Abstract
Although granulopoiesis is accelerated to suppress bacteria during infection, some bacteria can still cause life-threatening infections, but the mechanism behind this remains unclear. In this study, we found that mature neutrophils in bone marrow cells (BMCs) were decreased in C. perfringens-infected mice and also after injection of virulence factor α-toxin. C. perfringens infection interfered with the replenishment of mature neutrophils in the peripheral circulation and the accumulation of neutrophils at C. perfringens-infected sites in an α-toxin-dependent manner. Measurements of bacterial colony-forming units in C. perfringens-infected muscle revealed that α-toxin inhibited a reduction in the load of C. perfringens. In vitro treatment of isolated BMCs with α-toxin (phospholipase C) revealed that α-toxin directly decreased mature neutrophils. α-Toxin did not influence the viability of isolated mature neutrophils, while simultaneous treatment of BMCs with granulocyte colony-stimulating factor attenuated the reduction of mature neutrophils by α-toxin. Together, our results illustrate that impairment of the innate immune system by the inhibition of neutrophil differentiation is crucial for the pathogenesis of C. perfringens to promote disease to a life-threatening infection, which provides new insight to understand how pathogenic bacteria evade the host immune system.
Neutrophils play an important role in the elimination of pathogenic bacteria by phagocytosis, killing and digesting them, which is the first line of defense of the innate immune system1,2,3. Normally, a certain number of neutrophils are sustained in a steady state through granulopoiesis, while the acceleration of granulopoiesis occurs during bacteremia to overwhelm the infection, which is termed emergency granulopoiesis4,5,6.
During infection with a gram-negative bacteria, endothelial cells in various organs sense lipopolysaccharide (LPS) from the bacteria through the MyD88-dependent Toll-like receptor 4 signaling pathway, leading to the activation of granulocyte colony-stimulating factor (G-CSF) release into the systemic circulation7. Endothelial cell-derived G-CSF acts on myeloid precursors, resulting in acceleration of the production of neutrophils in bone marrow and spleen7,8,9. Thus, the host innate immune system is precisely regulated to defeat pathogenic bacteria. Nevertheless, some bacteria cause neutropenia leading to serious and life-threatening infections in a clinical context. As a possible mechanism of neutropenia, blockage of myeloid differentiation during lethal sepsis due to Pseudomonas aeruginosa has been reported10. However, it is less well understood whether blockage of myeloid differentiation by bacterial toxins contributes to the progression of the infection, especially in the early phase of infection.
Clostridium perfringens is a gram-positive, anaerobic pathogenic bacterium11. Of five distinct subgroups, types A to E, C. perfringens type A causes gas gangrene in humans12. Gas gangrene caused by C. perfringens type A is accompanied by the destruction of muscle, shock, multiple organ failure, and death of patients13. C. perfringens infection progresses so rapidly that death precedes diagnosis in some patients, suggesting that C. perfringens can evade host innate immunity. Moreover, C. perfringens infection is characterized by an absence of polymorphonuclear leukocytes at the site of the infection14,15, but it has not been elucidated whether granulopoiesis is properly maintained during the infection.
Of the many toxins produced by C. perfringens, α-toxin is known to be a major virulence factor during infection and has two well-known enzyme activities: phospholipase C (PLC) and sphingomyelinase (SMase)13,16. In this study, to clarify whether the innate immune system is interfered with during C. perfringens type A infection, leading to rapid progression of the disease state, we evaluated granulopoiesis in C. perfringens-infected mice. This study demonstrated that mature neutrophils were decreased by infection in an α-toxin-dependent manner. Here, we demonstrate that C. perfringens α-toxin impairs innate immunity via the inhibition of neutrophil differentiation, which provides a new mechanism to explain how pathogenic bacteria evade the host immune system.
Results
Infection with C. perfringens diminishes mature neutrophils in bone marrow
In mouse bone marrow, granulocytes can be categorized into increasingly mature subsets by the expression of CD11b and Gr-1, as previously described17. Expression profiling of CD11b and Gr-1 in bone marrow cells (BMCs) from naive C57BL/6 mice identified three distinct populations defined as CD11b+Gr-1high, CD11b+Gr-1low, and CD11b−Gr-1+ (Fig. S1A). Giemsa staining of the sorted cells showed that the CD11b+Gr-1high cell population contained mature neutrophils with segmented nuclei, the CD11b+Gr-1low cell population represented an intermediate stage of maturation with incompletely condensed nuclei, and the CD11b−Gr-1+ cell population contained primitive precursor cells with a myeloblast-like morphology (Fig. S1A). Compared with the CD11b+Gr-1high cell population, the CD11b+Gr-1low and CD11b−Gr-1+ cell populations contained cells expressing a high level of cKit, a marker of immaturity18, which is consistent with the results of morphological analysis (Fig. S1B). Thus, the expression levels of CD11b and Gr-1 can be used to represent the stages of neutrophil maturation.
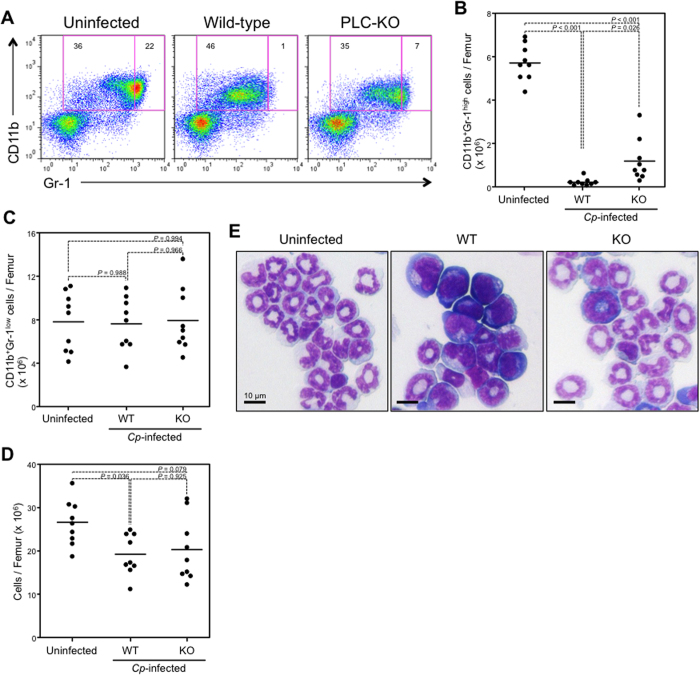
To investigate whether C. perfringens infection affects the maturation of neutrophils, BMCs were isolated from mice intramuscularly injected with C. perfringens type A, and flow cytometry analysis was performed. There were notable decreases in the proportion and number of CD11b+ Gr-1high cells in C. perfringens-infected wild-type strain (Strain 13) mice compared with TGY medium-treated BMCs, whereas the decreases were attenuated in a plc gene-knockout mutant (PLC-KO) C. perfringens-infected mice (Fig. 1A,B). On the other hand, the number of CD11b+Gr-1low cells was not affected by C. perfringens infection (Fig. 1C). Bone marrow cellularity was slightly decreased by infection, reflecting the loss of CD11b+Gr-1high cells (Fig. 1D). Giemsa staining of isolated Gr-1+ cells from C. perfringens-infected BMCs showed that mature neutrophils with segmented nuclei had disappeared in the presence of α-toxin (Fig. 1E). These results indicated that C. perfringens infection preferentially decreases mature neutrophils in bone marrow.
Figure 1. Mature neutrophils in bone marrow are decreased in Clostridium perfringens-infected mice.
Mice were intramuscularly injected with 1 × 107 colony-forming units (CFUs) of C. perfringens Strain 13 (WT, n = 9), PLC-KO (KO, n = 9), or TGY medium as a control (uninfected, n = 9), bone marrow cells (BMCs) were isolated from mice after 24 hours, and flow cytometry analysis was performed using a Guava easyCyte. Representative flow cytometry profile (A), the absolute number of CD11b+Gr-1high mature neutrophils per femur (B), the absolute number of CD11b+Gr-1low immature neutrophils per femur (C), and bone marrow cellularity (D) are shown. Magnetically isolated Gr-1+ cells from BMCs were stained with Giemsa (E). One-way analysis of variance was employed to assess statistical significance.
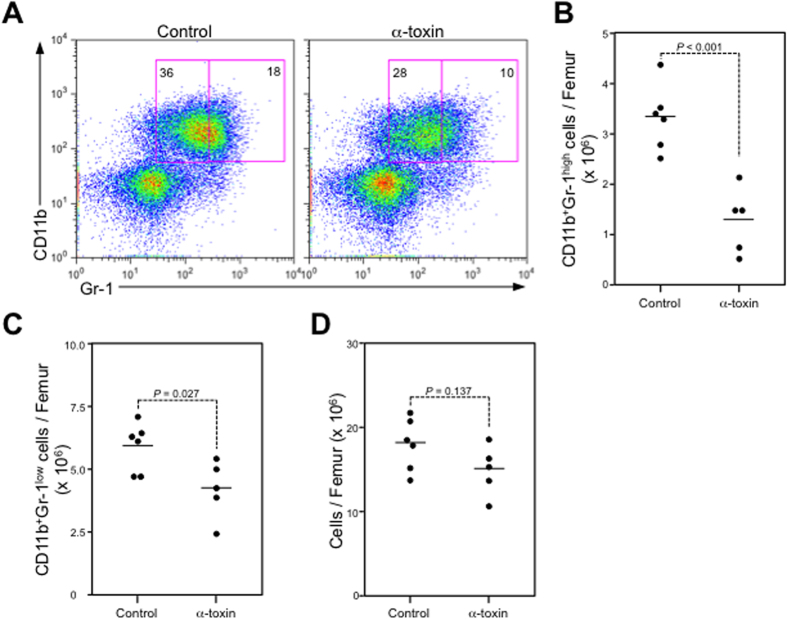
Next, we tested whether α-toxin was sufficient to induce a decrease in mature neutrophils in bone marrow. A single intravenous injection of purified α-toxin greatly decreased the proportion and number of CD11b+Gr-1high cells, whereas those of CD11b+Gr-1low cells were only slightly decreased in α-toxin-injected mice (Fig. 2A–C). Bone marrow cellularity was slightly decreased, but this was not significant (Fig. 2D). These results indicated that α-toxin plays an important role in the marked diminution of mature neutrophils by C. perfringens infection.
Figure 2. Mature neutrophils are decreased after injection with α-toxin.
Mice were injected intravenously with 100 ng of purified α-toxin (α-toxin, n = 5) or Tris-buffered saline containing 0.25% gelatin as a control (control, n = 6), and flow cytometry analysis of BMCs isolated from the mice was performed with a Guava easyCyte. A representative flow cytometry profile is shown (A). The absolute number of CD11b+Gr-1high cells (B), the absolute number of CD11b+Gr-1low cells (C), and bone marrow cellularity (D) were determined. Two-tailed Student’s t-tests were used to assess statistical significance.
α-Toxin interferes with the replenishment of mature neutrophils in the peripheral circulation
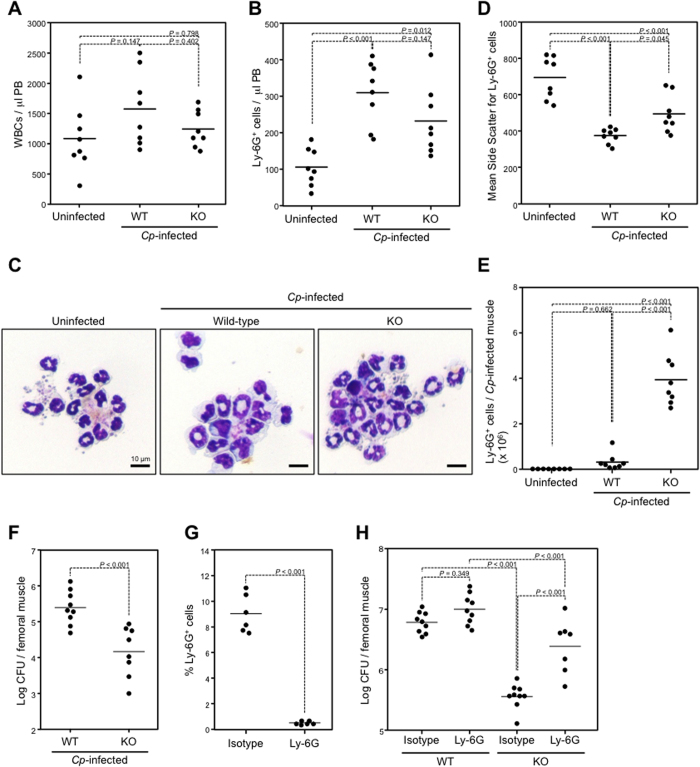
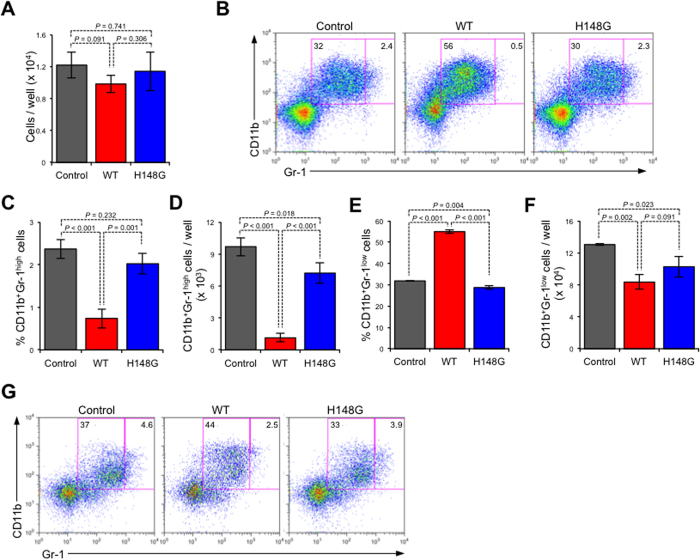
Next, we investigated whether C. perfringens infection influenced the replenishment of mature neutrophils in the peripheral circulation. Although the white blood cell (WBC) number in peripheral blood was not affected by C. perfringens infection, the number of Ly-6G+ neutrophils was increased compared with in uninfected mice (Fig. 3A,B). To assess the maturity of the neutrophils morphologically, we isolated peripheral Ly-6G+ cells from C. perfringens-infected mice and performed Giemsa staining. Figure 3C shows that the cells from uninfected control mice and PLC-KO-infected mice were mature neutrophils, whereas those from Strain 13-infected mice were immature cells with incompletely condensed nuclei. A comparison of side scatter intensity for Ly-6G+ cells, which is proportional to cell granularity, revealed that granulation was reduced by α-toxin producing C. perfringens infection (Fig. 3D). To evaluate the accumulation of neutrophils in C. perfringens-infected femoral muscle, we dissociated the muscle and determined the number of Ly-6G+ neutrophils. The numbers of neutrophils were similar in uninfected and Strain 13-infected muscles, whereas the cells dramatically increased in PLC-KO-infected muscle (Fig. 3E). Moreover, measurements of bacterial colony-forming units (CFUs) revealed that PLC-KO-infected mice were more efficient at reducing the load of C. perfringens compared with Strain 13-infected mice (Fig. 3F). By utilizing an antibody against Ly-6G, neutrophils can be specifically depleted in vivo19,20. To clarify that the accumulated neutrophils in PLC-KO-infected muscle contributed to the reduction in bacterial CFUs, mice were intraperitoneally administrated the antibody 1 day prior to C. perfringens infection. Figure 3G shows that administration of the antibody depleted Ly-6G+ neutrophils in peripheral blood. The depletion of neutrophils did not affect bacterial CFUs in Strain 13-infected muscle, whereas it greatly increased bacterial CFUs in PLC-KO-infected muscle, demonstrating that the accumulated neutrophils in PLC-KO-infected muscle were involved in the reduction of bacterial CFUs (Fig. 3H). Together, these results suggested that α-toxin interferes with the replenishment of mature neutrophils in the peripheral circulation, resulting in host innate immune deficiency.
Figure 3. Clostridium perfringens infection interferes with replenishment of mature neutrophils in peripheral blood.
Mice were injected intramuscularly with 1 × 107 colony-forming units (CFUs) of C. perfringens Strain 13 (WT, n = 8–9), PLC-KO (KO, n = 8–9), or TGY medium as a control (uninfected, n = 8). (A–D) Peripheral white blood cells (WBCs) were isolated 24 hours after the infection. The number of WBCs was determined (A). WBCs were labeled, and the number of Ly-6G+ cells was determined (B). Magnetically isolated Ly-6G+ cells from WBCs were stained with Giemsa (C). Mean side-scatter intensity in the Ly-6G+ cell population is shown (D). (E,F) C. perfringens-infected femoral muscle was dissociated. The number of Ly-6G+ cells (E) and C. perfringens CFUs (F) in the suspension were determined. Mice were administrated intraperitoneally with an antibody against Ly-6G (Ly-6G) (G,H). The next day, the proportion of Ly-6G+ cells in peripheral WBCs was determined (G), and the mice were injected intramuscularly with 1 × 107 CFUs of C. perfringens Strain 13 (WT) or PLC-KO (KO). C. perfringens CFUs in the muscle were determined 24 hours after infection (H). Rat IgG2a was used as an isotype control antibody (Isotype). One-way analysis of variance (A,B,D,E,H) or two-tailed Student’s t-test (F,G) were employed to assess statistical significance.
α-Toxin directly affects mature neutrophils in vitro
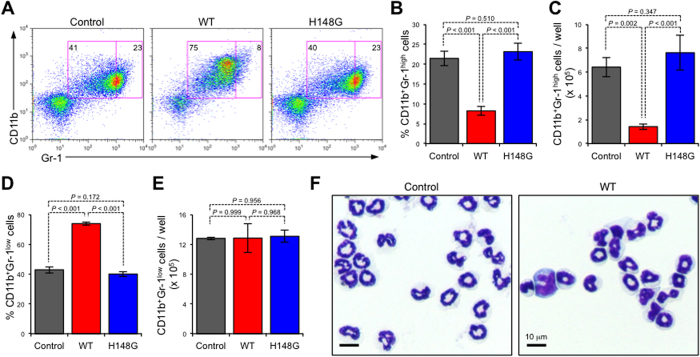
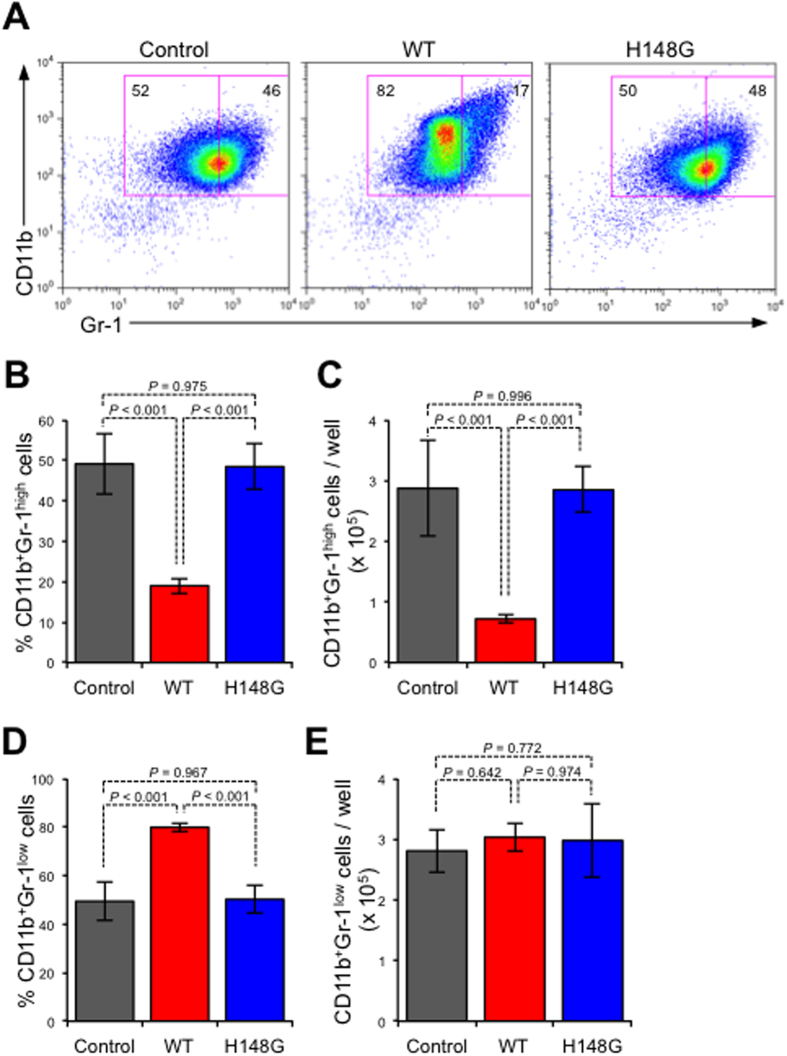
It has been reported that granulopoiesis could be ectopically affected by bacterial components during infection7. To test whether mature neutrophils were directly affected by α-toxin, we treated isolated BMCs with α-toxin in vitro. In α-toxin-treated BMCs, the proportion and number of CD11b+Gr-1high cells were significantly decreased, whereas the proportion of CD11b+Gr-1low cells was increased (Fig. 4A–D). Because the absolute numbers of CD11b+Gr-1low cells were similar between α-toxin-treated and untreated conditions, the increase in the CD11b+Gr-1low cell population in α-toxin-treated BMCs reflected the loss of CD11b+Gr-1high cells (Fig. 4D,E). Previously, we reported that a variant α-toxin (H148G) lacked PLC and SMase activities21. The H148G variant α-toxin lost the ability to decrease the proportion and number of CD11b+Gr-1high cells, demonstrating that α-toxin affects neutrophils in its enzyme activities-dependent manner (Fig. 4A–C). The disappearance of morphologically mature neutrophils in isolated Gr-1+ cells from α-toxin-treated BMCs was consistent with the results of the flow cytometry analysis (Fig. 4F). These results indicated that α-toxin directly influences BMCs to decrease the number of mature neutrophils.
Figure 4. α-Toxin directly affects bone marrow cells.
A total of 5 × 106 bone marrow cells (BMCs; n = 3 per condition) were cultured for 24 hours in the presence or absence (control) of 100 ng/ml α-toxin (WT) or a variant α-toxin (H148G). Representative flow cytometry profile (A), the frequency and absolute number of CD11b+Gr-1high neutrophils per culture well (B,C), and the frequency and absolute number of CD11b+Gr-1low neutrophils per culture well (D,E) are shown. Magnetically isolated Gr-1+ cells from cultured BMCs were stained with Giemsa (F). Values are mean ± standard deviation. One-way analysis of variance was employed to assess statistical significance.
Next, we investigated the effect of α-toxin on isolated Gr-1+ cells. Almost all of the isolated Gr-1+ cells co-expressed CD11b, which means that the cells were CD11b+Gr-1+ cells (Fig. S2). When the CD11b+Gr-1+ cells were treated with α-toxin, the proportion and number of CD11b+Gr-1high cells, but not CD11b+Gr-1low cells, were decreased (Fig. 5). Also, the H148G variant α-toxin did not affect the proportion and number of CD11b+Gr-1high cells.
Figure 5. α-Toxin directly affects neutrophils.
A total of 1 × 106 magnetically isolated Gr-1+ cells (n = 6 per condition) were cultured for 24 hours in the presence or absence (Control) of 100 ng/ml α-toxin (WT) or a variant α-toxin (H148G). Representative flow cytometry profile (A), the frequency and absolute number of CD11b+Gr-1high neutrophils per culture well (B,C), and the frequency and absolute number of CD11b+Gr-1low neutrophils per culture well (D,E) are shown. Values are mean ± standard deviation. One-way analysis of variance was employed to assess statistical significance.
To test whether α-toxin spreads to bone marrow and binds to neutrophils in C. perfringens-infected mice, we immunostained Gr-1+ cells isolated from mice with an antibody against α-toxin. Figure S3 shows that α-toxin was detected in cells from Strain 13-infected mice but not uninfected or PLC-KO-infected mice. Together, these results indicated that α-toxin directly affects neutrophils.
α-Toxin inhibits neutrophil differentiation
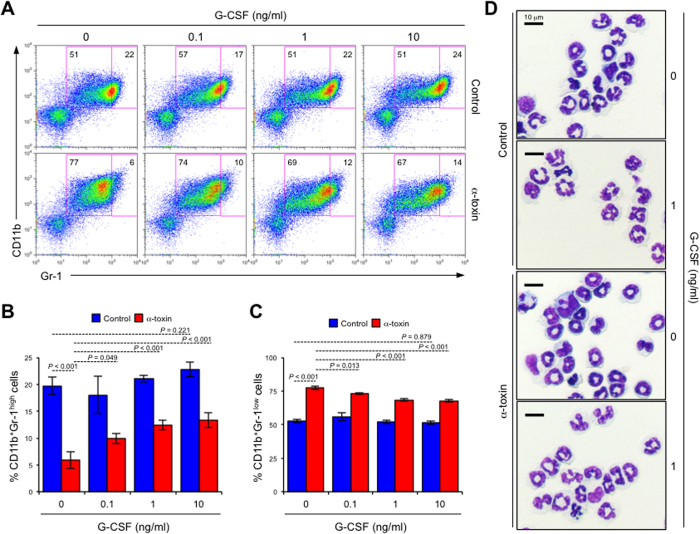
At least two possible reasons for the α-toxin-induced decrease in mature neutrophils were suggested. One is that α-toxin specifically leads to cell death in CD11b+Gr-1high cells, and the other is that it blocks the differentiation of immature neutrophils. We evaluated the number of viable cells in sorted CD11b+Gr-1high cells after α-toxin treatment by counting and found that α-toxin did not influence the viability of the cells, showing that α-toxin has no apparent cytotoxicity with mature neutrophils (Fig. 6A). Next, we tested whether α-toxin blocked the production of CD11b+Gr-1high cells using CD11b+Gr-1high cell-depleted BMCs. As shown in Fig. S2, CD11b+Gr-1high cells had been successfully removed from whole BMCs. After a 24-hour incubation of the cells, approximately 2.4% of CD11b+Gr-1high cells emerged in the control medium, whereas cells treated with α-toxin contained less than 1% CD11b+Gr-1high cells (Fig. 6B,C). The proportion and number of CD11b+Gr-1high cells were not affected by treatment of the H148G variant α-toxin (Fig. 6B,C). The absolute number of CD11b+Gr-1high cells decreased markedly in α-toxin-treated cells, but not in the H148G variant α-toxin-treated cells (Fig. 6D). On the other hand, α-toxin treatment induced an increase in the proportion of CD11b+Gr-1low cells, while the absolute number of CD11b+Gr-1low cells was decreased slightly (Fig. 6E,F). A similar result was obtained by using sorted CD11b−Gr-1+ cells (Fig. 6G). Furthermore, simultaneous treatment of BMCs with α-toxin and G-CSF, which is known to promote neutrophil differentiation22,23, attenuated the reduction in the proportion of CD11b+Gr-1high cells or the increase in that of CD11b+Gr-1low cells by α-toxin in a dose-dependent manner (Fig. 7A–C). Morphological analysis of the isolated Gr-1+ cells revealed that cells treated with α-toxin and G-CSF were more-fully differentiated than those treated with α-toxin alone (Fig. 7D). These results demonstrated that G-CSF induces the differentiation of immature neutrophils treated with α-toxin. Together, our results strongly suggested that blockage of neutrophil differentiation is involved in the reduction of mature neutrophils by α-toxin. Because the H148G variant α-toxin lost the ability to inhibit the differentiation of neutrophils, enzyme activities are necessary for α-toxin to impair neutrophil differentiation.
Figure 6. α-Toxin reduces the production but not cell viability of mature neutrophils in vitro.
A total of 5 × 104 sorted CD11b+Gr-1high cells (A), n = 6 per condition), 7.5 × 105 CD11b+Gr-1high-cell-depleted bone marrow cells (BMCs) (B–F), n = 3 per condition), or 1 × 105 CD11b−Gr-1+ cells (G) were cultured for 24 hours in the presence or absence (Control) of 100 ng/ml α-toxin (WT) or a variant α-toxin (H148G). The number of viable cells per culture well was counted after trypan blue staining (A). Representative flow cytometry profile (B,G), the frequency and absolute number of CD11b+Gr-1high neutrophils per culture well (C,D), and the frequency and absolute number of CD11b+Gr-1low neutrophils per culture well (E,F) are shown. Freshly isolated BMCs were used as the gating reference (B). Values are mean ± standard deviation. One-way analysis of variance was employed to assess statistical significance.
Figure 7. G-CSF induces differentiation of immature neutrophils treated with α-toxin.
A total of 5 × 106 BMCs (n = 4 per condition) were cultured for 24 hours in the presence or absence (control) of 100 ng/ml α-toxin (α-toxin) and the indicated concentration of purified mouse G-CSF. Representative flow cytometry profile (A), the frequencies of CD11b+Gr-1high neutrophils (B) and CD11b+Gr-1low neutrophils (C) are shown. Magnetically isolated Gr-1+ cells from cultured BMCs were stained with Giemsa (D). Values are mean ± standard deviation. One-way analysis of variance was employed to assess statistical significance.
Discussion
The clinical situation in C. perfringens-infected patients is complex. Various pathogenic bacteria are occasionally identified in C. perfringens-infected patients, meaning that polymicrobial infection is likely to occur clinically24. In addition, C. perfringens infection is characterized by an absence of polymorphonuclear leukocytes at the site of the infection14,15. In this study, we found that C. perfringens infection reduced mature neutrophils in bone marrow, peripheral blood, and C. perfringens-infected muscle in an α-toxin-dependent manner. These results suggested that α-toxin impairs granulopoiesis and interferes with the replenishment of mature neutrophils in the peripheral circulation leading to reduced recruitment of neutrophils to the C. perfringens infection site. Thus, C. perfringens infection impairs the host immune system via the systemic reduction of mature neutrophils, which could explain polymicrobial infection in patients and the absence of polymorphonuclear leukocytes at the site of infection.
C. perfringens infection markedly reduced the number of mature neutrophils in bone marrow, while a plc gene-knockout only partially attenuated the reduction. These results suggested that some other bacterial components also contribute to this phenomenon. Perfringolysin O, a cholestrerol-dependent cytolysin, is known as a major toxin produced by C. perfringens type A strains25,26. Purified perfringolysin O has been shown to be cytotoxic to polymorphonuclear leukocytes and macrophages27,28,29. C. perfringens is also known to produce other toxins and enzymes including a collagenase, hyaluronicdase, sialidases and the cysteine protease α-clostripain25,30. In the present study, we have not tested whether these bacterial components affect the number of mature neutrophils in bone marrow. Therefore, the possibility cannot be excluded that not only α-toxin but also the other bacterial components affect production or cell viability of neutrophils in C. perfringens-infected mice.
C. perfringens infection slightly increased the number of Ly-6G+ neutrophils in peripheral blood, but morphological analysis of the Ly-6G+ cells revealed that the increased neutrophils were immature cells with incompletely condensed nuclei. Also, a comparison of side scatter intensity for Ly-6G+ cells revealed that granulation was reduced in C. perfringens-infected peripheral neutrophils, and a plc gene-knockout slightly attenuated the reduction. Notably, the numbers of infiltrated neutrophils were similar in uninfected and Strain 13-infected muscles, whereas the cells dramatically increased in PLC-KO-infected muscle, demonstrating that the infiltration of neutrophils into C. perfringens-infected muscle was almost completely inhibited by α-toxin. These results suggested that α-toxin-induced impairment of neutrophil maturation might be insufficient to account for the dramatic inability of the cells to migrate to the infected sites. It has been reported that α-toxin mediates formation of platelet-leukocyte aggregates leading to vascular occlusion and induces a marked reduction in microvascular perfusion31,32. The platelet-leukocyte aggregates were also reported to impede neutrophil extravasation33. Therefore, the possibility cannot be excluded that the dramatic reduction of infiltrated neutrophils into C. perfringens-infected muscle is due not only to the impairment of neutrophil maturation, but also to reductions in microvascular perfusion and neutrophil extravasation.
In the present study, we demonstrated that the absolute number of CD11b+Gr-1high cells was greatly decreased by α-toxin in vitro, whereas α-toxin did not influence the viability of the cells. Stevens et al. reported that α-toxin had no apparent toxic effect on human polymorphonuclear leukocytes27. A possible explanation for the decrease in mature neutrophils by α-toxin with no direct cytotoxicity is that the half-life of neutrophils is very short. As shown in Fig. 6A, a total of 5 × 104 sorted CD11b+Gr-1high cells were cultured in the experiment, and only around 25% of the cells were still alive after 24 hours in control medium, suggesting that the half-life of mature neutrophils is much less than 24 hours. Basu et al. reported that the half-life of peripheral neutrophils was 11.4 hours in mouse blood34, which is consistent with our result. These results suggested that a certain number of mature neutrophils are sustained through granulopoiesis in vivo and in vitro. Therefore, the blockage of neutrophil differentiation by α-toxin can cause a decrease in the absolute number of mature neutrophils without cytotoxicity. The reduction in mature neutrophils in bone marrow by C. perfringens infection could be explained by the same reason. Together, continuous granulopoiesis is necessary to sustain a certain number of mature neutrophils during bacterial infection, and the findings in this study suggested that the blockage of differentiation leads to a reduction in cells numbers in a short period of time.
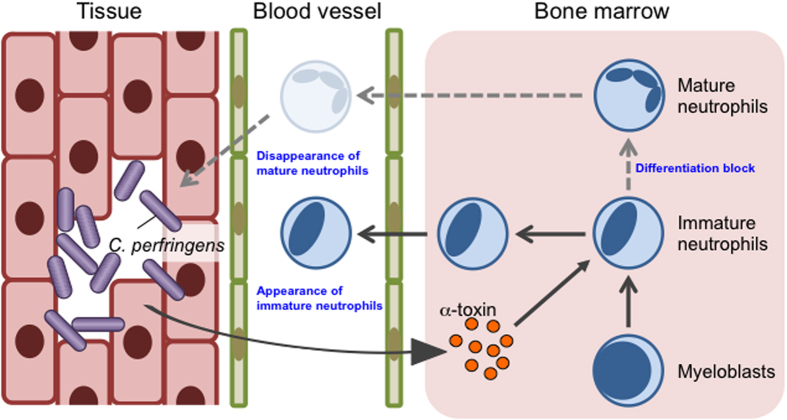
A growing body of scientific evidence has indicated that the host innate immune system is precisely regulated via the activation of granulopoiesis in the fight against infection4; however, the mechanisms by which some bacteria overwhelm the immune system to cause serious and life-threatening infection are still poorly understood. Here, we demonstrated that C. perfringens type A infection impaired neutrophil differentiation to cause a reduction in mature neutrophils in bone marrow in an α-toxin dependent manner. Moreover, we found that α-toxin interfered with the replenishment of mature neutrophils in the peripheral circulation accompanied by decreased efficiency at reducing the load of C. perfringens in infected muscle, suggesting that the innate immune system is impaired by α-toxin. Together, our results illustrate that the impairment of the innate immune system by the inhibition of granulopoiesis is crucial for the pathogenesis of C. perfringens to promote disease to a life-threatening infection (Fig. 8). We hope that our results provide new insight to understand how pathogenic bacteria evade the host immune system.
Figure 8. Model of impaired innate immunity by inhibition of granulopoiesis in a Clostridium perfringens-infected host.
Infection with C. perfringens diminishes mature neutrophils in bone marrow leading to impairment of replenishment of mature neutrophils in the peripheral circulation, resulting in host innate immune deficiency. The major virulence factor of C. perfringens, α-toxin, plays an important role in this phenomenon by directly blocking neutrophil differentiation. Together, impairment of the innate immune system by the inhibition of granulopoiesis is crucial for the pathogenesis of C. perfringens.
Methods
Mice
C57BL/6J mice were purchased from Charles River Laboratories Japan, Inc. The mice were kept in a specific pathogen-free animal facility at Tokushima Bunri University. For all experiments, mice aged more than 8 weeks old were used. Animal experiments were approved by the Animal Care and Use Committee of Tokushima Bunri University, and procedures were performed in accordance with institutional guidelines (approval numbers: 14-2 and 15-3). The institutional guidelines conform to the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, 2006.
Reagents and strains
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or allophycocyanin (APC)-conjugated specific antibodies against mouse CD11b (clone M1/70), Ly-6G/6C (Gr-1, clone RB6-8C5), Ly-6G (clone 1A8), or CD117 (cKit, clone 2B8), and purified rat anti-mouse CD16/CD32 (Fc Block) were purchased from BD Biosciences. A specific antibody against C. perfringens α-toxin was prepared as described previously35. Giemsa’s azur eosin methylene blue solution was purchased from Merck. Mouse G-CSF was from Miltenyi Biotec. All other chemicals were of the highest grade available from commercial sources. C. perfringens wild-type Strain 13 and Bacillus subtilis ISW1214 were used in this study.
Construction of mutant strain
The EcoRI-HindIII fragment containing the plc gene was cloned into pUC19 (pOT01). A 2.1 kb BamHI-BglII fragment containing the tetracycline resistance gene was inserted into the BamHI site of pOT01 located in the internal region of the plc gene36. The resultant plasmid, pOT11, was used to transform C. perfringens Strain 13. Transformants resulting from homologous recombination were screened on blood agar plates containing 2.5 μg/ml tetracycline, and α-toxin negative colonies were picked up by checking hemolysis. Allelic-exchange mutation of the plc gene due to a double cross-over recombination was confirmed by Southern blotting. Then, transformants were cultured on egg-yolk agar plates and lecithinase activity was checked.
Purification of wild-type and variant α-toxin
Purification of wild-type or H148G variant α-toxin was performed as described previously21,37. Briefly, recombinant forms of pHY300PLK harboring the structural genes of wild-type or H148G variant α-toxin were introduced into B. subtilis ISW1214 by transformation, and the transformants were cultured in Luria-Bertani broth containing 50 μg/ml ampicillin with continuous aeration. Wild-type or H148G variant α-toxin secreted into the culture medium was purified chromatographically.
Bone marrow cell isolation and culture
BMCs were isolated by crushing femurs and tibias in phosphate-buffered saline (PBS) supplemented with 2% heat-inactivated fetal bovine serum (FBS; AusGeneX), and filtered through a 40 μm mesh. Red blood cells were hemolyzed with lysis buffer (ACK lysing buffer, GIBCO). The number of living cells was counted after trypan blue staining. Isolated BMCs were cultured at 37 °C in RPMI 1640 medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Bacterial culture and infection
C. perfringens Strain 13 or PLC-KO was grown in TGY medium in anaerobic conditions at 37 °C. Exponentially growing bacteria were harvested, washed, re-suspended in TGY medium, and injected into the left femoral muscle of mice. To quantify CFUs, residual bacteria were serially diluted, plated on BHI agar plates, and cultured anaerobically at 37 °C. BMCs were isolated from the right femur of the mice 24 hours after injection.
Dissociation of C. perfringens-infected femoral muscle
C. perfringens-infected femoral muscle was isolated 24 hours after the infection. To quantify C. perfringens CFUs, isolated muscle was cut into small pieces of 2–4 mm in TGY medium and dissociated in a gentleMACS C tube (Miltenyi Biotec) using a gentle MACS dissociator (Miltenyi Biotec). The supernatant was serially diluted, plated on BHI agar plates, and cultured anaerobically at 37 °C.
To determine the number of Ly-6G+ cells in the infected muscle, isolated muscle was dissociated using Skeletal Muscle Dissociation Kit (Miltenyi Biotec) in accordance with the manufacturer’s protocol. Briefly, the muscle was cut into small pieces in Dulbecco’s modified Eagle’s medium (DMEM) medium containing Enzyme A, D, and P, and dissociated using a gentle MACS dissociator. The cell suspension was filtered through a 40 μm mesh after red blood cells were hemolyzed with lysis buffer. Flow cytometry analysis was performed as described below.
Neutrophil depletion
Depletion of neutrophils in mice was performed as described previously19. An antibody (500 μg) against mouse Ly-6G (clone 1A8) (Bio X Cell) was administered intraperitoneally into mice prior to infection with C. perfringens. Rat IgG2a (clone 2A3) (Bio X Cell) was used as an isotype control antibody.
Flow cytometry analysis
Antibodies described in the Reagents and Strains section were diluted with PBS containing 2% FBS and used to label cells after blocking Fc-receptors with purified rat anti-mouse CD16/CD32. The labeled cells were analyzed or sorted using a FACS Aria II (BD Biosciences) or a Guava easyCyte (Millipore). Data were analyzed using FlowJo (Tree Star) software.
Magnetic cell isolation
Gr-1+ or Ly-6G+ cell isolation were performed using an EasySep system (StemCell Technologies) in accordance with the manufacturer’s protocol, with some modifications. In brief, cells were labeled with PE-conjugated specific antibodies against Gr-1 or Ly-6G. Antibody conjugation to magnetic nanoparticles was achieved through incubation with EasySep PE Selection cocktail followed by additional incubation with EasySep Magnetic Nanoparticles. The cells were separated using EasySep Magnet (StemCell Technologies). To deplete CD11b+Gr-1high cells from BMCs, the sequential separation procedure was repeated twice.
Immunofluorescence microscopy
Isolated Gr-1+ cells were cytospinned onto microscopic glass slides, fixed with 4% paraformaldehyde in PBS at room temperature for 15 min, and blocked with Blocking One Histo (Nacalai Tesque, Inc.). The samples were then incubated with a primary antibody against α-toxin. After washing, samples were incubated with the secondary antibody conjugated with Alexa Fluor 488 (Molecular Probes). Nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI). Images were captured on a confocal laser-scanning fluorescence microscope (Nikon A1, Nikon instruments).
Statistical analysis
All statistical analyses were performed with Easy R (Saitama Medical Center, Jichi Medical University)38. Differences between two groups were evaluated using two-tailed Student’s t-test. One-way analysis of variance (ANOVA) followed by the Tukey test was used to evaluate differences among three or more groups. Differences were considered to be significant for values of P < 0.05.
Additional Information
How to cite this article: Takehara, M. et al. Clostridium perfringens α-Toxin Impairs Innate Immunity via Inhibition of Neutrophil Differentiation. Sci. Rep. 6, 28192; doi: 10.1038/srep28192 (2016).
Supplementary Material
Acknowledgments
This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank Kyohei Oishi and Yoshino Fujihara for technical assistance. Some experiments with the FACS Aria II were performed at the Institute for Genome Research, The University of Tokushima. We thank the members of the Institute for helpful assistance.
Footnotes
Author Contributions M.T. and M.N. designed the study, supervised experiments, performed experiments and analyses, and wrote the manuscript. T.T. and S.S. performed murine infection studies. K.M. contributed to the design of the study. K.O. and T.S. constructed a mutant strain used in the study. K.K. performed in vitro experiments.
References
- Amulic B., Cazalet C., Hayes G. L., Metzler K. D. & Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30, 459–489 (2012). [DOI] [PubMed] [Google Scholar]
- Dale D. C., Boxer L. & Liles W. C. The phagocytes: neutrophils and monocytes. Blood 112, 935–945 (2008). [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E. & Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13, 159–175 (2013). [DOI] [PubMed] [Google Scholar]
- Manz M. G. & Boettcher S. Emergency granulopoiesis. Nat Rev Immunol 14, 302–314 (2014). [DOI] [PubMed] [Google Scholar]
- Wirths S., Bugl S. & Kopp H. G. Neutrophil homeostasis and its regulation by danger signaling. Blood 123, 3563–3566 (2014). [DOI] [PubMed] [Google Scholar]
- Nauseef W. M. & Borregaard N. Neutrophils at work. Nat Immunol 15, 602–611 (2014). [DOI] [PubMed] [Google Scholar]
- Boettcher S. et al. Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood 124, 1393–1403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burberry A. et al. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe 15, 779–791 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos A. D. & Watowich S. S. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42, 277–288 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S. et al. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood 114, 4064–4076 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer J. G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev 9, 216–234 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L., Gibert M. & Popoff M. R. Clostridium perfringens: toxinotype and genotype. Trends Microbiol 7, 104–110 (1999). [DOI] [PubMed] [Google Scholar]
- Bryant A. E. Biology and pathogenesis of thrombosis and procoagulant activity in invasive infections caused by group A streptococci and Clostridium perfringens. Clin Microbiol Rev 16, 451–462 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. L., Tweten R. K., Awad M. M., Rood J. I. & Bryant A. E. Clostridial gas gangrene: evidence that alpha and theta toxins differentially modulate the immune response and induce acute tissue necrosis. The Journal of infectious diseases 176, 189–195 (1997). [DOI] [PubMed] [Google Scholar]
- Bahl H. & Dürre P. Clostridia: biotechnology and medical applications. (Wiley-VCH, 2001). [Google Scholar]
- Sakurai J., Nagahama M. & Oda M. Clostridium perfringens alpha-toxin: characterization and mode of action. J Biochem 136, 569–574 (2004). [DOI] [PubMed] [Google Scholar]
- Ueda Y., Kondo M. & Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med 201, 1771–1780 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edling C. E. & Hallberg B. c-Kit–a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol 39, 1995–1998 (2007). [DOI] [PubMed] [Google Scholar]
- Carr K. D. et al. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. European journal of immunology 41, 2666–2676 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley J. M., Thomay A. A., Connolly M. D., Reichner J. S. & Albina J. E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of leukocyte biology 83, 64–70 (2008). [DOI] [PubMed] [Google Scholar]
- Nagahama M., Okagawa Y., Nakayama T., Nishioka E. & Sakurai J. Site-directed mutagenesis of histidine residues in Clostridium perfringens alpha-toxin. J Bacteriol 177, 1179–1185 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokowa J. et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med 15, 151–158 (2009). [DOI] [PubMed] [Google Scholar]
- von Vietinghoff S. & Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol 181, 5183–5188 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley A. J. Necrotizing soft tissue infections: a primary care review. Am Fam Physician 68, 323–328 (2003). [PubMed] [Google Scholar]
- Rood J. I. Virulence genes of Clostridium perfringens. Annual review of microbiology 52, 333–360 (1998). [DOI] [PubMed] [Google Scholar]
- Verherstraeten S. et al. Perfringolysin O: The Underrated Clostridium perfringens Toxin? Toxins 7, 1702–1721 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. L., Mitten J. & Henry C. Effects of alpha and theta toxins from Clostridium perfringens on human polymorphonuclear leukocytes. The Journal of infectious diseases 156, 324–333 (1987). [DOI] [PubMed] [Google Scholar]
- Stevens D. L. & Bryant A. E. Role of theta toxin, a sulfhydryl-activated cytolysin, in the pathogenesis of clostridial gas gangrene. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 16 Suppl 4, S195–199 (1993). [DOI] [PubMed] [Google Scholar]
- O’Brien D. K. & Melville S. B. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infection and immunity 72, 5204–5215 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T. et al. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proceedings of the National Academy of Sciences of the United States of America 99, 996–1001 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant A. E. et al. Clostridial gas gangrene. II. Phospholipase C-induced activation of platelet gpIIbIIIa mediates vascular occlusion and myonecrosis in Clostridium perfringens gas gangrene. The Journal of infectious diseases 182, 808–815 (2000). [DOI] [PubMed] [Google Scholar]
- Hickey M. J. et al. Molecular and cellular basis of microvascular perfusion deficits induced by Clostridium perfringens and Clostridium septicum. PLoS pathogens 4, e1000045 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant A. E. et al. Clostridium perfringens phospholipase C-induced platelet/leukocyte interactions impede neutrophil diapedesis. Journal of medical microbiology 55, 495–504 (2006). [DOI] [PubMed] [Google Scholar]
- Basu S., Hodgson G., Katz M. & Dunn A. R. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 100, 854–861 (2002). [DOI] [PubMed] [Google Scholar]
- Nagahama M., Nakayama T., Michiue K. & Sakurai J. Site-specific mutagenesis of Clostridium perfringens alpha-toxin: replacement of Asp-56, Asp-130, or Glu-152 causes loss of enzymatic and hemolytic activities. Infection and immunity 65, 3489–3492 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Ba-Thein W., Tamaki M. & Hayashi H. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J Bacteriol 176, 1616–1623 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M. et al. A recombinant carboxy-terminal domain of alpha-toxin protects mice against Clostridium perfringens. Microbiol Immunol 57, 340–345 (2013). [DOI] [PubMed] [Google Scholar]
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48, 452–458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.