Abstract
Innate immune cells have a critical role in defense against infection and disease. Central to this is the broad specificity with which they can detect pathogen-associated patterns and danger-associated patterns via the pattern recognition receptors (PRRs) they express. Several families of PRRs have been identified including: Toll-like receptors (TLRs), C-type lectin-like receptors, retinoic acid-inducible gene-like receptors and nucleotide-binding oligomerization domain–like receptors. TLRs are one of the most largely studied families of PRRs. The binding of ligands to TLRs on antigen presenting cells (APCs), mainly dendritic cells, leads to APC maturation, induction of inflammatory cytokines and the priming of naive T cells to drive acquired immunity. Therefore, activation of TLRs promotes both innate inflammatory responses and the induction of adaptive immunity. Consequently, in the last two decades mounting evidence has inextricably linked TLR activation with the pathogenesis of immune diseases and cancer. It has become advantageous to harness these aspects of TLR signaling therapeutically to accelerate and enhance the induction of vaccine-specific responses and also target TLRs with the use of biologics and small molecule inhibitors for the treatment of disease. In these respects, TLRs may be considered a ‘Swiss Army' knife of the immune system, ready to respond in a multitude of infectious and disease states. Here we describe the latest advances in TLR-targeted therapeutics and the use of TLR ligands as vaccine adjuvants.
Toll-like receptors and signaling
Toll-like receptors (TLRs) are type I transmembrane proteins.1 10 human TLRs have been classified (TLR1–TLR10) and 12 in mouse (TLR1–9, TLR11–13).2 They form a part of the Toll/interleukin-1 (TIR) superfamily that includes the interleukin-1 receptors (IL-1Rs) because of the shared homology of their cytoplasmic domains. However, the extracellular domain of IL-1Rs consist of an immunoglobulin G (IgG) domain, while TLR extracellular domains are made up of tandem repeats of leucine-rich regions termed leucine-rich repeats. The arrangement of leucine-rich repeats confers a unique combinatorial code to each TLR allowing it to bind a specific ligand. They are expressed by a variety of cell types and are distinguished by their ligand specificity, signal transduction and cellular localization.3 TLRs are localized to either the cell surface (TLR1, TLR2, TLR4, TLR5 and TLR6) or intracellular compartments (TLR3, TLR7, TLR8 and TLR9). The location of any given TLR is related to the origin of the ligand it recognizes. TLRs on the cell surface are largely involved in the detection of bacterial products in the extracellular space, while endosomal TLRs detect nucleic acids of viral and bacterial origin. In addition, localization is also important for the discrimination between ‘self' and ‘non-self'. In contrast to most TLR ligands, nucleic acids can be of self and foreign origin. Studies have demonstrated that a chimeric TLR9 consisting of a transmembrane and cytoplasmic domain of TLR4 localizes to the plasma membrane.4 Here it is able to detect and respond to mammalian DNA, yet remain unresponsive to viral nucleic acids. Endogenous TLR9 is not exposed to mammalian DNA and can only be activated by viral DNA ingested and acidified within endosomes.
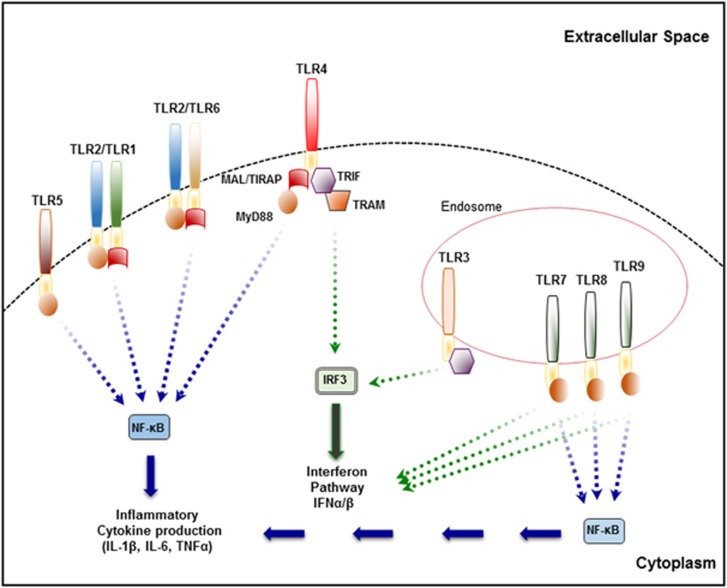
The recognition of pathogen-associated patterns or danger-associated patterns by TLRs results in the activation of signaling pathways that induce the upregulation of cytokines, chemokines and costimulatory molecules. Specifically, binding of ligand initiates the dimerization of two TLR receptor chains and conformational changes that allow the recruitment of TIR domain adaptor molecules to the cytoplasmic TIR domains of TLRs. Differential recruitment of specific adaptor proteins MyD88, MyD88 adaptor like (Mal, also known as TIR domain-containing adaptor protein, TIRAP),5 TIR-domain containing adaptor-inducing interferon-β (TRIF) or TRIF-related adaptor molecule drives subsequent signaling.6 This results in the activation of a number of downstream pathways, including nuclear factor kappa B (NF-κB), interferon regulatory factor (IRF) and mitogen-activated protein kinase (MAPK) pathways to induce type I interferons (IFNs), chemokines and cytokines.
The binding of ligand to a TLR receptor complex and subsequent recruitment of adaptors to active TIR domains of TLRs leads to the activation of two major signaling cascades, namely the MyD88-dependant and MyD88-independent pathways (also referred to as the TRIF-dependant pathway).7 The MyD88-dependent pathway results in nuclear translocation of NF-κB and induction of pro-inflammatory cytokines (for example, IL-6 and tumor necrosis factor-α (TNFα)), while the MyD88-independent pathway mediates induction of the anti-viral type I IFNs (for example, IFNα/β/γ) and IFN-inducible genes via IRFs (for example, IRF3/7). Therefore, the MyD88-dependant pathway is involved in inflammatory responses and the MyD88-independent pathway is predominantly responsible for anti-viral responses. All TLRs with the exception of TLR3 are known to recruit MyD88 and activate the MyD88-dependent pathway activating MAPKs and NF-κB.7 TLR3 typically activates IRFs and the expression of IFNs from endocytic compartments via TRIF. Signaling via TLR4 is unique in that it activates both the MyD88-dependant and –independent pathways. TLR4 utilizes MyD88 and Mal to activate NF-κB and yet can also activate IRFs via recruitment of TRIF-related adaptor molecule and TRIF (Figure 1). In addition to MyD88, TLR2 and TLR4 require Mal to activate the MyD88-dependant pathway.5, 8 MyD88 is essential for type 1 IFN production through the endosomal TLRs, TLR7 and TLR9.9 However, recent studies have highlighted a role for Mal in signaling via TLR7 and TLR9,10 although the mechanism of this signaling is not well understood.
Figure 1.
Toll-like receptor signaling.
Understanding the recognition and interaction of ligands by TLRs and their subsequent activation is important for designing therapeutic compounds that can target TLRs during inflammatory disease. Most ligands are by nature complex whether they are components from bacteria, viruses, fungi or the host. The potency of a ligand relies on its ability to induce homo- or hetero-dimerization and/or conformational change of receptor chains. Direct binding of several TLRs to their known ligands has been experimentally demonstrated including: TLR9,11 TLR1/2,12 TLR413 and TLR3,14 TLR5,15 TLR816 and TLR13.17 This work and the description of crystallographic structures of the extracellular domains of the TLR1/2 heterodimer,12 TLR318 and TLR4–MD213 in complex with ligands has enhanced the potential to identify novel small molecule inhibitors of TLRs and design better therapeutics.
Vaccines and adjuvants: TLRs lend a helping hand
Vaccination is a powerful tool for preventing infectious disease, allergy and cancer. The eradication of smallpox and the reduction of polio and measles are among the vaccination success stories. However, the development of improved vaccines is of great importance with the continued emergence of pathogenic viral strains (that is, influenza and HIV) and the existence of terminal cancers. Conventionally, vaccines were produced from either live attenuated or heat-inactivated organisms. In recent years, advances in molecular biology and our understanding of immune responses has made the development of ‘next generation' vaccines possible. Next-generation vaccines or subunit vaccines are based on recombinant proteins or naked DNA. This allows for the removal of non-immunogenic components of a pathogen that are unnecessary for the efficacy of the vaccine. It also has the added advantage of allowing discrimination between infected and vaccinated individuals/animals by serological examination, as individuals/animals that are infected with a pathogen will harbor components of that organism unrelated to the vaccine in their serum.
However, protein subunit vaccines can be less immunogenic than traditional vaccines and require co-administration with an immunological substance or ‘adjuvant' to boost the immune response. A good adjuvant works by promoting inflammatory responses at the site of antigen delivery to attract activated macrophage and dendritic cells (DCs) to improve antigen uptake and presentation. In doing so, a good adjuvant will facilitate co-stimulation of T and B cells to enhance production of antigen-specific acquired immunity. This distinguishing feature of an adjuvant makes TLRs prime candidates in vaccine development. Although not known at the time, the earliest vaccines actually contained bacterial contaminants that activated TLRs and served as adjuvants.19 This includes Edward Jenners use of lesions containing vaccinia virus from cowpox patients that activates TLR9, to ‘vaccinate' against smallpox the causative agent of which is variola virus. Similarly, heat-killed Mycobacterium tuberculosis in Freunds complete adjuvant developed in the late 1950s contained ligands for TLR2, TLR4 and TLR9.
TLRs and acquired immunity
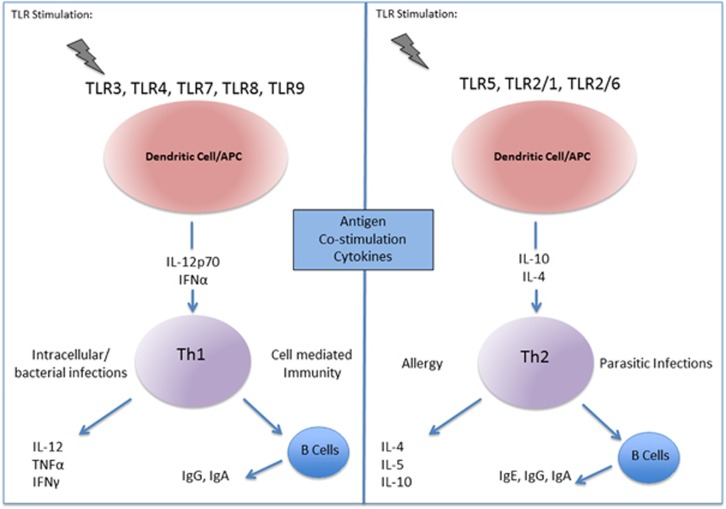
The activation of TLRs by their respective ligands has an informative role in directing acquired immunity. The generation of acquired immunity begins with the recognition of pathogen-associated patterns by TLRs on antigen presenting cells (APCs), mainly DCs in the peripheral tissues. From here DCs travel to the draining lymph nodes where they interact with T lymphocytes. Subsequently, a succession of events helps generate and differentiate T cells to establish adaptive responses. TLR activation upregulates expression of the costimulatory molecules CD80/CD86 and CD40 on the surface of DCs. The interaction between these molecules and CD28 and CD40 ligand on naive CD4+ T cells enhances APC activity. Secondly, ligation of T-cell receptors by the presentation of antigen peptides via major histocompatibility complex II on DCs is established. Finally and most importantly, the cytokine profile present within the microenvironment of T-cell activation finalizes T-cell differentiation.20 Cells of the innate immune system release these cytokines in a TLR-dependent manner. Hence recognition of pathogen-associated patterns via TLRs directs the main pathways by which DCs become activated and mature to provide signals to naive T cells and tailor responses. Naive T cells are primed toward specific T helper profiles: Th1, Th2, Th17 and T regulatory cell (Treg) subsets (Figure 2).21
Figure 2.
TLRs and T helper cell responses.
TLRs and T helper cells
Signaling via TLR3, TLR4, TLR7, TLR8 and TLR9 promotes Th1 type responses, whereas activation of TLR5, TLR2/1 and TLR2/6 favors Th2 type responses.20 Th1 cells aid responses to intracellular pathogens and bacteria, whereas Th2 cells are accountable for responses to parasitic infections and allergies. There is strong evidence that TLRs drive Th1 responses via the release of IL-12p70 and IFNα from APCs and an increased Th2 response is observed in MyD88-deficient mice with impaired TLR signaling.22, 23 Importantly, Th1 cells produce IL-12, TNFα and IFNγ and induce B cells to release high levels of IgG and IgA antibodies that drive phagocytic activation and defence against intracellular pathogens (that is, influenza). Dysregulation of Th1 type responses to self-antigen or commensal flora leads to tissue destruction and autoimmune disease. Differentiation to a Th2 lineage is driven by IL-10 and IL-4. Th2 cells release IL-4, IL-5 and IL-10 and induce the production of IgG, IgA and specifically IgE antibodies important for immunity to parasitic infections (that is, Schistosoma mansoni). Dysregulation of a Th2 type response is associated with allergy and asthma. Importantly, Th1 polarizing cytokines inhibit the differentiation of Th2 cells.21
Activation of TLRs on APCs also induces secretion of IL-21 and IL-23, which drive differentiation toward a Th17 phenotype. Th17 cells have a prominent role in bacterial and fungal infections particular at mucosal sites. Th17 type responses are characterized by the release of IL-17 and IL-23. These cytokines act by modulating B- and T-cell functions, particularly those of Treg cells. Treg cells are key players in maintaining peripheral T cell tolerance. These cells patrol the host organs against activation of self-reactive cells during steady state as well as during infections.20 TLR activation of DCs and other APCs can result in the production of anti-inflammatory cytokines such as IL-10, which promotes Treg cell proliferation and Treg-mediated suppression of the inflammatory cytokines IL-12p40, TNFα and IL-6. Current thinking proposes that the suppressive function of Treg cells can be augmented or attenuated depending on the nature of TLR stimulation in order to maximize responses against pathogens and minimize potential damage to the host. For example, increased activation of TLR2, TLR4 and TLR5 causes increased Treg function, while activation of TLR7, TLR8 and TLR9 can decrease Treg functions. Following maturation, DCs also have the ability to cross present antigen in the context of major histocompatibility complex class I molecules to CD8+ T cells. This aids the generation of a cytotoxic T lymphocyte (CTL) response, an important mechanism for effective vaccine responses against intracellular pathogens. CTL responses have been demonstrated in the context of TLR3 and TLR9 stimulation.24 Hence, the nature of a particular infection or disease state will influence the activation status of certain TLRs and ultimately direct the type of T-cell responses involved. This along with our understanding of signaling and the Th-biasing properties of TLRs is harnessed in the development of vaccines with TLR-based adjuvants.
TLR designer adjuvants and therapeutics
Many preclinical and clinical studies have demonstrated the use of purified TLR agonists as adjuvants. Equally, due to the significant role of TLRs in inflammation, molecules that antagonize or inhibit TLR signaling pathways have showed considerable potential as therapeutics against a variety of inflammatory diseases and autoimmune conditions. A major challenge in both areas is optimizing efficacy and required responses while keeping unwanted immune side effects at bay.
Toll-like receptor 4
TLR4 was the first TLR identified in mammals and is certainly the most studied member of the TLR family.25 The potency with which this TLR activates inflammatory pathways makes it an ideal target for therapeutic intervention and adjuvant development. A broad range of clinical studies have examined the adjuvant activity of TLR4 activators in vaccines against infectious and tumor antigens. TLR4 recognizes the active lipid A component of lipopolysaccharide (LPS) from Gram-negative bacteria in conjunction with MD2, CD14 and LPS-binding protein.26, 27 Although LPS is the most immunostimulatory and studied TLR4 ligand, it is a highly toxic molecule hindering its use as a vaccine adjuvant. Importantly, the lipid A portion of the parental LPS molecule mediates its potency and interaction with TLR4.28 As a result, most clinical trials involving TLR4 adjuvants examine LPS derivatives or lipid A analogs. One such derivative monophospholipid A (MPLA) is purified from LPS of Salmonella minnesota. It has 0.1% of the toxicity of LPS and is made up of a heterogeneous combination of MPLA molecules that vary in length and type of fatty acid acylation. MPLA can induce NF-κB activity, cytokine secretion and the maturation and trafficking of DCs to draining lymph nodes. It further induces cytokine cascades of both Th1 and Th2 type responses; however, a preferential bias toward induction of a Th2 phenotype has been reported.29
Pollinex Quattro (Allergy Therapeutics, West Sussex, UK) is a vaccine containing MPLA, combined with ragweed pollen extract and is used for the treatment of seasonal allergic rhinitis in Europe.30 As of March 2015, Allergy Therapeutics announced progression of Pollinex Quattro through to Food Drug and Administration approval and planned launch in the United States in 2019. MPLA has been combined with other adjuvants in several vaccines. Combinations include: MPLA in conjunction with quillaja saponaria 21 (QS21) and liposomes (AS01, GlaxoSmithKline GSK Vaccines, Middlesex, UK); QS21 and an oil-in-water emulsion (AS02 (GSK)); and alum (AS04 (GSK)). The addition of AS04 to a hepatitis B virus (HBV) vaccine lead to FENDrix, which proved very successful and the vaccine is in use. The formulation is well tolerated and induces higher antibody titers than previously licensed HBV vaccines (Engerix-B).31, 32 AS04 has also been studied in the formulation of cancer vaccines. Melacine consists of lysates from two melanoma cell lines combined with DETOX (also known as Enhanzyn), an adjuvant made up of AS04 and the cell wall products from Mycobacterium phlei.33 Melacine is approved by the Canadian Food Drug and Administration for the treatment of metastatic melanoma.
Cervarix is another AS04 containing vaccine against human pappilloma virus (HPV)34 and is used prophylactically for the treatment of cervical cancer following its authorization by the European Medicine Agency in 2007. Stimuvax is a cancer vaccine formulation containing MUC1, a heavily glycosylated tumor antigen and AS04, for prostate cancer or non-small cell lung carcinoma. While initial studies were promising, Stimuvax did not meet primary endpoints to show statistically significant improvement in a Phase III clinical trial of patients with non-small cell lung carcinoma. However, AS04 remains one of the most successful adjuvants to date and has also been tested in vaccines against other viral pathogens such as herpes simplex virus (HSV)35 and Epstein-Barr virus (EBV).36
Phase III clinical trials of AS01 as an adjuvant in Mosquirix for the treatment of malaria in young children were recently completed.37 In July 2015 the European Medicine Agency adopted a positive scientific opinion on the use of Mosquirix outside of the EU. AS01 is also demonstrated to induce Th1 type immunity and improve CD8+ T-cell responses and antibody titers to antigens from tuberculosis, varicella zoster virus and HIV.38, 39, 40 Similar to AS01, AS02 has been tested with antigens to HBV, HIV, tuberculosis and malaria. However, AS02 elicited more balanced Th1/Th2 responses with shorter-lived protection and lower lymphoproliferative responses detected over AS01.41 AS02 also induces higher CD8+ T-cell responses than AS04. More recently, a new class of synthetic lipid A mimetics have been developed, the aminoalkyl glucosaminide 4-phosphates.42 In contrast to MPLA, aminoalkyl glucosaminide 4-phosphates are synthesized at very high purity as single chemical entities and can be modified to alter biological activity. Ribi.529 (also known as RC-529) is a leading aminoalkyl glucosaminide 4-phosphate used in the human HBV recombinant antigen vaccine, Supervax®. The triggering of TLR4 via Ribi.529 (RC-529) has been used in experimental approaches to improve vaccines for HIV infection.43 Ribi.529 and another aminoalkyl glucosaminide 4-phosphate, E6020, have been tested against Listeria monocytogenes, influenza, respiratory syncytial virus,44 malaria and cardiac disease. In addition, the Infectious Disease Research Institute (IDRI), USA, have developed the adjuvant glucopyranosyl lipid A (GLA). This is a hexalyated lipid A derivative of MPLA. GLA is similar in its immunomodulatory activity but more potent at driving T cell activation than MPLA. A formulation of GLA in an oil-in-water emulsion (GLA-SE) has been most effective. GLA-SE has been tested in several preclinical subunit vaccine studies targeting herpes simplex virus-2 (HSV-2), Schistosomiasis, respiratory syncytial virus and the perioperative treatment of cancers to reduce metastasis to name a few.45, 46, 47, 48 All of which indicate GLA is safe and a viable adjuvant that is currently on a clinical path.
In terms of therapeutics, structural understanding of the interaction between TLR4 and lipid A analogs has been exploited to develop antagonists. Eritoran (E5564) is a synthetic analog of lipid A. Structural studies of TLR4–MD2 complexed with Eritoran determined that the interactions are mediated by a hydrophobic internal pocket in MD2,13 and as such it acts as a TLR4 antagonist. In human monocytes, Eritoran inhibited the production of LPS induced TNFα and IL-6.49 Eritoran progressed successfully to Phase III clinical trials for the treatment of sepsis. However, in 2011 Eritoran failed to meet clinical endpoints and Eisai Pharmaceuticals announced they would not be applying for marketing authorization for the drug in the treatment of severe sepsis. Given sepsis is a notoriously difficult indication perhaps with a more robust trial design, future Phase III trials utilizing drugs for sepsis treatment may have greater success. The TLR4-targeted antagonist AV411 (Ibudilast) has been identified as a potential treatment of pain associated with neurological indications. Opioids are demonstrated to possess TLR4 agonistic properties and TLR4 is implicated as a key microglial receptor for the initiation of neuropathic pain via the release of pro-inflammatory cytokines including TNFα and IL-1.50 A TLR4 antagonist such as AV411 may be used to block this. Phase II clinical trials looking at AV411 for treatment of neuropathic pain in 2008 demonstrated the antagonist was well tolerated in humans.51 AV411 is already used in Asia for the treatment of asthma and post-stroke disorders. A novel peptide inhibitor, VIPER (derived from vaccinia virus protein A46), has been described to inhibit TLR4-dependent signaling via blocking TIR–TIR domain interactions.52 Latest research with VIPER demonstrates the attenuation of blood pressure and inflammatory responses in hypertensive rats.53 TLR4 targeted monoclonal antibody NI-0101 (Novimmunne) binds an epitope on TLR4 and as a result inhibits dimerization reducing pro-inflammatory cytokine production. This antibody has several potential indications including asthma and rheumatoid arthritis. NI-0101 is the first antibody for TLR4 to pass Phase I clinical trials for safety and tolerability.54 Due to the potent inflammatory responses mediated via TLR4 activation, agonists and biologics targeting the receptor comprise a promising class of therapeutics.
Toll-like receptor 2
TLR2 forms functional heterodimers with TLR1, TLR6 and possibly TLR10 to recognize a variety of lipoproteins from microorganisms. TLR1/2 dimers are receptors for triacyl lipopeptides found in bacteria and mycobacteria.3 TLR6 associates with TLR2 to recognize diacyl lipopeptides such as M. fermentans macrophage-activating lipopeptide (MALP-2). The level of TLR2 activation is increased by the presence of CD14 and CD36. Synthetic triacylated and diacylated lipoproteins for TLR2 activation include Pam3Cys and Pam2Cys, respectively. The known Th2-bias of TLR2 activation and availability of highly specific synthetic ligands for TLR2 provides great opportunities to harness TLR2-induced immune responses for adjuvant purposes. Certainly, lipopeptides represent the most broadly used ligand to target TLR2 in this context.
Two strategies are taken when targeting TLR2 in vaccine development; (1) bacterial or synthetic lipopeptides are conjugated to peptides or (2) palmitic acid is covalently linked to peptide antigens. Bacterial lipopeptides are most frequently used as they contain an acylated s-glycerylcysteine moiety to which a peptide can be conjugated via a cysteine residue. LYMErix is a vaccine containing Pam3Cys linked to the C terminus epitope of outer surface protein A (OspA) of Borrelia burgdorferi, the causative agent of Lime disease. Three doses of the vaccine improve protective immunity by >75% and the vaccine was licensed in 1998. However, following concerns over skewed Treg responses towards a Th17 profile and autoimmune side effects the vaccine was withdrawn from the market.55 Recently, while addressing different adjuvants effects of influenza subunit vaccines, a role for TLR2 activation in leukocyte migration to inflammation foci was suggested. Pam3Cys was more efficient than TLR9 and TLR7/8 ligands, CpG and resiquimod respectively, at inducing early recruitment of leukocytes, mainly neutrophils, to the site of injection. This observation correlated with a higher capacity of Pam3Cys to induce antibody responses against influenza antigens.56 An experimental vaccine of a saturated fatty acid and TLR2 activator, palmitic acid conjugated to a HBV core antigen and T helper lymphocyte peptide known as Theradigm-HBV has been trialed. The vaccine induced higher HBV-specific CTL responses and longer term immunity than its un-palmitolated counterpart.57 Similarly, a vaccine against HIV-1 Tat protein in conjunction with lipopeptides as an adjuvant also showed long-lived antigen-specific CTL and humoral responses in preclinical tests.58 Furthermore, bacterial porins are candidate molecules currently in vaccine development. Examples include the outer membrane proteins OmpS1 and OmpS2 from Salmonella Typhi, which activate TLR2 and TLR4 for development in a vaccine to typhoid fever.59 Similarly, PorB from Neisseria meningitides has been shown to promotes Th2-driven cellular immune response in a TLR2-dependent manner.60 The incorporation of TLR2 ligands during vaccine development is relatively easy, allowing simplified production of completely synthetic vaccines. TLR2 adjuvants also boost cell-mediated responses and high antibody titers that would prove beneficial in preventing disease. However, the ability of TLR2-adjuvant vaccines to produce CTL responses is less impressive. As a result the benefits of targeting TLR2 for vaccine development are still debatable and more studies are required.
Multiple studies have implicated a role for TLR2 in tissue damage caused by ischemia/reperfusion injury associated with stroke, myocardial infarction and solid organ transplantation. This has provided rationale for the development of TLR2-targeted biologics. Opsona Therapeutics, Dublin, Ireland. have developed OPN-305, a humanized monoclonal TLR2 antibody with the ability to block both TLR1/2- and TLR2/6-mediated signaling and reduce TLR2-mediated pro-inflammatory cytokine production. In March of 2015 OPN-305 progressed into the second part of phase II clinical trials for prevention of delayed renal graft function. Phase I/II clinical trials also began in early 2015 for prevention of low- and intermediate-risk myelodysplastic syndrome, a diseases that affects normal blood cell production in the bone marrow.
On the other hand, the TLR2 agonist SMP105 (Dainippon Sumitomo Pharma) a preparation of the cell wall skeleton components of Mycobacterium bovis, BCG, Tokyo, Japan, has been investigated for potential therapeutic benefits in cancer. SMP105 was demonstrated to activate NFκB in a TLR2- and MyD88-dependent manner. Administration of SMP105 enhanced levels of CTLs and IFN-producing cells and reduced tumor growth in mice.61 Arabinomycolates (glycolipids) isolated from SMP105 are also demonstrated to possess adjuvant activity similar to SMP105 itself.62 Recent studies indicate great potential for SMP105 as an immunotherapy in delayed-type hypersensitivity and in an anti-tumor model in rats.63 However, SMP105 is not yet in clinical use. OM-174 is a lipid A analog with dual action on TLR2 and TLR4. In a Phase I trial completed in 2013 the biologic was tested for efficacy against refractory solid tumors in adults.64 The therapy was well tolerated at biologically active concentrations and further studies are planned in combination with chemotherapy, as animal models suggest a strong synergistic anti-tumor effect. The wide variety of studies investigating TLR2-targeted therapeutics and adjuvants highlight that targeting TLR2 may be beneficial for a wide range of indications.
Toll-like receptor 3
TLR3 ligands are considered excellent candidates as vaccine adjuvants. TLR3 binds dsRNA of viral origin while two synthetic TLR3 ligands mimicking dsRNA, polyriboinosinic-polyribocytidylic acid (poly I:C) and polyadenylic-polyuridylic acid (polyA:U), have also been described.3 Importantly, it has been demonstrated extensively that TLR3 activation leads to cross-priming of CD8+ T cells via major histocompatibility complex I for the generation of CTL responses. Furthermore, TRIF-dependant signaling following TLR3 activation results in the upregulation of type I IFNs, which further enhances this process. The synthetic TLR3 ligand, poly I:C activates mucosal and systemic humoral responses when co-administered intranasally with an inactivated influenza vaccine. This results in complete protection against homologous and heterologous influenza viruses, including the highly pathogenic H5N1 avian influenza virus.65 However, it is known that the adjuvant effects of poly I:C require cooperative activation of TLR3 and cytoplasmic RNA helicase MDA5 pathways.66
Murine models studying the efficacy of peptide-based cancer vaccines have demonstrated that poly I:C enhances tumor-specific T-cell responses. However, unfortunately this anti-tumor effect was not observed in humans due to degradation by nucleases.67 To prevent this derivatives were produced: polyICLC and poly I:C12U (known as Ampligen), which retain the immunostimulatory properties and exhibits less toxicity than poly I:C. Ampligen is currently in Phase I/II clinical trials to test its immunogenicity and safety when administered with the approved and currently used intranasal flu vaccine FluMist. Ampligen Phase II and III clinical trials have also been performed for cancer and HIV vaccine studies, respectively, and it has been deemed safe to use and induces secretion of IL-12, maturation of DCs and enhances antigen-specific CTL responses and CD4+ T cell responses. Ampligen also has therapeutic potential for other indications including: Chronic Fatigue Syndrome, Hepatitis and HPV. A cationic adjuvant formulation known as CAF01, incorporates two liposomal components with synthetic mycobacterial cord factor.68 CAF01 can prime protective Th1, Th17 and antibody responses in animal models of bacterial, viral and parasitic infections. Phase I clinical trials have been conducted for Tuberculosis using CAF01. Poly I:C has been combined with CAF01 to produce CAF05, which enhances CD8+ T cells in addition to the adjuvant effects of CAF01. These types of combinational adjuvant trials highlight the promise TLR3 agonists possess to activate broad, systemic immune responses.
Toll-like receptor 5
TLR5 is expressed on the surface of most immune cells and recognizes flagellin, a constituent protein of bacterial flagella.3 Flagellin interacts with TLR5 via a conserved region within flagellin, the D1 portion/domain. Both soluble monomeric and polymeric forms of the ligand have been shown in experimental models to interact with TLR5 and have adjuvant properties, including the upregulation of costimulatory molecules on DCs, subsequent DC maturation/activation, T-cell proliferation and secretion of IL-10 and TNFα by monocytes.69 A synthetic derivative of flagellin, Entolimod (CBLB502) is being developed by Cleveland BioLabs New York, NY, USA. Entolimod was studied in a murine model of acute ischemic renal failure where administration within 30 min of reperfusion of the ischemic kidney provided protection from injury, attenuated leukocyte and neutrophil infiltration and reduced elevated diagnostic markers of renal dysfunction normally associated with renal failure.70 The biologic was also shown to elicit protective effects in radiation induced injury. It does so by inducing factors that aid in suppressing apoptosis, scavenging reactive oxygen species and promoting tissue regeneration. The company has recently released results of a Phase I trial with Entolimod in advanced cancer patients, where it was well tolerated with safety and immunological data suggesting it could be combined with chemotherapeutic or other immunotherapeutic anticancer agents or vaccines. A second Phase I study is currently ongoing in patients with advanced cancer in the Russian Federation to expand on clinical observations using higher doses than used in the initial trial. More generally, immunization of flagellin containing vaccines induces the secretion of antigen-specific IgG and local IgA responses. VAX102 (produced by VaxInnate) is a recombinant fusion protein linking four copies of the ectodomain of influenza virus matrix protein 2 (M2e) antigen to Salmonella typhimurium flagellin. The addition of flagellin enhanced immune responses compared to those without flagellin and thus VAX102 may be useful for the broad protection of influenza A. Phase I trials yielded promising results, inducing a fourfold rise in anti-M2e antibodies in humans.71 VaxInnate also have other influenza vaccines in development. VAX125, which contains the H1 hemagglutinin antigen linked to flagellin, which is hoped will be useful for elderly patients72 and VAX2012Q comprised of four seasonal influenza strains, each fused to a flagellin protein. Both adjuvants have progressed to Phase II trials. Recent studies have made significant advances in our understanding of the binding of TLR5 to its ligand and will aid the development of TLR5 agonists as adjuvants.15
TLR7 and TLR8
TLR7 and TLR8 are structurally related, expressed on endosomes and recognize microbial nucleic acids. This includes single-stranded RNA from viruses enriched for U or GU-rich oligoribonucleotides such as RNA40 from the U5 region of HIV-1 RNA.73 TLR7/8 activation is also achieved by synthetically complexed single-stranded RNAs known as Imidazoquinolines such as R-848 (Resiquimod) or R-837 (Imiquimod). Imiquimod predominantly activates TLR7 and Resiquimod triggers both TLR7 and TLR8. As of yet no TLR7/8 agonists have been approved as vaccine adjuvants; however, most preclinical studies have utilized Imiquimod. Of note, activation of murine TLR7 but not human TLR7 can be achieved using Resiquimod. Both Imiquimod and Resiquimod have undergone extensive clinical testing as TLR agonist treatment (Aldara) for topical treatment of HPV-induced warts and other indications including basal cell and squamous cell carcinoma, lentigo maligna and actinic keratosis.74 This treatment induces a cytotoxic T-cell response and local secretion of IFNα, TNFα and IL-6 and drives DC activation. Indeed, activators of TLR7/8 induce not only the upregulation of costimulatory molecules and DC maturation, TLR7/8 also stimulate the production of IgG and induction of cytokines from B cells (for example, IL-6 and TNFα), and secretion of IFNγ by natural killer cells. In preclinical studies targeting HIV, Imiquimod enhanced antigen-specific T-cell responses and antibody secretion.75 In addition, enhanced macrophage–dependent killing and resolution of lesions has been reported following use of Imiquimod during Leishmania infections. Systemic administration of Imiquimod is highly toxic and studies looking at the safety and efficacy of TLR7/8 adjuvants have been limited by the unresponsiveness of mice to TLR8 agonists for human use.73 However, while TLR8 was initially considered only to be active in humans and not in mice, activation of murine TLR8 has been achieved with combination treatments of Imidazoquinolines and polyT oligodeoxynucleotides and this may help negate this problem in the future.76 Encouragingly, Resiquimod (3M Pharmaceuticals Minnesota, MN, USA) has demonstrated positive results in a Phase II clinical trial of genital HSV. The major benefit of TLR7/8 agonists as adjuvants is their simultaneous stimulation of several cell types. Future trials will help clarify whether or not TLR7/8 agonists can fulfill their promise as effective anti-tumor treatments and/or vaccine adjuvants.
Toll-like receptor 9
TLR9 is an intracellular TLR involved in the recognition of DNA from bacterial and viral origin but also of self DNA in immune-complexes. Non-self DNA is detected by the presence of unmethylated CpG motifs. TLR9 is expressed by B cells and plasmacytoid DCs (pDCs). TLR9 stimulation induces activation of natural killer cells, T cells, B cells, monocytes and macrophage.3 The resulting immune responses include induction of pro-inflammatory and Th1 cytokines (for example, IL-6, IL-1, TNFα, IFNγ and IL-12). In particular, IL-12 and Type I IFNs induced by pDCs via TLR9 induce strong Th1 type immunity and CTL cytotoxicity. Indeed, stimulating endosomal TLRs is particularly effective at promoting the generation of CTL responses capable of eliminating viral pathogens and cancer. Furthermore, TLR9 activation on B cells is involved in IgG class switching and humoral responses.77
The immunostimulatory activity of bacterial DNA is mimicked by synthetic oligonucleotides (ODN) harboring CpG motifs. CpG–ODNs are used in vaccines and immunotherapies, as similar to native CpGs, they have strong CD8+ T-cell responses. To date four classes of ODNs with different immunostimulatory CpG motifs have been characterized. Class A have a mixed phopsphodiester and phosphorothioate backbone, contain polyG motifs and form large structures. Class A ODNs activate pDCs to induce high levels of IFNα yet have weak NFκB stimulating capability. Class B ODNs contain one or more CpG motifs, do not form palindromic structures, have a purely phosphorothioate backbone and activate B cells. Class C ODNs have intermediate stimulatory properties as they form palindromic motifs and contain only a phosphorothioate backbone. With regard to adjuvant development, the phopsphodiester backbone of ODNs is susceptible to degradation by nucleases, while a phosphorothioate backbone can bind non-specifically to proteins in serum causing unwanted side effects. As a result efforts are made to develop CpG–ODNs with a backbone solely consisting of phopsphodiester and that are also resistant to nuclease activity and retain the immunostimulatory properties of naturally occurring ODNs.78 To this effect the addition of a polyG run to the 3'-end of a CpG–ODN with a phopsphodiester backbone has proven effective.
Although co-administration of antigen with a given TLR agonist can induce protective responses, agonist–antigen conjugates work particularly well at boosting immunity. This is certainly the case for TLR9, were murine studies show a 100-fold greater boost in immunity with CpG–ODN conjugated to apoptotic tumor cells compared with antigen and CpG–ODN mixtures. This works by directly delivering both antigen and TLR activation to each APC/DC and CpG–ODN administration was found to induce high numbers of tumor-specific CD8+ T cells when co-administered with HPV and melanoma tumor antigens. The ability of CpG–ODN to induce strong CTL responses is harnessed in vaccines targeting cancer. An initial pilot study in 2008 supported preclinical finding's to demonstrate that CpG–ODN conjugated or co-administered with tumor antigen had the ability to boost cytotoxic CD8+ T cells in melanoma patients.79 Furthermore, subsequent vaccine trials made use of CpG–ODN as an adjuvant with the synthetic tumor peptide melanoma-associated antigen recognized by T cells 1 (MART1) and with NY-ESO-1 peptide antigen, and demonstrated enhanced antigen-specific CD8+ T cell responses. The use of CpG 7909 (ProMune) as an adjuvant in a melanoma vaccine comprising the synthetic tumor peptide melanoma-specific antigen A3 (MAGE-A3) showed partial success and was boosted by the addition of MPLA and QS21 in a liposomal formulation to CpG 7909, known as the AS15 adjuvant system.80 However, the administration of CpG 7909 in conjunction with chemotherapy had no benefit for progression free survival of patients with non-small-cell lung cancer compared to those that received chemotherapy alone.81, 82
Although TLR9 adjuvants certainly serve to reduce tumor growth and size they rarely lead to complete tumor eradication. A key explanation for this is the microenvironment of the tumors in question. These environments are rich in factors that drive Tregs and monocyte-derived suppressor cells that have the ability to suppress the host's tumor-specific immunity. A means of averting this problem is the local delivery of TLR9 agonists as this promotes the infiltration of the CD8+ T cells to the tumor site.83 Murine studies have demonstrated reduced frequency of immunosuppressive Treg cells within the tumor microenvironment following local delivery of CpG ODN. Therefore, current thinking favors the local delivery of CpG–ODN conjugated antigens for the treatment of cancer (Table 1).
Table 1. Clinical development: vaccines/vaccine adjuvants/prophylactics.
| Toll-like receptor | Indication | Mechanism of action | Compound | Commercial name/company | Reference |
|---|---|---|---|---|---|
| TLR4 | Seasonal allergic rhinitis | Agonist | MPLA | Pollinex Quattro (Allergy Therapeutics)a | 30 |
| HBV | Adjuvant | AS04 and HBV antigen | FENDrix (GlaxoSmithKline)a | 31, 32 | |
| HSV, EBV | Adjuvant | AS04 | AS04 (GlaxoSmithKline)a | — | |
| Melanoma | Adjuvant | AS04 and cell wall products of Mycobacterium phlei | Enhanzyn | 33 | |
| Metastatic melanoma | Adjuvant | Enhanzyn and melanoma cell lysate | Melacinea | — | |
| HPV | Adjuvant | AS04 | Cervarixa | 34 | |
| Prostate cancer, non-small cell lung carcinoma | Adjuvant | AS04 and MUC1 tumor antigen | Stimuvax | — | |
| TB, VZV, HIV | Adjuvant | AS01 | — | — | |
| Malaria | Adjuvant | AS01 | Mosquirixa | 37 | |
| HBV | Adjuvant | Ribi.529 (lipid A mimetic) | Supervax | — | |
| Influenza (IAV), RSV, Malaria | Adjuvant | E6020 (lipid A mimetic) | E6020 (Eisai Pharmaceuticals) | 44 | |
| HSV, RSV | Adjuvant | lipid A derivative of MPLA in oil-in-water emulsion | GLA-SE IDRI | 45, 46, 47, 48 | |
| Neuropathic pain | Agonist | AV411 | Ibudilast | — | |
| Blood pressure | Antagonist | Peptide from vaccinia virus protein A46 | VIPER | 52 | |
| Asthma | |||||
| Rheumatoid arthritis | Antibody | NI-0101 | NI-0101 (Novimmunne)a | — | |
| Septic shock | Antagonist | Synthetic Lipopolysaccharide | Eritoran (Eisai Pharmaceuticals) | 49 | |
| TLR2 | Lime disease | Adjuvant | Pam3Cys linked to OspA of Borrelia burgdorferi | LYMErixa | 55 |
| HBV | Adjuvant | Palmitic acid conjugated to HBV core antigen | Theradigm-HBV | — | |
| HIV-1 | Adjuvant | Lipopeptides | — | 58 | |
| myocardial ischemia/reperfusion injury | Antibody | OPN-305 | OPN-305 (Opsona Therapeutics) | ||
| Cancer/anti-tumor models | Agonist | Components of Mycobacterium bovis BCG Tokyo | SMP105 (Dainippon Sumitomo Pharma) | 61 | |
| TLR3 | Influenza | Adjuvant | polyICLC /poly I:C12U (Ampligen) | FluMista | — |
| Chronic fatigue syndrome (CFS), hepatitis and HPV | Adjuvant | Ampligen | — | — | |
| TLR5 | myocardial ischemia/ reperfusion injury | Adjuvant | CBLB502 (synthetic flagellin) | Entolimod (Cleveland BioLabs) | 70 |
| Influenza | Adjuvant | Influenza antigen, M2e linked to Salmonella typhimurium flagellin. | VAX102 (VaxInnate) | 71 | |
| Influenza | Adjuvant | Influenza H1 hemagglutinin (HA) antigen linked to flagellin | VAX125 (VaxInnate) | 72 | |
| TLR7/8 | Basal cell and squamous cell carcinoma, lentigo maligna, actinic keratosis, HPV | Adjuvant | Imiquimod and Resiquimod | Aldarab | 74 |
| HSV, HIV and Leishmania infections | Adjuvant | Imiquimod | — | 75 | |
| Genital herpes | Adjuvant | Resiquimod | 3M Pharmaceuticals | — | |
| TLR9 | Tumors | Adjuvant | CpG–ODN | — | — |
| Melanoma vaccine Non-small-cell lung cancer | Adjuvant | CpG 7909 | ProMune/ Energix | — |
Abbreviations: AS01, MPLA, QS21 and liposomes; AS02, MPLA and QS21 in oil-in-water emulsion; AS04, MPLA, QS21 and alum; EBV, epstein-barr virus; HBV, hepatitis B virus; HPV, human pappilloma virus; HSV, herpes simplex virus; MPLA, monophospholipid A; RSV, respiratory syncytial virus; TB, tuberculosis; TLR, Toll-like receptor; VZV, varicella zoster virus.
Denotes adjuvants in clinical use.
In use for the topical treatment of HPV-induced warts.
Conclusion
TLR agonists have complex pleiotropic effects on the immune system and are demonstrated to boost antigen-specific cellular and humoral immunity. Following their initial discovery, the specificity with which TLRs tailor responses was not fully appreciated. Today targeting specific TLRs allows for the precise activation or inhibition of immune responses with fewer side effects compared with drugs of alternative mechanisms. In many respects this unique family of receptors acts as a ‘Swiss Army' knife of the immune system with the capability of responding in a multitude of infectious and disease states. This trait has been exploited successfully and led to the development of many small molecule biologics and vaccine adjuvants. However, challenges certainly remain within the field. For example, large tumor microenvironments are infiltrated with sizeable numbers of immunosuppressive cells that have the ability to hinder the tumoricidal activity of CTL cells induced by TLR adjuvants. It is worth noting that targeting multiple PRRs families may provide improved protective responses in this context. Indeed, examples of synergy and crosstalk between TLR and NOD agonists have already been reported. Activation of TLR4 can synergize with NOD1 and NOD2 agonists to induce DC maturation.84 These strategies are an example of how improved adjuvant design is an exciting ongoing endeavor. Encouragingly, continued advances in understanding TLR and innate immune signaling will ultimately help improve strategies for the prevention, treatment and cure of cancers, infectious and autoimmune diseases.
Acknowledgments
Work carried out in the Pattern Recognition Receptors and Inflammation Research group is supported by the Victorian Government's Operational Infrastructure Support Program and funding from NHMRC GNT1107804 and GNT1062721.
The authors declare no conflict of interest.
References
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 2004; 6: 1382–1387. [DOI] [PubMed] [Google Scholar]
- Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem 2007; 76: 141–165. [DOI] [PubMed] [Google Scholar]
- Dowling JK, Dellacasagrande J. Toll-like receptors: ligands, cell-based models and readouts for receptor action. Methods Mol Biol 2016; 1390: 3–27. [DOI] [PubMed] [Google Scholar]
- Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol 2006; 7: 49–56. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 2001; 413: 78–83. [DOI] [PubMed] [Google Scholar]
- Akira S, Yamamoto M, Takeda K. Role of adapters in Toll-like receptor signalling. Biochem Soc Trans 2003; 31: 637–642. [DOI] [PubMed] [Google Scholar]
- Brikos C, O'Neill LA. Signalling of toll-like receptors. Handb Exp Pharmacol 2008; 183: 21–50. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 2002; 420: 324–329. [DOI] [PubMed] [Google Scholar]
- Dai J, Megjugorac NJ, Amrute SB, Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J Immunol 2004; 173: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W et al. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 2014; 156: 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz M, Metzger J, Gellert T, Luppa P, Lipford GB, Wagner H et al. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur J Immunol 2004, 2541–2550. [DOI] [PubMed]
- Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007; 130: 1071–1082. [DOI] [PubMed] [Google Scholar]
- Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007; 130: 906–917. [DOI] [PubMed] [Google Scholar]
- Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 2008; 320: 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL et al. Structural basis of TLR5-flagellin recognition and signaling. Science 2012; 335: 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science 2013; 339: 1426–1429. [DOI] [PubMed] [Google Scholar]
- Oldenburg M, Kruger A, Ferstl R, Kaufmann A, Nees G, Sigmund A et al. TLR13 recognizes bacterial 23 S rRNA devoid of erythromycin resistance-forming modification. Science 2012; 337: 1111–1115. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu L, Davies DR, Segal DM. Dimerization of Toll-like receptor 3 (TLR3) is required for ligand binding. J Biol Chem 2010; 285: 36836–36841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser DW. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature 1961; 191: 1169–1171. [DOI] [PubMed] [Google Scholar]
- Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T-cell development and differentiation. ClinDevImmunol 2012; 2012: 836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010; 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddis DE, Michalek SM, Katz J. TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B. J Immunol 2011; 186: 5772–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int Immunol 2002; 14: 695–700. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Storni T, Manolova V, Didierlaurent A, Sirard JC, Rothlisberger P et al. Role of Toll-like receptors in costimulating cytotoxic T cell responses. Eur J Immunol 2003; 33: 1465–1470. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997; 388: 394–397. [DOI] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 1999; 189: 1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland TN, Finley F, Leturcq D, Moriarty A, Lee JD, Ulevitch RJ et al. Analysis of lipopolysaccharide binding by CD14. J Biol Chem 1993; 268: 24818–24823. [PubMed] [Google Scholar]
- Johnson AG, Gaines S, Landy M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med 1956; 103: 225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007; 316: 1628–1632. [DOI] [PubMed] [Google Scholar]
- Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy 2001; 56: 498–505. [DOI] [PubMed] [Google Scholar]
- Boland G, Beran J, Lievens M, Sasadeusz J, Dentico P, Nothdurft H et al. Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine 2004; 23: 316–320. [DOI] [PubMed] [Google Scholar]
- Thoelen S, De Clercq N, Tornieporth N. A prophylactic hepatitis B vaccine with a novel adjuvant system. Vaccine 2001; 19: 2400–2403. [DOI] [PubMed] [Google Scholar]
- Mitchell MS, Harel W, Kempf RA, Hu E, Kan-Mitchell J, Boswell WD et al. Active-specific immunotherapy for melanoma. J Clin Oncol 1990; 8: 856–869. [DOI] [PubMed] [Google Scholar]
- Bhatla N, Suri V, Basu P, Shastri S, Datta SK, Bi D et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine in healthy Indian women. J Obstet Gynaecol Res 2010; 36: 123–132. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347: 1652–1661. [DOI] [PubMed] [Google Scholar]
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis 2007; 196: 1749–1753. [DOI] [PubMed] [Google Scholar]
- Penny MA, Verity R, Bever CA, Sauboin C, Galactionova K, Flasche S et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet 2015; 387: 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A, McCarthy L, Mills KH. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 1999; 17: 2517–2527. [DOI] [PubMed] [Google Scholar]
- Pichyangkul S, Gettayacamin M, Miller RS, Lyon JA, Angov E, Tongtawe P et al. Pre-clinical evaluation of the malaria vaccine candidate P. falciparum MSP1(42) formulated with novel adjuvants or with alum. Vaccine 2004; 22: 3831–3840. [DOI] [PubMed] [Google Scholar]
- Lal H, Zahaf T, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella zoster virus subunit candidate vaccine (HZ/su): a phase-I, open-label study in Japanese adults. Hum Vaccin Immunother 2013; 9: 1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini F, Audran R, Lurati F, Ofori-Anyinam O, Zysset F, Vandepapeliere P et al. The candidate tuberculosis vaccine Mtb72F/AS02 in PPD positive adults: a randomized controlled phase I/II study. Tuberculosis (Edinb) 2013; 93: 179–188. [DOI] [PubMed] [Google Scholar]
- Baldridge JR, Cluff CW, Evans JT, Lacy MJ, Stephens JR, Brookshire VG et al. Immunostimulatory activity of aminoalkyl glucosaminide 4-phosphates (AGPs): induction of protective innate immune responses by RC-524 and RC-529. J Endotoxin Res 2002; 8: 453–458. [DOI] [PubMed] [Google Scholar]
- Wong YN, Rossignol D, Rose JR, Kao R, Carter A, Lynn M. Safety, pharmacokinetics, and pharmacodynamics of E5564, a lipid A antagonist, during an ascending single-dose clinical study. J Clin Pharmacol 2003; 43: 735–742. [PubMed] [Google Scholar]
- Morefield GL, Hawkins LD, Ishizaka ST, Kissner TL, Ulrich RG. Synthetic Toll-like receptor 4 agonist enhances vaccine efficacy in an experimental model of toxic shock syndrome. Clin Vaccine Immunol 2007; 14: 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner P, Sorski L, Shaashua L, Elbaz E, Lavon H, Melamed R et al. Perioperative treatment with the new synthetic TLR-4 agonist GLA-SE reduces cancer metastasis without adverse effects. Int J Cancer 2016; 138: 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Flynn PA, Campbell DJ, Robbins SH, Dong L, Wang K et al. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. Vaccine 2016; 34: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton K, Aslam S, Shambaugh C, Lin R, Heeke D, Frantz C et al. Enhanced immunogenicity of a respiratory syncytial virus (RSV) F subunit vaccine formulated with the adjuvant GLA-SE in cynomolgus macaques. Vaccine 2015; 33: 4472–4478. [DOI] [PubMed] [Google Scholar]
- Santini-Oliveira M, Coler RN, Parra J, Veloso V, Jayashankar L, Pinto PM et al. Schistosomiasis vaccine candidate Sm14/GLA-SE: Phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine 2016; 34: 586–594. [DOI] [PubMed] [Google Scholar]
- Czeslick E, Struppert A, Simm A, Sablotzki A. E5564 (Eritoran) inhibits lipopolysaccharide-induced cytokine production in human blood monocytes. Inflamm Res 2006; 55: 511–515. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA 2005; 102: 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan P, Gibbons JA, He L, Chang E, Jones D, Gross MI et al. Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br J Clin Pharmacol 2008; 66: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakova-Devine T, Keogh B, Harrington B, Nagpal K, Halle A, Golenbock DT et al. Viral inhibitory peptide of TLR4, a peptide derived from vaccinia protein A46, specifically inhibits TLR4 by directly targeting MyD88 adaptor-like and TRIF-related adaptor molecule. J Immunol 2010; 185: 4261–4271. [DOI] [PubMed] [Google Scholar]
- Dange RB, Agarwal D, Teruyama R, Francis J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation 2015; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet E, Shang L, Lapeyre G, De Graaf K, Hatterer E, Wambiekele G et al. Translational data and phase 1 study results of a new monoclonal antibody targeting Toll like receptor 4 (TLR4) developed for rheumatoid arthritis (RA) treatment with a potential for personalized medicine. Arthritis Rheumatol 2015; 67 Abstract 1661. [Google Scholar]
- Abbott A. Lyme disease: uphill struggle. Nature 2006; 439: 524–525. [DOI] [PubMed] [Google Scholar]
- Caproni E, Tritto E, Cortese M, Muzzi A, Mosca F, Monaci E et al. MF59 and Pam3CSK4 boost adaptive responses to influenza subunit vaccine through an IFN type I-independent mechanism of action. J Immunol 2012; 188: 3088–3098. [DOI] [PubMed] [Google Scholar]
- Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A et al. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer 2001; 3: 49–57. [DOI] [PubMed] [Google Scholar]
- Borsutzky S, Ebensen T, Link C, Becker PD, Fiorelli V, Cafaro A et al. Efficient systemic and mucosal responses against the HIV-1 Tat protein by prime/boost vaccination using the lipopeptide MALP-2 as adjuvant. Vaccine 2006; 24: 2049–2056. [DOI] [PubMed] [Google Scholar]
- Moreno-Eutimio MA, Tenorio-Calvo A, Pastelin-Palacios R, Perez-Shibayama C, Gil-Cruz C, Lopez-Santiago R et al. Salmonella Typhi OmpS1 and OmpS2 porins are potent protective immunogens with adjuvant properties. Immunology 2013; 139: 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Ganley-Leal LM, Khatri A, Wetzler LM. Neisseria meningitidis PorB, a TLR2 ligand, induces an antigen-specific eosinophil recall response: potential adjuvant for helminth vaccines? J Immunol 2007; 179: 3222–3230. [DOI] [PubMed] [Google Scholar]
- Murata M. Activation of Toll-like receptor 2 by a novel preparation of cell wall skeleton from Mycobacterium bovis BCG Tokyo (SMP-105) sufficiently enhances immune responses against tumors. Cancer Sci 2008; 99: 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi M, Murata M, Shibuya K, Koga-Yamakawa E, Uenishi Y, Kusunose N et al. Arabino-mycolates derived from cell-wall skeleton of Mycobacterium bovis BCG as a prominent structure for recognition by host immunity. Drug Discov Ther 2011; 5: 130–135. [DOI] [PubMed] [Google Scholar]
- Miyauchi M, Murata M, Fukushima A, Sato T, Nakagawa M, Fujii T et al. Optimization of cell-wall skeleton derived from Mycobacterium bovis BCG Tokyo 172 (SMP-105) emulsion in delayed-type hypersensitivity and antitumor models. Drug Discov Ther 2012; 6: 218–225. [PubMed] [Google Scholar]
- Isambert N, Fumoleau P, Paul C, Ferrand C, Zanetta S, Bauer J et al. Phase I study of OM-174, a lipid A analogue, with assessment of immunological response, in patients with refractory solid tumors. BMC Cancer 2013; 13: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Tamura S, Kawaguchi A, Ninomiya A, Imai M, Itamura S et al. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis 2007; 196: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol 2008; 180: 683–687. [DOI] [PubMed] [Google Scholar]
- McFarlin DE, Bever CT, Salazar AM, Levy HB. A preliminary trial of poly(I,C)-LC in multiple sclerosis. J Biol Response Mod 1985; 4: 544–548. [PubMed] [Google Scholar]
- van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014; 32: 7098–7107. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Carvalho FA, Aitken JD, Fifadara NH, Gewirtz AT. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur J Immunol 2010; 40: 3528–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa N, Petro M, Baldwin WM 3rd, Gudkov AV, Fairchild RL. A TLR5 agonist inhibits acute renal ischemic failure. JImmunol 2011; 187: 3831–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 2011; 29: 5145–5152. [DOI] [PubMed] [Google Scholar]
- Taylor DN, Treanor JJ, Strout C, Johnson C, Fitzgerald T, Kavita U et al. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI). Vaccine 2011; 29: 4897–4902. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004; 303: 1526–1529. [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Dinehart S, Whiting D, Lee PK, Tawfik N, Jorizzo J et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol 2004; 50: 714–721. [DOI] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA 2005; 102: 15190–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol 2006;6584–6587. [DOI] [PubMed]
- Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med 1997; 3: 849–854. [DOI] [PubMed] [Google Scholar]
- Meng W, Yamazaki T, Nishida Y, Hanagata N. Nuclease-resistant immunostimulatory phosphodiester CpG oligodeoxynucleotides as human Toll-like receptor 9 agonists. BMC Biotechnol 2011; 11: 88–6750-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade J, Kudela P, Andrade Filho PA, Janjic B, Land SR, Sander C et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother 2008; 31: 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol 2013; 31: 2413–2420. [DOI] [PubMed] [Google Scholar]
- Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska-Morawiec M et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 2012; 23: 72–77. [DOI] [PubMed] [Google Scholar]
- Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011; 29: 2667–2674. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol 2012; 188: 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M et al. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol 2005; 35: 2459–2470. [DOI] [PubMed] [Google Scholar]