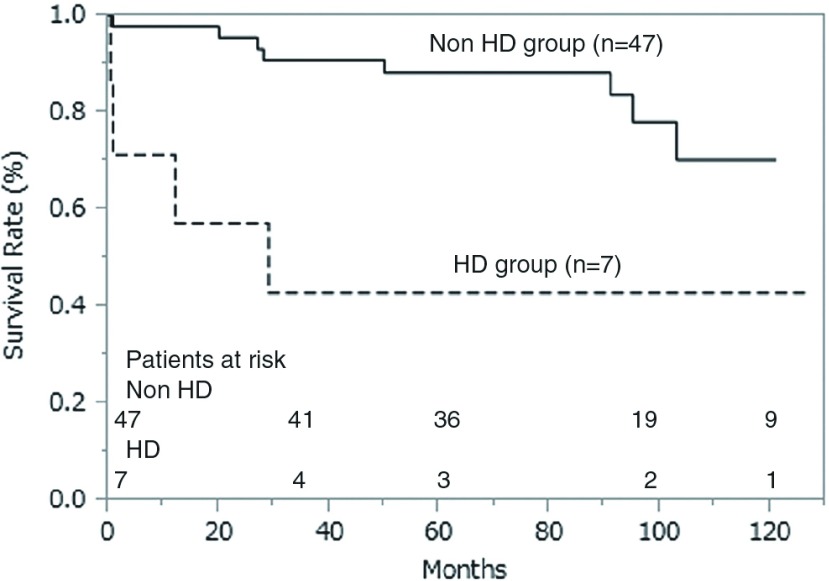
Follow-up for all patients in both groups was complete, except for four patients in the non-HD group (follow-up rate, 93%). Mean follow-up period was 68.5 ± 38.5 months. The Kaplan-Meier survival curve is shown in Fig. 1; actuarial survival in the HD and non-HD groups at 3, 5, and 10 years was 57% vs. 89%, 43% vs. 87%, and 43% vs. 76%, respectively (p = 0.007).
Fig. 1.
Actuarial survival curve and patients at risk. HD: hemodialysis
Discussion
Reported mortality of HD patients who underwent operation during the active phase of IE is high,2–4) and perioperative management should be carefully planned based on previous experience. In our present study, clinical characteristics specific to our HD patients were (1) methicillin-resistant Staphylococcus aureus (MRSA) was the most common causative microorganism; (2) the aortic valve was the most frequent site of involvement; and (3) there was a high incidence of annular abscess formation and postoperative AV block. Additionally, two of our HD patients experienced early prosthetic dysfunction, which resulted in early postoperative death. Early postoperative outcome and long-term survival were poorer in the HD patients than the other patients with active IE.
The higher rates of MRSA infection in our HD group might have been associated with heightened susceptibility to infection and frequent blood access. Even if complete debridement of infective intra-cardiac tissue is achieved, the effectiveness of postoperative antibiotic therapy for postoperative infection control should be carefully evaluated, and where necessary, alternative antibiotics should be selected, such as daptomycin or linezoid.
The lower incidence of double valve IE in HD patients than non-HD patients might have resulted from the close attention of attending physicians or nurses in the HD center to the slightest clinical manifestation, such as sustained low grade fever. Although the aortic valve was the most frequently affected in our study, the significance of this result is doubtful. In their series of 11156 HD patients hospitalized due to IE, of whom 1267 (11.4%) underwent valve surgery, Leither et al. had reported that 588 (46%) had aortic valve operations, 565 (45%) had mitral valve operations, and 114 (9%) underwent both aortic and mitral valve operations.8) Our results might therefore have resulted from the small number of patients.
We experienced a high incidence of postoperative complete AV block in the HD group, which might have been associated with the higher incidence of annular abscess formation. If complete AV block is seen during weaning from cardiopulmonary bypass, secure placement of multiple (more than two) pacing wires is warranted. If permanent pacemaker implantation is predicted after weaning from cardiopulmonary bypass (CPB), epicardial permanent pacing wires (atrial and ventricular) should be implanted intra-operatively, because a permanent intravascular device is a potential risk for recurrent IE in HD patients.9) The generator would be implanted after the operation when the infection is controlled and the IE is considered to be healed in order to prevent electrical device infection.
Evidence to guide the selection of prostheses for HD patients with active IE is scarce. We generally select the prosthesis according to patient age and risk of bleeding complications. Although accelerated bioprosthesis calcification and structural valve dysfunction have been demonstrated in HD patients,10) the incidence of late bleeding or stroke was higher in HD patients who underwent mechanical valve replacement.11) We experienced a stuck disk with a mechanical valve and structural valve dysfunction in a bioprosthesis in the early postoperative period (POD 4 and POD 11), neither of which was seen in the non-HD group. The mechanism was unclear in both cases. Possible causes include coagulation disorders, malnutrition, or highly toxic MRSA (the causative bacteria of IE was MRSA in both cases). Frequent assessment of cardiac and prosthetic function by echocardiogram is warranted in the postoperative care of HD patients.
There were several limitations of this study. First, sample size was small. While the incidence of peripheral arterial disease in the HD patients was high, other factors might have reached statistical significance if the sample size was larger: specifically, while the incidence of diabetes mellitus (28.5% vs. 12.5%), hypertension (57.1% vs. 20.0%) and a preoperative history of atrial fibrillation (28.6% vs. 11.7%) was higher in the HD group, none of these differences reached statistical significance. Although the small number of cases in the HD group may have limited statistical power, the single-center design of our study minimized variable bias in the treatment strategy of IE, i.e., surgical indications and perioperative management, including antibiotic therapy. We therefore consider that our clinical results are valuable in guiding perioperative care. Second, selection bias for surgical intervention is undeniable. This study retrospectively reviewed the cases of patients undergoing surgical intervention, and did not examine patients who received medical therapy only.
Conclusions
In this series of HD patients who underwent surgery during the active phase of IE, the most frequent causative microorganism was MRSA. Additionally, annular abscess formation and postoperative complete AV block were both frequent. We experienced two early dysfunctions of prostheses in HD patients, one in a mechanical valve and the second in a bioprosthesis.
Disclosure Statement
The authors have no conflict of interest.
References
- 1).Abbott KC, Agodoa LY. Hospitalizations for bacterial endocarditis after initiation of chronic dialysis in the United States. Nephron 2002; 91: 203-9. [DOI] [PubMed] [Google Scholar]
- 2).Jones DA, McGill LA, Rathod KS, et al. Characteristics and outcomes of dialysis patients with infective endocarditis. Nephron Clin Pract 2013; 123: 151-6. [DOI] [PubMed] [Google Scholar]
- 3).Kamalakannan D, Pai RM, Johnson LB, et al. Epidemiology and clinical outcomes of infective endocarditis in hemodialysis patients. Ann Thorac Surg 2007; 83: 2081-6. [DOI] [PubMed] [Google Scholar]
- 4).Rekik S, Trabelsi I, Hentati M, et al. Infective endocarditis in hemodialysis patients: clinical features, echocardiographic data and outcome: a 10-year descriptive analysis. Clin Exp Nephrol 2009; 13: 350-4. [DOI] [PubMed] [Google Scholar]
- 5).Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96: 200-9. [DOI] [PubMed] [Google Scholar]
- 6).Shang E, Forrest GN, Chizmar T, et al. Mitral valve infective endocarditis: benefit of early operation and aggressive use of repair. Ann Thorac Surg 2009; 87: 1728-33; discussion 1734. [DOI] [PubMed] [Google Scholar]
- 7).Omoto T, Tedoriya T, Oi M, et al. Significance of mitral valve repair for active-phase infective endocarditis. Asian Cardiovasc Thorac Ann 2011; 19: 149-53. [DOI] [PubMed] [Google Scholar]
- 8).Leither MD, Shroff GR, Ding S, et al. Long-term survival of dialysis patients with bacterial endocarditis undergoing valvular replacement surgery in the United States. Circulation 2013; 128: 344-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Tompkins C, McLean R, Cheng A, et al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovascular Electrophysiol 2011; 22: 1099-104. [DOI] [PubMed] [Google Scholar]
- 10).Kaul TK, Fields BL, Reddy MA, et al. Cardiac operations in patients with end-stage renal disease. Ann Thorac Surg 1994; 57: 691-6. [DOI] [PubMed] [Google Scholar]
- 11).Bianchi G, Solinas M, Bevilacqua S, et al. Are bioprostheses associated with better outcome than mechanical valves in patients with chronic kidney disease requiring dialysis who undergo valve surgery? Interact Cardiovasc Thorac Surg 2012: 15: 473-83. [DOI] [PMC free article] [PubMed] [Google Scholar]