SUMMARY
Splenic infarcts are rare cases. It may not be noticed in the emergency department because the clinical picture is likely to mimic various acute abdominal pains. The splenic infarct is often the result of systemic thromboembolism associated with cardiovascular disorders. The aim of this study is to present an evaluation of the patients that presented to the emergency department (ED) with abdominal pain and were diagnosed with splenic infarct.
Key words: Emergency department, D-dimer, infarct, spleen
Introduction
Splenic infarction is a rare clinical condition. The presentation can mimic other causes of acute abdominal pain. The diagnosis is based on clinical presentation and imaging studies. It is mostly seen in conjunction with hematologic diseases, vascular and thromboembolic disorders. In this cases series we reported splenic infarcts presented with abdominal pain in emergency department.
Case Report
In this study, patients that presented to the adult ED (for patients over 18) of our hospital and were diagnosed with a splenic infarct between January 1, 2009 and January 1, 2013 were analyzed retrospectively. Age, gender, case history, triage category, complaints, pain characteristics, time when the pain started, vital and physical examination findings as well as the results of electrocardiogram (ECG), laboratory tests and radiological imaging were recorded.
Three female and three male patients were included in this study. The average age of patients was 62.17±12.28 (range: 22–90). Table 1 provides the details of case history, examination, laboratory tests, imaging and clinical results.
Table 1.
Demographic and clinical characteristics of patients
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Age | 79 | 26 | 90 | 83 | 22 | 73 |
| Sex | Male | Female | Male | Female | Male | Female |
| Triage category | 2 | 3 | 1 | 1 | 3 | 1 |
| Symptoms | Abdominal pain, constipation | Abdominal pain | Abdominal pain | Abdominal pain, nausea, vomiting and constipation | Abdominal pain | Abdominal pain, nausea, vomiting and constipation |
| Onset of the pain | 4 hours | 3 days | 1 day | 30 days | 2 days | 5 days |
| Medicine/drugs/other | Smoke/alcohol | − | − | − | Smoke, Coumadin | |
| Tension arterial (mmHg) | 140/80 | 110/80 | 120/70 | 110/70 | 80/50 | 110/60 |
| Pulse (/min.) | 79 | 80 | 61 | 101 | 78 | 92 |
| Respiratory rate (/min.) | 24 | 18 | 28 | 22 | 26 | 19 |
| Fever ('C) | 36.3 | 36.5 | 36.1 | 36.4 | 39.6 | 36.3 |
| Past medical history | Asthma, Hypertension | C/S operation (6 months ago), rubella | COPD, DM, CHF | Anaemia (AA) | Aorta valve replacement | CHD, HT |
| EKG | Atrial fibrillation | NSR | NSR | NSR | NSR | NSR, LVH |
| Abdomen examination | Epigastric tenderness rebound and defence | Left upper quadrant tenderness | Diffuse tenderness | Diffuse tenderness | Diffuse tenderness | Epigastric tenderness and defence |
| Leukocytes (/mm3) | 22.000 | 3800 | 12800 | 19680 | 5600 | 6400 |
| D-dimer (μg/dl) | 2047 | 7780 | − | − | − | − |
| Amylase (U/L) | 109 | − | 316 | 164 | 91 | 31 |
| CT findings | Infarct in upper and middle pole | Splenomegaly, infarct in lower and middle pole | Infarct in middle pole | Multiple infarcts in parenchyma | Infarct in middle inferior pole | Infarct in middle upper pole |
| Admission time (day) | 8 | 4 | 12 | 5 | 13 | 7 |
| Conclusion | Discharged | Discharged | Discharged | Discharged | Discharged | Discharged |
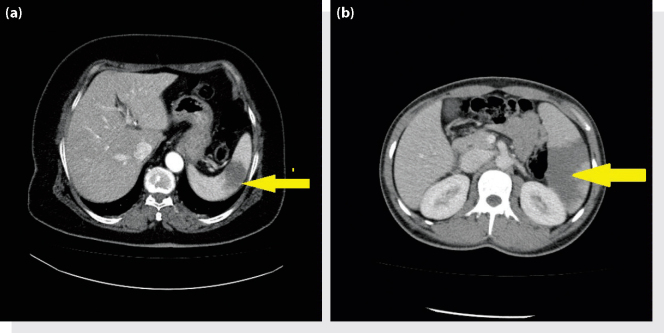
All patients had abdominal pain when they first presented to the ED. Abdominal pain was accompanied by nausea and vomiting in four patients, and by constipation in three patients. The pain started on average 7±4.64 (range: 1–30) days before the patients presented to the hospital. The evaluation of vital findings showed that four patients had tachypnea and one patient had a high fever. The medical history of patients included chronic obstructive pulmonary disease, congestive heart failure, mitral valve replacement, diabetes mellitus, asthma and brucella. According to the ECG results, one patient had atrial fibrillation. The abdominal examination showed three patients had sensitivity throughout, two patients had epigastric sensitivity and one patient had sensitivity in the left upper quadrant while two patients had rebound tenderness and guarding. The laboratory test results of three patients revealed high leucocyte values. The D-dimer test was performed on two patients, which revealed high values (see Table 1). The bedside ultrasound imaging did not indicate any pathological results apart from a hypoechoic lesion in spleen in one patient. All patients underwent an abdominal computerized tomography (CT) scan in the ED, the results of which were evaluated in the same unit. As a result of the CT scan, the radiology specialists reported a significant number of hypodense lesions that were likely associated with an infarct, and these findings were considered to confirm the presence of a splenic infarct (Figure 1a, b). All patients diagnosed with a splenic infarct were hospitalized. The average period of stay in the hospital was 8.16±1.49 (range: 4–13) days. The patients were discharged after conservative treatment, since none of them developed severe complications or mortality.
Figure 1.
(a, b) Splenic infarction.
Discussion
Splenic infarction occurs as a result of tissue necrosis that develops due to parenchymal ischemia, which is a result of the interruption of arterial blood supply to the spleen. The infarct may occur in a segment of the spleen or in the complete spleen. Infiltrative hematologic disorders that cause the congestion of splenic circulation with abnormal cells or thromboembolism constitute the most common (88%) causes of an infarct.[1] It was reported that the rate of splenic infarction development ranges from 50 to 72% in CML and myelofibrosis patients.[1] Splenic infarction may also develop secondary to cardiovascular disorders, autoimmune/collagen tissue diseases, trauma, surgery (pancreatectomy or liver transplantation) or an infection. In 16.6% of patients, it is the first symptom of an underlying disease.[2] In the present study, four patients had a risk factor for thromboembolism (patients taking warfarin due to coronary artery disease, congestive heart failure, diabetes mellitus, chronic obstructive pulmonary disease, hypertension and valve replacement). No hematological diseases were detected according to medical history or clinical follow-up of any patients. As reported in the literature as a rare occurrence, one patient had a active brucella infection.[3]
In patients with a splenic infarct, the clinical presentation may be in the form of non-specific abdominal pain or hemorrhagic shock resulting from massive subscapular bleeding. In some cases, the clinical picture does not provide any indications, and the diagnosis is based on imaging, laparoscopy or laparotomy. The overall symptom is abdominal pain or abdominal pain in the left upper quadrant in two thirds of the patients.2, 4 Nausea and vomiting are also among early symptoms. Abdominal pain can be accompanied by fever, shivering, pleuritic chest pain and left shoulder pain (Kehr finding). Pain often has been occurring for at least one week in half of patients.[2] The most common symptom found on physical examination was left upper quadrant pain. All patients presenting to our clinics had abdominal pain and sensitivity in abdomen. In five patients, the pain lasted for less than one week.
There is no disease-specific laboratory test used for diagnosis. The leucocyte count may be over 12000/m3 in 50% of patients, and 7% of patients may have thrombocytosis.4, 5 In concordance with the literature, the leucocyte values of three patients in our study were high. The D-dimer test was performed for two patients with the pre-diagnosis of mesenteric embolism, and the value was high for both patients. Given that there are a limited number of studies related to the link between D-dimer and splenic infarct in the literature, D-dimer values of cases seems to be high.2, 6 This result leads us to think that because etiology is generally secondary to thromboembolism, D-dimer, a fibrin degradation product found in clotting disorders, may be useful for excluding splenic infarction. However, there is a need for further research. The other laboratory test results did not present any pathological findings.
CT scan with contrast is the best method and option for the diagnosis of splenic infarct. It is also more advantageous than other diagnostic methods for the identification of other pathologies. The possibility of splenic infarct should be considered in patients at risk and with non-specified left upper quadrant pain, and a CT scan should be performed. Magnetic resonance imaging by intravenously injected gadolinium contrast medium is another option. Research on splenic infarcts indicates that ultrasound imaging is a method that may be preferred.[7] Ultrasound is useful if the spleen parenchyma can be identified.[8] In the acute phase of infarction, the incidence of negative imaging is high in B-mod ultrasound scan,[9] and another research puts that its diagnostic value is 18%.[1] In cases where the infarction area is large, color Doppler ultrasound may show the area without blood.[9] Recent studies suggest that in patients with suspected splenic infarct, the incidence of imaging the infarct increases up to 100% with the use of second-generation ultrasound contrast agents.10, 11 In the present case, an abdominal CT scan was used for imaging since the patients’ pain did not alleviate during the follow-up period; the left upper quadrant pain continued on physical examination and the laboratory findings did not provide any pathologies that explained the clinical situation. Before the CT, the patients underwent bedside ultrasound scan, which showed one patient had a hypoechoic lesion with an irregular contour. The results for the other patients did not present any pathological findings or abdominal free fluid. The fact that all patients were discharged from the hospital after conservative treatment may indicate that the clinical situation and lesions were in a mild form. This may explain why the ultrasound imaging failed to show the lesions in other five patients.
The diagnosis of splenic infarct has increased because of using abdominal radiological imaging techniques more frequently[12] and opting for angiographic embolization more commonly in vascular injuries of the spleen.[13]
The present guidance approaches suggest conservative follow-ups in cases of uncomplicated and asymptomatic splenic infarcts. Nevertheless, surgery is preferred in case of complications such as resistant symptoms, bleeding, rupture, abscess and pseudocyst.
Conclusion
Splenic infarction, a cause of abdominal pain, is rarely encountered and often unnoticed. The diagnosis is based on clinical suspicion and imaging. Abdominal CT is the first option for diagnosis. Splenic infarction should certainly be considered in differential diagnosis of patients presenting to the emergency department due to abdominal pain, in view of underlying and risk-posing diseases. A D-dimer test may be used for excluding the diagnosis of splenic infarct; however, there is a need for further research.
Conflict of Interest
The authors declare that there is no potential conflicts of interest.
Footnotes
Published online: March 02, 2015
References
- 1.Nores M, Phillips EH, Morgenstern L, Hiatt JR. The clinical spectrum of splenic infarction. Am Surg. 1998;64:182–188. [PubMed] [Google Scholar]
- 2.Antopolsky M, Hiller N, Salameh S, Goldshtein B, Stalnikowicz R. Splenic infarction: 10 years of experience. Am J Emerg Med. 2009;27:262–265. doi: 10.1016/j.ajem.2008.02.014. CrossRef. [DOI] [PubMed] [Google Scholar]
- 3.Jae Hoon Lee, Yu Min Lee, Chang Hoon Lee, Chang Soo Choi, Tae Hyeon Kim. Splenic Infarction Associated with Brucellosis in a Non-Endemic Area. Infect Chemother. 2010;42(1):48–50. [Google Scholar]
- 4.Jaroch MT, Broughan TA, Hermann RE. The natural history of splenic infarction. Surgery. 1986;100:743–750. [PubMed] [Google Scholar]
- 5.Dahlberg PJ, Frecentese DF, Cogbill TH. Cholesterol embolism: experience with 22 histologically proven cases. Surgery. 1989;105:737–746. [PubMed] [Google Scholar]
- 6.Tomoya Hara, Yamaguchi Koji, Sata Masataka. Massive splenic infarction due to left ventricular apical. Heart Asia. 2012;4:53. doi: 10.1136/heartasia-2011-010070. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goerg C, Schwerk WB. Splenic infarction: sonographic patterns, diagnosis, follow-up, and complications. Radiology. 1990;174:803–807. doi: 10.1148/radiology.174.3.2406785. CrossRef. [DOI] [PubMed] [Google Scholar]
- 8.Goerg C, Schwerk WB. Splenic infarction: sonographic patterns, diagnosis, follow-up, and complications. Radiology. 1990;174:803–807. doi: 10.1148/radiology.174.3.2406785. CrossRef. [DOI] [PubMed] [Google Scholar]
- 9.Caremani M, Occhini U, Caremani A, Tacconi D, Lapini L, Accorsi A. Focal splenic lesions: US findings. J Ultrasound. 2013;16:65–74. doi: 10.1007/s40477-013-0014-0. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen MJ, Huang MJ, Chang WH, Wang TE, Wang HY, Chu CH. Ultrasonography of splenic abnormalities. World J Gastroenterol. 2005;11:4061–4066. doi: 10.3748/wjg.v11.i26.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Görg C, Bert T. Second-generation sonographic contrast agent for differential diagnosis of perisplenic lesions. AJR Am J Roentgenol. 2006;186:621–626. doi: 10.2214/AJR.04.1900. CrossRef. [DOI] [PubMed] [Google Scholar]
- 12.Joshi SC, Pant I, Shukla AN, Anshari MA. Splenic infarct as a diagnostic pitfall in radiology. J Cancer Res Ther. 2008;4:99–101. doi: 10.4103/0973-1482.42262. [DOI] [PubMed] [Google Scholar]
- 13.Pachter HL, Guth AA, Hofstetter SR, Spencer FC. Changing patterns in the management of splenic trauma: the impact of nonoperative management. Ann Surg. 1998;227:708–719. doi: 10.1097/00000658-199805000-00011. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]