Abstract
Cardiovascular disease (CVD) remains the leading cause of mortality worldwide. Atherosclerosis is the most common form of CVD, which is complex and multifactorial with an elevated risk observed in people with either metabolic or inflammatory diseases. Accumulating evidence now links obesity with a state of chronic low-grade inflammation and has renewed our understanding of this condition and its associated comorbidities. An emerging theme linking disease states with atherosclerosis is the increased production of myeloid cells, which can initiate and exacerbate atherogenesis. Although anti-inflammatory drug treatments exist and have been successfully used to treat inflammatory conditions such as rheumatoid arthritis (RA), a commonly observed side effect is dyslipidemia, inadvertently, a major risk factor for the development of atherosclerosis. The mechanisms leading to dyslipidemia associated with anti-inflammatory drug use and whether CVD risk is actually increased by this dyslipidemia are of great therapeutic importance and currently remain poorly understood. Here we review recent data providing links between inflammation, hematopoiesis, dyslipidemia and CVD risk in the context of anti-inflammatory drug use.
Cardiovascular disease (CVD) is currently the leading cause of death worldwide. Atherosclerosis is the major form of CVD and is characterized by a chronic inflammatory build up, driven largely by lipid accumulation within the artery wall. Unlike acute inflammation, atherosclerosis is hallmarked by a state of unresolved low-grade chronic inflammation. Importantly, low-grade inflammation is also a feature of several diseases known to increase the risk of CVD. Obesity is a prime example of a low-grade chronic inflammatory disease that can promote insulin resistance and type 2 diabetes (T2D), and can increase the risk of CVD.1 Indeed, people with T2D have up to fourfold the risk of developing CVD compared with non-diabetic individuals. Hence, strategies targeting insulin resistance and glucose homeostasis through inflammation modulation or other mechanisms are important for the treatment of CVD. Conversely, therapies that are associated with triggering known CV risk factors including weight gain, insulin resistance and dyslipidemia are met with caution.
There has been a significant amount of research examining the immunological changes that occur within metabolically important tissues such as the adipose tissue, liver, muscle and the atherosclerotic lesion during CVD and T2D. In addition, recent advances have highlighted a significant role of the hematopoietic system in the context of metabolic diseases, which likely contributes to the elevated CVD risk due to an increase in production of white blood cells (WBCs) that feed the atherosclerotic lesion.2 In this review we will briefly outline the current understanding of the inflammatory processes linked with metabolic diseases. We will also discuss how the use of current and potential anti-inflammatories in the treatment of inflammatory diseases, such as rheumatoid arthritis (RA; a pathology associated with an increased risk of CVD), alters metabolic pathways particularly in relation to cholesterol homeostasis and whether this influences CVD risk.
Targeting inflammation for the treatment of insulin resistance and CVD: current therapies
Since the seminal finding that tumor necrosis factor (TNF)-α causes metabolic dysfunction and the discovery of macrophages within the obese adipose tissue,3, 4 it has become apparent that most cells of the innate and acquired immune systems are altered in obesity.1 Adipose tissue pro-inflammatory macrophages have received the greatest amount of attention, as these are the predominant leukocytes that accumulate in obese adipose tissue. These CD11c+F4/80+ macrophages appear to be one of the main sources of the elevated cytokines TNF-α, interleukin (IL)-6 and IL-1β observed in obesity and thought to directly contribute to insulin resistance both locally and in peripheral tissues through the activation of stress-signaling pathways such as Janus N-terminal Kinase (JNK) and IκB Kinase.5 Whereas the activation of inflammatory pathways leading to insulin resistance and enhanced atherosclerosis has been demonstrated consistently in rodent models,6, 7 it is less clear whether insulin resistance and CVD can be targeted therapeutically with anti-inflammatory drugs in humans. To date, several clinical trials have attempted to address this proposition with various anti-inflammatory regimes with varying success. We have summarized the key findings from some of these anti-inflammatory trials described below.
Aspirin/salsalate
Aspirin (acetylated salicylate) is an anti-inflammatory drug, which inhibits cylo-oxygenase (Cox) enzymes Cox-1 and Cox-2 in the prostaglandin synthesis pathway and at higher doses inhibits the IκB Kinase-β/NF-κB pathway. Aspirin reduces CVD risk by altering platelet reactivity and preventing clot formation,8 but also appears to lower CVD risk through decreased levels of C-reactive protein.9 Unlike aspirin, salsalate is a non-acetylated salicylate and, as such, is not a cyclo-oxygenase inhibitor, nor does it influence hemostasis, but it works through inhibition of the NFκB pathway. Nonetheless, salsalate is still an effective anti-inflammatory treatment option. In the Targeting Inflammation using Salsalate in T2D (TINSAL-T2D) clinical trial, treatment of patients with T2D with salsalate consistently lowers fasting glycemia and/or HbA1c levels.10, 11, 12 It must be highlighted that, in these trials, salsalate impaired insulin clearance; thus, tissue exposure to circulating insulin was prolonged and could account for at least some of the improvements in glycemic control.12, 13 However, a more recent 12-week salsalate clinical trial was found not to improve insulin sensitivity in people with glucose intolerance despite reducing fasting glycemia.13
Anti-cytokine therapies
The cytokines IL-1β and TNF-α have been therapeutically targeted in clinical trials for the treatment of insulin resistance.
Anakinra, a recombinant IL-1Ra (IL-1 receptor antagonist) molecule, has been effective in treating a wide range of inflammatory conditions. In addition, specific anti-IL-1β monoclonal antibodies such as canakinumab appear equally efficacious.14 Consistent with its appreciated role in preserving pancreatic β-cell function, the blockade of IL-1 signaling with anakinra reduced glucose intolerance in obese mice. Importantly, it also improved glucose metabolism in people with the metabolic syndrome or T2D by enhancing pancreatic function and not necessarily by insulin sensitivity.15, 16 Furthermore, large clinical trials such as the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) have been initiated to assess whether IL-1β blockade will improve cardiovascular (CV) outcomes in patients with T2D.17
TNF-α has been shown to induce insulin resistance in animal models.18 However, translating these findings into the clinic is yet to show any efficacy. In people with T2D or the metabolic syndrome, TNF-α neutralization failed to improve insulin sensitivity.19, 20, 21 Unfortunately, many of these studies contained major limitations such as an insufficient statistical power and short study duration, which potentially explains their lack of impact. However, a longer study in obese, insulin-resistant (non-diabetic) subjects revealed that TNF-α antagonism reduced fasting glycemia.22 Whether improvements in metabolism in these studies are because of a direct effect or whether they are because the underlying disease burden is reduced remains unknown. More studies are warranted to assess whether TNF-α remains a valuable target for metabolic diseases and their associated complications.
Peroxisome Proliferator Activator Receptor gamma agonism
Thiazolidinediones (TZDs) such as rosiglitazone and pioglitazone are insulin-sensitizing Peroxisome Proliferator Activator Receptor gamma (PPARγ) agonists. The complete actions of PPARγ with respect to metabolism remain incompletely understood. However, TZDs are currently thought to improve insulin sensitivity by reducing lipotoxicity in tissues such as the liver and skeletal muscle, in addition to promoting lipid partitioning into adipocytes.23 It has become well appreciated that TZDs also elicit some anti-inflammatory effects pertaining to reductions in macrophages and/or stimulation of regulatory T cells within the adipose tissue as well as an alteration in circulating monocytes24 and immunomodulatory proteins.25 However, TZDs have also been associated with weight gain, bone fractures and heart failure because of an appearance of cardiac edema, making it a drug to prescribe with caution according to the patient history.26
Can anti-inflammatory therapies for the treatment of metabolic diseases also promote dyslipidemia?
The number of clinical studies targeting aspects of inflammation for the treatment of metabolic diseases continues to expand. However, it is becoming a frequent observation that some anti-inflammatory treatments can cause perturbations in cholesterol homeostasis. Notwithstanding the potential benefits on fasting glycemia, salsalate treatment consistently appears to increase either low-density lipoprotein (LDL) or total cholesterol levels.10, 11, 13, 27, 28 Furthermore, in patients with T2D, treatment with TZDs also appears to alter circulating cholesterol concentrations29 and lipoprotein particle size,30 which may be specific to the type of TZD used. For example, rosiglitazone seems to increase total and LDL cholesterol, whereas pioglitazone does not alter total or LDL cholesterol and increases high-density lipoprotein (HDL).30, 31, 32 In preclinical models of diabetes and atherosclerosis, rosiglitazone does appear to reduce CVD.24 Surprisingly, there is a lack of data in the context of diabetes regarding the effect of TNF-α blockade on cholesterol homeostasis in humans with metabolic diseases. Studies either do not report cholesterol levels20, 21, 33 or report unaltered levels.19, 22, 34, 35 Blocking TNF-α activity in patients with RA also appears to improve indices of metabolism36 while concurrently increasing cholesterol levels.37 Recently, researchers from the Interleukin-1 Genetics Consortium analyzed genetic variants involved in IL-1Ra production to assess CV risk associated with long-term IL-1 inhibition. This consortium generated a genetic score for IL-1Ra gene variants and, surprisingly, the genetic score predicted a greater risk for coronary heart disease with increasing IL-1Ra concentration. Interestingly, the genetic score was also associated with increases in circulating LDL cholesterol, total cholesterol and triglycerides.38 Even though this study does not allow for the discrimination between IL-1α and IL-1β signaling, it is interesting to note that canakinumab—allowing for specific IL-1β antagonism—was recently shown to increase triglycerides (but not LDL cholesterol).39 Taken together, these data reveal that alterations in cholesterol homeostasis can be a side effect of treatments that target inflammation in people with metabolic diseases (Table 1).
Table 1. Current anti-inflammatory therapeutics and their CVD impact.
| Drug | Disease | Target | Mode of action | Metabolic impact | CVD risk | References |
|---|---|---|---|---|---|---|
| Salsalate | IR/T2D | NFκB pathway | Inhibition of the NFκB pathway | ↑ LDL ↑TC | Unknown | 10, 11, 13, 27, 28 |
| Thiazolidinediones | IR/T2D | PPARγ | PPARγ agonist | ↑ Weight ↑ LDL ↑TC ↑ HDL | ↓↑ | 29, 30, 31, 32 |
| Anakinra | T2D | IL-1 receptor | Recombinant IL-1R antagonist | → LDL | Unknown | 15 |
| Canakinumab | T2D | IL-1β | Anti IL-1β monoclonal antibody | ↑TG | Unknown | 39 |
| Methotrexate | RA | T cells/B cells | Multiple mechanisms including inhibition of folate pathway/purine metabolism/T-cell activation/IL-1β/IL-1βR interaction | ↑ LDL ↑ HDL ↑TC | ↓ | 40, 41 |
| Infliximab | RA | TNF-α signaling | Anti TNF monoclonal antibody | ↑TC ↑ LDL ↑ HDL ↑ TG | ↓↑→ | 42, 43, 44, 45, 46 |
| Tocilizumab | RA | IL-6 receptor | Anti IL-6R monoclonal antibody | ↑TC ↑ LDL | ↓→ | 47, 48, 49, 50, 51, 68 |
| Sgp130Fc | RA | IL-6 trans-signaling | Inhibits soluble form of IL-6R | Unknown | Unknown | |
| Rituximab | RA | B cells | Anti-CD20 monoclonal antibody | ↑TC ↑ LDL | → | 60, 61, 62, 63 |
| Tofacitinib | RA | JAK-STAT signaling | Inhibitor of the JAK1 and 3 kinases | ↑TC | Unknown | 58, 59 |
Abbreviations: CVD, cardiovascular disease; HDL, high-density lipoprotein; IL, interleukin; JAK, Janus N-terminal Kinase; IR, insulin resistance; LDL, low-density lipoprotein; PPARγ, Peroxisome Proliferator Activator Receptor gamma; RA, rheumatoid arthritis; TC, total cholesterol; T2D, type 2 diabetes; TG, triglycerides; TNF-α, tumor necrosis factor-α.
Dyslipidemia and CVD risk in inflammatory diseases: the example of RA
CV risk and dyslipidemia are elevated in patients with inflammatory conditions such as chronic kidney disease, recurrent infections, myeloproliferative neoplasms, RA and systemic lupus erythematous. However, unlike traditional metabolic diseases in which elevated CVD risk is associated with elevated cholesterol, in diseases such as RA, cancer, sepsis and immediately post myocardial infaction, the relationship between elevated CVD risk and cholesterol is less clear. Often in these patients, increased C-reactive protein (indicative of CVD risk) is associated with reduced circulating cholesterol. This inverse relationship between increased CVD risk and reduced lipids is termed the ‘lipid paradox'. Using RA as an example inflammatory disease that is associated with a two- to threefold elevated risk of CVD, many studies have found cholesterol levels to be increased with the successful treatment of RA with anti-inflammatory drugs, suggesting a link between inflammation and cholesterol homeostasis. Hence, anti-inflammatory treatments in people with RA allow for a better understanding of the cross-talk between inflammation and CVD risk and are an ideal example to discuss in the context of this review. Below we have summarized the data from some of the more recent targeted anti-inflammatories used in RA and their metabolic effects.
Disease-modifying anti-rheumatic drugs
Methotrexate is the frontline treatment from all existing disease-modifying anti-rheumatic drugs and has been observed to increase cholesterol and triglyceride levels in patients with RA.40 Importantly, CVD risk appears to be reduced with methotrexate41 and, although the mechanism by which this occurs is not well understood, it is likely to be through a reduction in inflammation.
TNF-α antagonists
TNF-α antagonism in RA patients is associated with significant alterations in circulating lipids. From meta-analyses, it is generally reported that increases in total, LDL and HDL cholesterol and triglycerides occur with TNF-α antagonism.42, 43 Despite these changes in circulating lipids, a meta-analysis of 3 randomized control trials and 13 observational cohort studies found that anti-TNF-α therapy in RA patients was associated with a reduction in the risk of all CV events, including myocardial infaction and stroke in the cohort studies but not the randomized clinical trials, probably because of being statistically underpowered.44 In patients with heart failure, however, blockade of TNF-α with infliximab adversely affected patient outcomes.45 More recently, data from a German biologics registry revealed reduced mortality rates in RA patients treated with anti-TNF-α compared with those treated with conventional disease-modifying anti-rheumatic drugs. However, whether the reduction in mortality was due to a reduction in CVD was not specifically assessed in this study.46
IL-6 receptor blockade
Targeting IL-6 in RA is achieved with tocilizumab, an anti-IL-6 receptor monoclonal antibody. Although tocilizumab is generally well tolerated and is efficacious in reducing disease severity, it also causes significant perturbations to lipid and cholesterol homeostasis such as increases in both LDL and total cholesterol.47, 48, 49, 50, 51 IL-6 biology is complex with signaling permitted through a membrane-anchored (leading to classical signaling) or soluble IL-6 receptor (termed IL-6 trans-signaling). A naturally occurring soluble glycoprotein 130 (sgp130) molecule specifically antagonizes IL-6 trans-signaling, and work over the past decade has established IL-6 trans-signaling as the major inflammatory signaling paradigm in IL-6-associated pathologies.52 Indeed, blockade of IL-6 trans-signaling with sgp130 was able to reduce inflammation and disease severity in experimental models of arthritis.53, 54 Whether the blockade of IL-6 trans-signaling alters circulating lipids in the context of RA remains unknown. However, transgenic mice that overexpress a human sgp130Fc molecule display an IL-6 trans-signaling ‘knockout' phenotype.55 These sgp130Fc transgenic mice are protected from obesity-induced white adipose tissue macrophage accumulation without having an impact on circulating lipids.56 Furthermore, sgp130Fc administration reduces atherosclerosis in high-fat-fed Ldlr−/− mice without altering body mass or the blood lipid profile, suggesting that sgp130Fc may be a therapeutic target for the treatment of atherosclerosis.57 As bacterial infections and hyperlipidemia are major consequences of global IL-6 blockade in RA patients treated with tocilizumab, it is hypothesized that IL-6-trans-signaling antagonism with sgp130Fc may provide a more specific treatment option.
JAK inhibitors
Tofacitinib, a janus kinase inhibitor (JAK), has been developed as an orally administered anti-inflammatory. In adjuvant-induced arthritic rats, tofacitinib successfully reduced disease severity but also induced hypercholesterolemia.58 Recently, tofacitinib was Food And Drug Administration-approved for the treatment of RA. Similar to rodents, JAK inhibition in humans also increases circulating cholesterol.59 The hypercholesterolemia observed with tofacitinib is similar to that observed by IL-6 blockade with tocilizumab, which is perhaps not surprising, given that the JAK signaling pathway is activated by IL-6.
B-cell therapies
Rituximab is a monoclonal antibody that depletes B cells by targeting CD20, and it appears to be a promising therapeutic for the treatment of RA. Administration of rituximab can achieve sustained reductions in inflammation; however, this is at the expense of increasing total and LDL cholesterol.60, 61 However, alterations in cholesterol are not always consistent with rituximab.62 Pooled data from clinical trails have revealed that people with RA treated with rituximab do not have an increased CVD risk.63
Taken together, these studies reveal that significant perturbations in lipid homeostasis occur in RA patients successfully treated with anti-inflammatories. However, despite the increase in circulating lipids, it is generally accepted that CVD risk is either reduced or not affected (Table 1). Interestingly, it has been observed that in people who develop RA (compared with those who do not), a significant reduction in total and LDL cholesterol occurs in the 5 years preceding RA diagnosis.64 As such, it is now thought that the increase in cholesterol levels with anti-inflammatory treatments reflects a normalization of lipid levels to those seen in the general population, potentially explaining why CVD is not elevated in successfully treated patients.65 This also highlights the fact that in RA patients low levels of LDL and total cholesterol are associated with worsened CV outcomes, challenging the dogma that cholesterol levels are a reflection of the CVD risk.
Anti-inflammatory therapies and dyslipidemia: dissecting the mechanisms
Association between anti-inflammatory therapies and changes in lipoprotein subclasses
A critical question that remains is whether the reduction in CVD risk is because of beneficial alterations in composition and subclasses of lipids or inflammation reduction per se. Recent evidence from the clinical trial MEASURE suggests that IL-6 blockade with tocilizumab induces beneficial alterations intrinsic to the cholesterol subfractions. In this trial, plasma lipid subfractions from RA patients treated with methotrexate alone, or with add on tocilizumab for 12 weeks, were analyzed using nuclear magnetic resonance. Despite increasing total and LDL cholesterol, the concentration of the pro-atherogenic small, dense LDL particles was not increased with tocilizumab treatment. Although levels of HDL cholesterol concentration were unchanged with tocilizumab treatment, the HDL particles themselves shifted toward an anti-inflammatory phenotype,66 a result consistent with TNF-α antagonism.67 Furthermore, several proxy markers of vascular risk such as Lipoprotein(a), D-Dimer and fibrinogen were reduced in RA patients treated with tocilizumab. In a comprehensive review of the long-term safety profile of tocilizumab, data from over 4000 patients collected from five randomized, controlled tocilizumab trials were analyzed. With a mean follow-up of 4.6 years, tocilizumab improved disease severity, with no significant safety concerns over and above the ‘all-control' population.68 Furthermore, tocilizumab did not increase the carotid intima-media thickness,69 suggesting that the hypercholesterolemia associated with IL-6 blockade does not increase CVD risk. Although these findings taken together are promising, they do not prove that CVD risk is reduced per se with tocilizumab treatment. Similar changes in HDL phenotypes were observed in the HDL particles in responders to rituximab treatment.70 Interestingly, the major lipoprotein of HDL, apoA-I, has potent anti-inflammatory anti-atherogenic effects, and recent evidence suggests that apoB/apoA-I ratio is a stronger predictor of CVD risk than lipid levels.71 These observations add credence to the notion that inflammation is the major driver of atherogenesis and CVD risk in RA. However, this hypothesis is yet to be tested formally as confounding influences such as previous drug treatments and lipid-lowering effects of other anti-inflammatories can influence the contribution of CVD risk attributable to inflammation alone.
Hematopoiesis, the missing link?
How circulating lipids are altered by the modulation of inflammation remain incompletely understood. Several mechanisms have been proposed to explain how cholesterol levels may be reduced in states of high inflammation including enhanced hepatic LDL uptake or an impairment in cholesterol efflux from foam cells, both mechanisms being influenced by the immune system. Indeed, cells from adaptive immunity have been found to interact with cholesterol metabolism. Klingenberg et al.72 showed that regulatory T cells can affect lipid levels via modulation of the liver transcriptome. This leads to reduced clearance of very-low-density lipoprotein and chylomicron remnants, which ultimately increased atherosclerotic lesion formation. Moreover, recent work from the McInnes laboratory has also begun to shed some light on this topic. Using isotopically labeled cholesterol and leucine infusions, cholesterol and lipoprotein kinetics were determined in people with RA before and after 6 weeks of tofacitinib treatment and were compared with non-treated matched healthy controls.73 It was revealed that the reduced cholesterol levels observed in RA were because of increased cholesterol ester catabolism. Interestingly, tofacitinib administration increased cholesterol levels to those of the healthy controls.73 However, they did not elucidate in this study the mechanism by which cholesterol catabolism is enhanced in the context of RA. In this review, we propose that alterations in hematopoiesis could be responsible for enhanced cholesterol catabolism, which will be discussed below.
CVD risk and inflammation: focus on enhanced hematopoiesis
Hematopoiesis in CVD
Hematopoiesis is the process by which blood cells are formed, and it occurs primarily within the bone marrow after birth. Hematopoiesis is a highly regulated, hierarchical and efficient process that is able to produce millions of cells daily to maintain the immune system.74 Hematopoiesis is initiated by hematopoietic stem cells, which are lowly dividing cells able to proliferate and differentiate into lineage-committed stem and progenitor cells. Hematopoietic stem and multipotential progenitor cells (HSPCs) are extremely sensitive to extrinsic cues from endocrine factors such as cytokines and chemokines as well as signals from adjacent cells within the bone marrow niche such as endothelial cells, mesenchymal stem cells, osteoblasts and macrophages.75 In times of stress or disease, factors such as granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor, C-X-C motif chemokine (CXCL-12), monocyte chemoattractant protein-1, stem cell factor, IL-6 and thrombopoietin are critical in modulating an appropriate immune response.76 As such, the measurement of circulating WBCs is routinely performed to aid in the diagnosis of a wide range of pathologies and stress states from infection and inflammatory diseases to hematological malignancies.
A strong association between elevated WBCs and CVD has been identified in numerous epidemiological studies.77, 78, 79 Preclinical studies of atherosclerosis have revealed that circulating monocytes, neutrophils and platelets have an important role in disease initiation and progression.80, 81 Leukocyte abundance is also known to influence heart failure after a myocardial infaction82 and may also contribute to secondary CV events.83 Of the circulating leukocytes, cells of the myeloid compartment, namely monocytes and neutrophils, are the most highly associated ones with cardiac events.84, 85, 86, 87 However, an important relationship between adaptive immune cells and CVD has emerged over the past decade (see reviews Hedrick88, Wigren et al.89 and Ammirati et al.90).
The balance of different leukocyte subsets has a critical role in CVD. Indeed, in the context of atherosclerosis, hematopoiesis and, in particular, WBC production outside the bone marrow (extramedullary hematopoiesis) is skewed toward the production of pro-inflammatory monocyte subsets (Ly6-Chi in mice and CD14++CD16−/+ in humans91), which are known to be strongly pro-atherogenic. Alongside the Ly6-Chi/Ly6-Clo monocyte balance, many different T-cell subsets have been shown to participate in atherosclerotic lesion development. Most of these T-cell subpopulations prove to be inflammatory, accelerating lesion development and stability.90, 92 Regulatory T cells are the main cells from adaptive immunity that have been shown to have an atheroprotective effect in CVD mostly through the secretion of anti-inflammatory cytokines and the inhibition of some inflammatory T-cell subsets.72, 93 Over the past decade, a number of discoveries have allowed a better understanding of the inflammatory pathways involved in promoting enhanced hematopoiesis and its impact on WBC homeostasis in the context of CVD.94 This knowledge will be useful in targeting these pathways when developing novel or prescribing existing anti-inflammatory therapies to treat CVD.
Cholesterol homeostasis and hematopoiesis
A direct correlation between hyperlipidemia and leukocyte number is routinely reported in humans.95, 96 Furthermore, lowering LDL and increasing HDL with pravastatin treatment in people with coronary artery disease was associated with reductions in monocytes and neutrophils.97 These effects with statins have also been modeled in hypercholesterolemic Apoe−/− mice where statin use reduced inflammatory Ly6-Chi monocytes.98 Thus, a close relationship exists between cholesterol homeostasis and circulating leukocytes. Recent work has begun to unravel an important role for intrinsic cholesterol-handling in bone marrow HSPCs in modulating leukocyte production (reviewed recently99). Cellular cholesterol levels are determined by the balance between de novo synthesis and cellular flux (that is, uptake versus efflux). Critical regulators of cellular cholesterol levels are the ATP-binding Cassette Transporters (ABC)A1 and ABCG1, which transport cholesterol from membranes to HDL particles. In a hallmark study, Yvan-Charvet et al.100 revealed that mice deficient of ABCA1 and ABCG1 develop prominent leukocytosis and accelerated atherosclerosis. It was found that leukocytosis was due to altered proliferation of HSPCs in the bone marrow and in extramedullary locations.101 In these mice the hyperproliferation of the HSPCs was linked with increased plasma membrane lipid rafts and levels of the common β-subunit of the receptor for IL-3 and GM-CSF. Thus, these data reveal that impaired cholesterol efflux and the subsequent cholesterol accumulation in HSPCs result in enhanced proliferation because of enhanced sensitivity to growth factor signaling. A similar mechanism was also discovered in western-type-diet-fed Apoe−/− mice.102 The proliferative defects in both the Abca1−/−Abcg1−/− and Apoe−/− mice were cell-intrinsic, meaning that it was not simply because of changes in systemic inflammation. Importantly, restoring the cholesterol efflux in the Abca1−/−Abcg1−/− and Apoe−/− mice restored this proliferative defect.100, 102 It is also of interest that the cholesterol transporter ABCG4 is highly expressed in bone marrow megakaryocyte progenitors and has been found to regulate thrombopoiesis.103 Thus, this newly discovered link between intrinsic cellular cholesterol metabolism in regulating hematopoiesis and atherosclerosis highlights the potential importance of maintaining efflux potential in HSPCs.
Hematopoiesis and CVD risk: lessons learned from RA
Reductions in neutrophil numbers are consistently reported in RA patients treated with tocilizumab, which coincides with increases in cholesterol.48, 50, 51 Whether the increase in cholesterol by IL-6 blockade is caused by a suppression of hematopoiesis remains to be tested experimentally. However, IL-6 has been shown to stimulate granulopoiesis in the absence of G-CSF and GM-CSF in times of increased neutrophil need,104, 105 an effect most likely regulated by IL-6 trans-signaling.106 Furthermore, two studies in the New England Journal of Medicine revealed that treatment of RA with the JAK inhibitor tofacitinib caused reductions in neutrophils, which mirrored increases in LDL cholesterol.107, 108 Whereas these data reveal that inhibitors of JAK and IL-6 can reduce neutrophil levels, they do not show that WBC production is enhanced in RA. However, it is well recognized that patients with RA generally present with leukocytosis due primarily to monocytosis and neutrophilia.109 There remains, however, a relative paucity of data, directly assessing hematopoiesis and cholesterol homeostasis in RA. Looking at another pathology, people with myeloproliferative diseases also exhibit hypocholesterolemia which, importantly, is reversed with successful disease treatment.110 Furthermore, the administration of the hematopoietic growth factors macrophage colony-stimulating factor or GM-CSF into non-human primates and rabbits or humans, respectively, causes a reduction in cholesterol levels.111, 112 Conversely, immunosuppressants required for successful hematopoietic stem cell transplantation and solid organ transplantation cause dyslipidemia.113, 114 Collectively, these studies reveal that altered hematopoiesis (by either pathological or therapeutically induced means) can have a direct impact on cholesterol homeostasis and provide a potential mechanism by which cholesterol levels are reduced in states of high inflammatory burden. To date, very few studies have directly investigated whether anti-inflammatory treatments alter RA-induced leukocytosis. In one particular study, a significant increase in circulating neutrophils, monocytes and platelets was found utilizing a rodent model of adjuvant-induced arthritis. Importantly, the treatment of these arthritic rodents with tofacitinib caused a normalization of platelet, reticulocyte and neutrophil levels in the blood, which was associated with an increase in cholesterol levels.58 Taken together, these data reveal an important link between arthritis-induced hematopoiesis and perturbations in circulating cholesterol levels.
Metabolic dysfunctions and hematopoiesis: consequences on atherosclerosis
Circulating myeloid cells are also elevated in states of metabolic dysfunction such as obesity, insulin resistance and diabetes,115, 116, 117, 118 and are thought to contribute directly to the enhanced atherosclerosis observed in patients with these conditions. Importantly, even when lipids are controlled with statins, significant residual CVD risk remains in people with diabetes.119 Indeed, it was found in streptozotocin-treated diabetic mice that hyperglycemia per se promoted monocytosis by inducing bone marrow myeloid progenitor cell expansion and proliferation. In addition, it was shown that diabetic animals have an impaired potential for atherosclerotic lesion regression.120 Importantly, it seems that in diabetic animals, monocytosis is mostly linked to hyperglycemia as monocyte levels were not affected by changes in circulating cholesterol and insulin levels and was resolved following glucose normalization.
Interestingly, a recent clinical trial of over 7000 patients with T2D (EMPA-REG OUTCOME) showed that a glucose-lowering treatment (using empagliflozin, a sodium glucose co-transporter 2 inhibitor (SGLT2i) that blocks renal re-absorption of glucose) was associated to lower rates of death from CV causes and from any cause compared with placebo,121 making this the first antidiabetic drug to lower CV events to date. However, this was primarily because of a reduction in heart failure through an unknown mechanism, and no readouts on inflammatory profiles were reported. A recent meta-analysis has suggested that there is also a reduction in myocardial infarction in SGLT2i-treated diabetic patients.122 Hence, further longitudinal studies are still required to investigate the anti-atherogenic effects of glucose-lowering with SGLT2is in people with diabetes.
Interestingly, prominent monocytosis and neutrophilia due to altered hematopoiesis are also observed in mouse models of obesity.123 Unlike the streptozotocin model, hyperglycemia was moderate and glucose-lowering had no effect on monocytosis, signifying an alternative mechanism of action.123 Together, these data provide mechanistic evidence by which diabetes and obesity can promote atherosclerosis by modulating hematopoiesis and monocytosis.
Should we tolerate dyslipidemia as an acceptable side effect when suppressing inflammation to treat CVD?
Having discussed the importance of hematopoiesis in metabolic disease and its impact on CVD, it appears that, despite promoting dyslipidemia, some of the current anti-inflammatory treatments for metabolic diseases are able to correct the hematopoietic dysfunctions. In T2D patients treated with salsalate, increased total and LDL cholesterol was associated with significant reductions in neutrophils and lymphocytes.10, 11 Recently, IL-1β was identified in preclinical studies as a contributor to obesity-induced monocytosis by directly modulating bone marrow myeloid progenitor cell expansion and proliferation via the IL-1 receptor.123 When obese mice were treated with the IL-1R antagonist anakinra, circulating monocytes and neutrophils were reduced. Similarly, anakinra reduces blood cell counts in humans with T2D, neonatal-onset multisystem inflammatory disease and following a stroke.15, 124, 125 Similarly, an anti-IL-1β antibody LY2189102 reduces neutrophils and WBCs in patients with T2D.126 In the EMPA-REG OUTCOME trial, despite a reduction in Hba1c with empagliflozin treatment, an increase in circulating LDL and HDL cholesterol was observed. Similar increases in cholesterol have been observed with canagliflozin, another SGLT2i.127 Unfortunately, in the study by Zinman et al.121 WBCs were not reported; however, based on the study by Nagareddy et al.,128 it could be expected that leukocytosis would be reduced by SGLT2i in the context of hyperglycemia-induced inflammation, but this pathway would likely be over-ridden if obesity was also present.123
What is the future for current therapies?
One of the staple treatments in people with CVD is statins. This class of drugs effectively lowers plasma cholesterol level by blocking endogenous cholesterol synthesis, primarily in the liver, and by causing the upregulation of the LDL receptor. By targeting endogenous cholesterol pathways and the formation of synthesis intermediaries, statins also show some anti-inflammatory properties; however, this is probably a mild effect compared with targeted anti-inflammatory therapies. Thus, now that CVD is defined as a chronic inflammatory disease, the question is how important is plasma cholesterol levels versus inflammation in the pathogenesis of CVD (Figure 1). The link between elevated cholesterol levels and CVD risk is well appreciated and should not be lightly dismissed. Considerable epidemiological and experimental evidence has reinforced the mechanisms by which elevated cholesterol (particularly, LDL cholesterol) drives atherogenesis and its associated CV complications. As such, statins remain the frontline drug treatment for the treatment of hypercholesterolemia, and their impact on the reduction of CVD risk and mortality cannot be understated with randomized clinical trials reporting a reduction of up to 30% in coronary events associated with statin use.129 Epidemiological studies also show that increased plasma cholesterol is associated with monocytosis,95 and this can be modeled in mice when manipulating genes involved in cholesterol metabolism (that is, Apoe, Abc transporters, Ldlr and Apoa-I).100, 102, 130 As previously discussed, mouse models advocate that, even with an adverse lipid profile, reduced monocytes clearly equate to smaller atherosclerotic lesions.131 Whereas statins or other lipid-modulating therapies will always remain staple drugs in the treatment of CVD, these genetic models do highlight the importance of also targeting inflammation in the etiology of CVD.
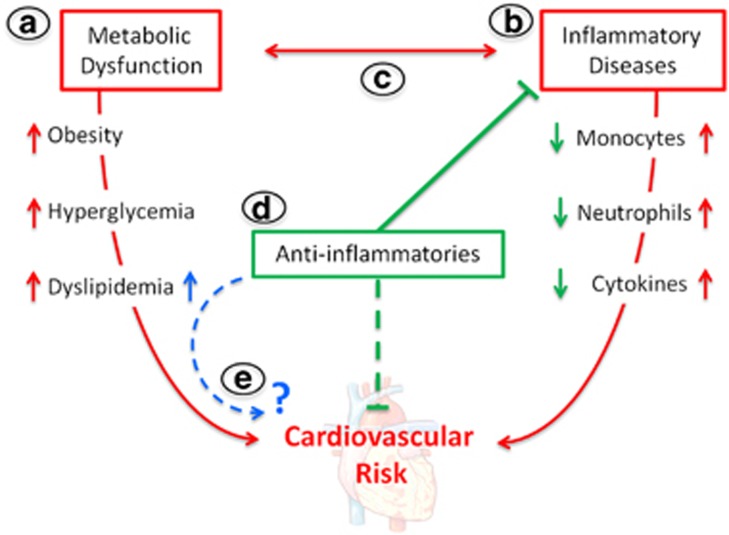
Figure 1.
Is cardiometabolic risk elevated with anti-inflammatory drug use? (a) Metabolic disorders such as obesity, hyperglycemia and dyslipidemia increase the risk of cardiovascular disease. (b) Inflammatory diseases are hallmarked by enhanced myelopoiesis and increased cytokine levels that can directly elevate cardiovascular risk. (c) Metabolic dysfunction and inflammation can also be interconnected to further drive cardiovascular risk. (d) Anti-inflammatory treatments reduce disease severity and dampen inflammation, which appears to lower cardiovascular risk. (e) However, anti-inflammatories are also associated with elevated lipid levels and could potentially have a negative impact on cardiovascular risk over time.
Conclusion
Inflammation clearly has a role in elevating CVD risk in disease populations irrespective of whether cholesterol levels are elevated (obesity, insulin resistance and diabetes) or reduced (RA, HIV, infection, sepsis, systemic lupus erythematous and several cancers). An emerging trend is that several anti-inflammatory treatments appear to increase cholesterol levels. Current evidence suggests that these increases in cholesterol are not associated with any obvious adverse CV effects in the context of sustained inflammation suppression. Whether this is because of a beneficial alteration in cholesterol subclasses, the reduction in inflammation or a combination of both remains unknown. In addition, understanding how anti-inflammatories promote increased cholesterol levels and whether this is due to the suppression of hematopoiesis and stem cell proliferation is a critical question with potential wide-reaching implications, and certainly warrants further investigation.
The long-term CV safety of anti-inflammatory therapies will become more apparent with data to emerge from current trials in future years and will allow us to decipher whether the contribution of inflammation to CVD is equally or more important than the metabolic abnormalities. However, until we better understand this relationship, a close screening of all patients receiving anti-inflammatory drugs should be mandate in order to closely monitor CVD risk in case of subsequent dyslipidemia.
Acknowledgments
AJM was supported by a Career Development Fellowship from the NHMRC, a Future Leader Fellowship from the NHF and Project grants from the NHMRC and Diabetes Australia. MJK is the Russell Berrie Foundation Scholar in Diabetes Research from the Naomi Berrie Diabetes Centre.
The authors declare no conflict of interest.
References
- Kammoun HL, Kraakman MJ, Febbraio MA. Adipose tissue inflammation in glucose metabolism. Rev Endocr Metab Disord 2014; 15: 31–44. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Tall AR. Disordered haematopoiesis and athero-thrombosis. Eur Heart J 2016; 37: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol 2015; 12: 15–28. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001; 293: 1673–1677. [DOI] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008; 322: 1539–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br Med J 2002; 324: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336: 973–979. [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Buck JS, Desouza C, Fonseca V, Chen YD, Shoelson SE et al. Targeting inflammation using salsalate in patients with type 2 diabetes: effects on flow-mediated dilation (TINSAL-FMD). Diabetes Care 2013; 36: 4132–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med 2013; 159: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci 2008; 1: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia 2013; 56: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011; 117: 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007; 356: 1517–1526. [DOI] [PubMed] [Google Scholar]
- van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2011; 96: 2119–2126. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011; 162: 597–605. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91. [DOI] [PubMed] [Google Scholar]
- Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 1996; 45: 881–885. [DOI] [PubMed] [Google Scholar]
- Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS et al. Effects of TNF-alpha neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab 2007; 293: E102–E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquot N, Castillo MJ, Lefebvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocrinol Metab 2000; 85: 1316–1319. [DOI] [PubMed] [Google Scholar]
- Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab 2011; 96: E146–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med 2013; 19: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C, Jandeleit-Dahm KA, Sheehy K, Murphy A, Chin-Dusting J, Kling D et al. Reduced plaque formation induced by rosiglitazone in an STZ-diabetes mouse model of atherosclerosis is associated with downregulation of adhesion molecules. Atherosclerosis 2008; 199: 55–64. [DOI] [PubMed] [Google Scholar]
- Esterson YB, Zhang K, Koppaka S, Kehlenbrink S, Kishore P, Raghavan P et al. Insulin sensitizing and anti-inflammatory effects of thiazolidinediones are heightened in obese patients. J Invest Med 2013; 61: 1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta 2007; 1771: 1065–1081. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Jablonski KA, Fonseca V, Shoelson SE, Goldfine AB, Strauch C et al. The impact of salsalate treatment on serum levels of advanced glycation end products in type 2 diabetes. Diabetes Care 2014; 37: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes. Endocr Pract 2012; 18: 826–833. [DOI] [PubMed] [Google Scholar]
- Spanheimer R, Betteridge DJ, Tan MH, Ferrannini E, Charbonnel B. Investigators PR. Long-term lipid effects of pioglitazone by baseline anti-hyperglycemia medication therapy and statin use from the PROactive experience (PROactive 14). Am J Cardiol 2009; 104: 234–239. [DOI] [PubMed] [Google Scholar]
- Deeg MA, Buse JB, Goldberg RB, Kendall DM, Zagar AJ, Jacober SJ et al. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2007; 30: 2458–2464. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. Clinical trials with thiazolidinediones in subjects with Type 2 diabetes—is pioglitazone any different from rosiglitazone? Expert Opin Pharmacother 2008; 9: 405–420. [DOI] [PubMed] [Google Scholar]
- van Wijk JP, de Koning EJ, Martens EP, Rabelink TJ. Thiazolidinediones and blood lipids in type 2 diabetes. Arterioscler Thromb Vasc Biol 2003; 23: 1744–1749. [DOI] [PubMed] [Google Scholar]
- Wascher TC, Lindeman JH, Sourij H, Kooistra T, Pacini G, Roden M. Chronic TNF-alpha neutralization does not improve insulin resistance or endothelial function in "healthy" men with metabolic syndrome. Mol Med 2011; 17: 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res 2005; 42: 517–525. [DOI] [PubMed] [Google Scholar]
- Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med 2006; 166: 902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huvers FC, Popa C, Netea MG, van den Hoogen FH, Tack CJ. Improved insulin sensitivity by anti-TNFalpha antibody treatment in patients with rheumatic diseases. Ann Rheum Dis 2007; 66: 558–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol 2007; 26: 1495–1498. [DOI] [PubMed] [Google Scholar]
- The Interleukin 1 Genetics Consortium. Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol 2015; 3: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 2012; 126: 2739–2748. [DOI] [PubMed] [Google Scholar]
- Navarro-Millan I, Charles-Schoeman C, Yang S, Bathon JM, Bridges SL Jr., Chen L et al. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum 2013; 65: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010; 49: 295–307. [DOI] [PubMed] [Google Scholar]
- Daien CI, Duny Y, Barnetche T, Daures JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis 2012; 71: 862–868. [DOI] [PubMed] [Google Scholar]
- Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis 2009; 68: 460–469. [DOI] [PubMed] [Google Scholar]
- Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011; 63: 522–529. [DOI] [PubMed] [Google Scholar]
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003; 107: 3133–3140. [DOI] [PubMed] [Google Scholar]
- Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFalpha inhibitors and rituximab. Ann Rheum Dis 2015; 74: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004; 50: 1761–1769. [DOI] [PubMed] [Google Scholar]
- Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006; 54: 2817–2829. [DOI] [PubMed] [Google Scholar]
- Jones G. The AMBITION trial: tocilizumab monotherapy for rheumatoid arthritis. Expert Rev Clin Immunol 2010; 6: 189–195. [DOI] [PubMed] [Google Scholar]
- Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008; 67: 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008; 58: 2968–2980. [DOI] [PubMed] [Google Scholar]
- Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 2015; 26: 475–487. [DOI] [PubMed] [Google Scholar]
- Nowell MA, Williams AS, Carty SA, Scheller J, Hayes AJ, Jones GW et al. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J Immunol 2009; 182: 613–622. [DOI] [PubMed] [Google Scholar]
- Richards PJ, Nowell MA, Horiuchi S, McLoughlin RM, Fielding CA, Grau S et al. Functional characterization of a soluble gp130 isoform and its therapeutic capacity in an experimental model of inflammatory arthritis. Arthritis Rheum 2006; 54: 1662–1672. [DOI] [PubMed] [Google Scholar]
- Rabe B, Chalaris A, May U, Waetzig GH, Seegert D, Williams AS et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood 2008; 111: 1021–1028. [DOI] [PubMed] [Google Scholar]
- Kraakman MJ, Kammoun HL, Allen TL, Deswaerte V, Henstridge DC, Estevez E et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab 2015; 21: 403–416. [DOI] [PubMed] [Google Scholar]
- Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2012; 32: 281–290. [DOI] [PubMed] [Google Scholar]
- Balague C, Pont M, Prats N, Godessart N. Profiling of dihydroorotate dehydrogenase, p38 and JAK inhibitors in the rat adjuvant-induced arthritis model: a translational study. Br J Pharmacol 2012; 166: 1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009; 60: 1895–1905. [DOI] [PubMed] [Google Scholar]
- Hsue PY, Scherzer R, Grunfeld C, Imboden J, Wu Y, Del Puerto G et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc 2014; 3: e001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juanatey C, Llorca J, Vazquez-Rodriguez TR, Diaz-Varela N, Garcia-Quiroga H, Gonzalez-Gay MA. Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor alpha blocker therapy. Arthritis Rheum 2008; 59: 1821–1824. [DOI] [PubMed] [Google Scholar]
- Kerekes G, Soltesz P, Der H, Veres K, Szabo Z, Vegvari A et al. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol 2009; 28: 705–710. [DOI] [PubMed] [Google Scholar]
- van Vollenhoven RF, Emery P, Bingham CO 3rd, Keystone EC, Fleischmann RM, Furst DE et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis 2013; 72: 1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasoedova E, Crowson CS, Kremers HM, Fitz-Gibbon PD, Therneau TM, Gabriel SE. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis 2010; 69: 1310–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao KP, Cai T, Gainer VS, Cagan A, Murphy SN, Liu C et al. Lipid and lipoprotein levels and trends in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken) 2013; 65: 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 2015; 74: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa C, van Tits LJ, Barrera P, Lemmers HL, van den Hoogen FH, van Riel PL et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis 2009; 68: 868–872. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Rubbert-Roth A, Smolen JS, Kremer J, Khraishi M, Gomez-Reino J et al. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J Rheumatol 2013; 40: 768–780. [DOI] [PubMed] [Google Scholar]
- Kume K, Amano K, Yamada S, Hatta K, Ohta H, Kuwaba N. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomized controlled trial. J Rheumatol 2011; 38: 2169–2171. [DOI] [PubMed] [Google Scholar]
- Raterman HG, Levels H, Voskuyl AE, Lems WF, Dijkmans BA, Nurmohamed MT. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis 2013; 72: 560–565. [DOI] [PubMed] [Google Scholar]
- Ohman M, Ohman ML, Wallberg-Jonsson S. The apoB/apoA1 ratio predicts future cardiovascular events in patients with rheumatoid arthritis. Scand J Rheumatol 2014; 43: 259–264. [DOI] [PubMed] [Google Scholar]
- Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest 2013; 123: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles-Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015; 67: 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger MA, Schroeder T. Hematopoiesis. Cold Spring Harb Perspect Biol 2012; 4: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014; 505: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Hematopoietic cytokines. Blood 2008; 111: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol 1997; 145: 416–421. [DOI] [PubMed] [Google Scholar]
- Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol 2001; 154: 758–764. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998; 279: 1477–1482. [DOI] [PubMed] [Google Scholar]
- Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost 2011; 106: 827–838. [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Swirski FK. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol Metab 2013; 24: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir TA, Afzal MN, Habib ur R. Baseline leukocyte count and acute coronary syndrome: predictor of adverse cardiac events, long and short-term mortality and association with traditional risk factors, cardiac biomarkers and C-reactive protein. J Ayub Med Coll Abbottabad 2009; 21: 46–50. [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS et al. Myocardial infarction accelerates atherosclerosis. Nature 2012; 487: 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares R, Ducimetiere P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol 1993; 137: 49–53. [DOI] [PubMed] [Google Scholar]
- Ganda A, Magnusson M, Yvan-Charvet L, Hedblad B, Engstrom G, Ai D et al. Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation 2013; 127: z988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzas P, Quiles J, Lopez de Sa E, Sanchez A, Rubio R, Garcia E et al. Neutrophil count and infarct size in patients with acute myocardial infarction. Int J Cardiol 2004; 97: 155–156. [DOI] [PubMed] [Google Scholar]
- Haumer M, Amighi J, Exner M, Mlekusch W, Sabeti S, Schlager O et al. Association of neutrophils and future cardiovascular events in patients with peripheral artery disease. J Vasc Surg 2005; 41: 610–617. [DOI] [PubMed] [Google Scholar]
- Hedrick CC. Lymphocytes in atherosclerosis. Arterioscler Thromb Vasc Biol 2015; 35: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigren M, Nilsson J, Kolbus D. Lymphocytes in atherosclerosis. Clin Chim Acta 2012; 413: 1562–1568. [DOI] [PubMed] [Google Scholar]
- Ammirati E, Moroni F, Magnoni M, Camici PG. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin Exp Immunol 2015; 179: 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: an expanded "cardiovascular continuum”. J Am Coll Cardiol 2016; 67: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 2000; 101: 2883–2888. [DOI] [PubMed] [Google Scholar]
- Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol 2015; 35: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet MJ, Nahrendorf M, Swirski FK. The journey from stem cell to macrophage. Ann N Y Acad Sci 2014; 1319: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe G, Gabriel H, Kovacs E, Klucken J, Stohr J, Kindermann W et al. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler Thromb Vasc Biol 1996; 16: 1437–1447. [DOI] [PubMed] [Google Scholar]
- Ates AH, Canpolat U, Yorgun H, Kaya EB, Sunman H, Demiri E et al. Total white blood cell count is associated with the presence, severity and extent of coronary atherosclerosis detected by dual-source multislice computed tomographic coronary angiography. Cardiol J 2011; 18: 371–377. [PubMed] [Google Scholar]
- Tani S, Nagao K, Anazawa T, Kawamata H, Furuya S, Takahashi H et al. Association of leukocyte subtype counts with coronary atherosclerotic regression following pravastatin treatment. Am J Cardiol 2009; 104: 464–469. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007; 117: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 2015; 15: 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010; 328: 1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 2012; 11: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 2011; 121: 4138–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A et al. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med 2013; 19: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F, Zhang HH, Matthews V, Weinstock J, Nice EC, Ernst M et al. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood 2008; 111: 3978–3985. [DOI] [PubMed] [Google Scholar]
- Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood 1997; 90: 2583–2590. [PubMed] [Google Scholar]
- Peters M, Muller AM, Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood 1998; 92: 3495–3504. [PubMed] [Google Scholar]
- van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012; 367: 508–519. [DOI] [PubMed] [Google Scholar]
- Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012; 367: 495–507. [DOI] [PubMed] [Google Scholar]
- Klimek E, Mikolajczyk T, Sulicka J, Kwasny-Krochin B, Korkosz M, Osmenda G et al. Blood monocyte subsets and selected cardiovascular risk markers in rheumatoid arthritis of short duration in relation to disease activity. Biomed Res Int 2014; 2014: 736853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HS, Ginsberg H, Fagerstrom R, Brown WV. Characterization of hypocholesterolemia in myeloproliferative disease. Relation to disease manifestations and activity. Am J Med 1981; 71: 595–602. [DOI] [PubMed] [Google Scholar]
- Stoudemire JB, Garnick MB. Effects of recombinant human macrophage colony-stimulating factor on plasma cholesterol levels. Blood 1991; 77: 750–755. [PubMed] [Google Scholar]
- Nimer SD, Champlin RE, Golde DW. Serum cholesterol-lowering activity of granulocyte-macrophage colony-stimulating factor. JAMA 1988; 260: 3297–3300. [PubMed] [Google Scholar]
- Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2002; 2: 807–818. [DOI] [PubMed] [Google Scholar]
- Griffith ML, Savani BN, Boord JB. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood 2010; 116: 1197–1204. [DOI] [PubMed] [Google Scholar]
- Ford ES. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of US adults. Am J Epidemiol 2002; 155: 57–64. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30,>or =30). Am J Cardiol 2002; 89: 1441–1443. [DOI] [PubMed] [Google Scholar]
- Ohshita K, Yamane K, Hanafusa M, Mori H, Mito K, Okubo M et al. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care 2004; 27: 491–496. [DOI] [PubMed] [Google Scholar]
- Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 2011; 31: 2322–2330. [DOI] [PubMed] [Google Scholar]
- Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M et al. Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome—serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN-ACS Trial). Circ J 2010; 74: 1165–1174. [DOI] [PubMed] [Google Scholar]
- Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ et al. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes 2011; 60: 1759–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- Sonesson C, Johansson PA, Johnsson E, Gause-Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysis. Cardiovasc Diabetol 2016; 15: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 2014; 19: 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med 2006; 355: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry 2005; 76: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Abu-Raddad E, Polzer J, Miller JW, Scherer JC, De Gaetano A et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care 2013; 36: 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode B, Stenlof K, Harris S, Sullivan D, Fung A, Usiskin K et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab 2015; 17: 294–303. [DOI] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013; 17: 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352: 29–38. [DOI] [PubMed] [Google Scholar]
- Tolani S, Pagler TA, Murphy AJ, Bochem AE, Abramowicz S, Welch C et al. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis 2013; 229: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M et al. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest 1998; 101: 2702–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]