Abstract
Prospective observational studies have shown inconsistent associations of dietary or circulating n-3 long-chain polyunsaturated fatty acids (LCPUFA) with risk of all-cause mortality. A meta-analysis was performed to evaluate the associations. Potentially eligible studies were identified by searching PubMed and EMBASE databases. The summary relative risks (RRs) with 95% confidence intervals (CIs) were calculated using the random-effects model. Eleven prospective studies involving 371 965 participants from general populations and 31 185 death events were included. The summary RR of all-cause mortality for high-versus-low n-3 LCPUFA intake was 0.91 (95% CI: 0.84–0.98). The summary RR for eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) intake was 0.83 (95% CI: 0.75–0.92) and 0.81 (95% CI: 0.74–0.95), respectively. In the dose-response analysis, each 0.3 g/d increment in n-3 LCPUFA intake was associated with 6% lower risk of all-cause mortality (RR = 0.94, 95% CI: 0.89–0.99); and each 1% increment in the proportions of circulating EPA and DHA in total fatty acids in blood was associated with 20% (RR = 0.80, 95% CI: 0.65–0.98) and 21% (RR = 0.79, 95% CI: 0.63–0.99) decreased risk of all-cause mortality, respectively. Moderate to high heterogeneity was observed across our anlayses. Our findings suggest that both dietary and circulating LCPUFA are inversely associated with all-cause mortality.
The potential benefits of marine derived n-3 long-chain polyunsaturated fatty acids (LCPUFA) (e.g., eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) have received great attention for over 40 years1. Prospective observational studies have shown consistent associations between intake of fish, a major dietary source of n-3 LCPUFA, and reduced risk of cardiovascular disease (CVD), in particular of coronary heart disease (CHD) mortality2. Experimental and human intervention studies have demonstrated that n-3 LCPUFA have various physiologic benefits, such as improvement of endothelial function3 and reductions of heart rate, blood pressure, and inflammation4,5. Some early randomized controlled trials (RCT) find significant reductions in cardiac death by n-3 LCPUFA supplementation6. The encouraging evidence led to the American Heart Association to recommendation to increase oily fish intake or n-3 LCPUFA supplementation for primary and secondary prevention of CHD7. Such a recommendation, however, has been questioned by several recent human intervention trials8,9 and meta-analyses10,11 of available RCTs. Potential explanations for the differences between observational and clinical studies, and between early and recent trials are addressed in greater depth below.
Circulating concentrations of n-3 LCPUFA represent a good indicator of dietary intakes and endogenous metabolism12. There have been a number of prospective observational studies that investigate the relationships between dietary or circulating n-3 LCPUFA and all-cause mortality, and the results have been inconsistent13,14,15,16,17,18,19,20,21,22. In an attempt to quantitatively summarize the evidence, a systematic review and meta-analysis was carried out in the present study.
Results
Study selection and characteristics
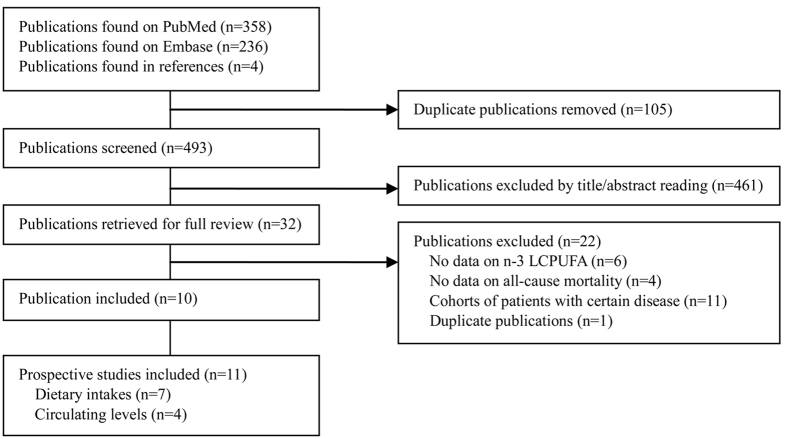
A flow chart of study selection is reported in Fig. 1. Briefly, a total of 493 independent citations were identified after duplicate exclusion, of which 32 were retrieved for full-text review. Ten publications were excluded because the exposure or outcome was not relevant to the topic we studied; 11 reports were excluded because they investigated dietary/circulating n-3 LCPUFA among patients with certain diseases, such as type 2 diabetes23,24, CHD25,26,27,28, heart failure29,30, and kidney disease31,32,33; further excluded was one publication34 which was an overlapping report of another35 with larger events. Finally, 10 publications13,14,15,16,17,18,19,20,21,22 including 11 independent prospective studies (2 cohorts were combined in one publication17) were included in this meta-analysis, involving 7 studies13,14,15,16,17,18 on dietary n-3 LCPUFA and 4 studies19,20,21,22 on circulating EPA/DHA in relation to all-cause mortality risk. These studies covered a total of 371 965 participants from general populations and 31 185 death events.
Figure 1. Literature search for the meta-analysis.
LCPUFA, long-chain polyunsaturated fatty acids.
The 7 studies on dietary n-3 LCPUFA were published between 2004 and 2015, covering 361 273 participants and 27 624 deaths during 5.0 to 24 years of follow-up. Dietary intakes were mostly assessed with self-administered food frequency questionnaires (FFQ), with the exception of one Japanese cohort15 in which food records were applied. N-3 LCPUFA consisted of both EPA and DHA in all studies and the intakes were estimated from food sources in 6 studies14,15,16,17,18 and from both food and supplementation in one13. In all four studies that were published between 2008 and 2015, circulating n-3 LCPUFA were measured by gas chromatography. A total of 3561 death events were identified from 10 692 participants during 9.6 to 30.7 years of follow-up. All of the 4 studies separately reported results for EPA and DHA. The characteristics of the included studies are summarized in Table 1. All of the included studies provided risk estimates that were controlled for multi-variables.
Table 1. Prospective studies that investigated the association of dietary and circulating EPA and/or DHA with risk of all-cause mortality.
| First author, year (Country/region) | Source of populations, duration | Participants | No. of deaths | Comparison | RR (95% CI) | Diet assessment | Adjustment for potential confounders | Quality scores |
|---|---|---|---|---|---|---|---|---|
| Dietary intake | ||||||||
| Folsom, 2004 (United States) | IWHS, 14yr | 41 836 F aged 55–69 yr | 4653 | Q5 vs. Q1 | 0.96 (0.86–1.06) | Self-reported FFQ | Age, BMI, WHR, education, physical activity, smoking, age at first live birth, estrogen use, vitamin use, diabetes, hypertension, and intake of energy, alcohol, whole grains, fruit and vegetables, red meat, cholesterol, and saturated fat. | 7 |
| Nagata, 2012 (Japan) | Takayama Study, 16 yr | 28 356 M/F aged ≥35 yr | 2499 M; 2117 F | Q5 vs. Q1 | 1.03 (0.86–1.23) (M) 1.04 (0.85–1.26) (F) | Self-reported FFQ, validated | Age, height, BMI, physical activity, smoking, education, marital status, histories of diabetes and hypertension, and intakes of energy, alcohol, protein, SFA, MUFA, non-long-chain n-3 PUFA, fruit, vegetables, fiber, and percent energy from carbohydrate in foods other than rice. | 9 |
| Takata, 2013 (China) | SMHS, 5.6 yr; SWHS, 11.2 yr | 61 137 M aged 40–74 yr; 73 159 F aged 40–70 yr | 2170 M; 3666 F | Q5 vs. Q1 | 0.79 (0.72–0.87) | Self-reported FFQ, validated | Age, income, occupation, education, comorbidity index, physical activity, smoking, and intakes of energy, alcohol (for M), red meat, poultry, fruit, and vegetable. | 8 |
| Miyagawa, 2014 (Japan) | NIPPON DATA80, 24 yr | 9190 M/F aged ≥30 yr | 2551 | Q5 vs. Q1 | 0.80 (0.66–0.96)a 0.97 (0.84–1.12)b | Food records | Age, sex, BMI, smoking, SBP, blood glucose, serum total cholesterol, antihypertensive medication status, residential area, intakes of alcohol SFA, n-6 PUFA, vegetable protein, total dietary fiber, and sodium. | 8 |
| Bell, 2014 (United States) | VITAL Study, 5.0 yr | 70 495 M/F aged 50–76 yr | 3051 | Q4 vs. Q1 | 0.84 (0.76–0.93) | Self-reported FFQ | Age, sex, BMI, smoking, race/ethnicity, marital status, education, physical activity, self-rated health, mammogram in, prostate- specific antigen test, sigmoidoscopy, uses of cholesterol-lowering medication, aspirin, non-aspirin NSAIDs, estrogen, and estrogen+progestin, morbidity score, age at menopause, age at death of father or mother, and intakes of total energy and energy from trans fat and SFA, alcohol, fruit, and vegetables, | 7 |
| Villegas, 2015 (United States) | SCCS, 5.5 yr | 77 100 M/F aged 40–79 yr | 6917 | Q5 vs. Q1 | 0.94 (0.86–1.03) | Self-reported FFQ, validated | Age, sex, BMI, smoking, physical activity, income, education, insurance coverage, race, and intakes of energy, alcohol, and total meat. | 8 |
| Circulating levels | ||||||||
| Warensjö, 2008 (Sweden) | ULSAM, 30.7yr | 1885 M aged 50 yr | 1012 | Per SDc increase | EPA: 1.00 (0.94–1.08) DHA: 0.95 (0.89–1.02) | Gas chromatography | Total cholesterol, BMI, smoking, physical activity, and hypertension. | 8 |
| Chien, 2013 (Taiwan) | Residents living in Chin-Shan Township, 9.6 yr | 1833 M/F aged >35 yr | 568 | Q4 vs. Q1 | EPA: 0.77 (0.59–1.00) DHA: 0.89 (0.68–1.18) | Gas chromatography | Age, sex, BMI, smoking, alcohol drinking, marital status, education, occupation, sports activity, hypertension, diabetes, LDL-C and HDL-C levels. | 8 |
| Mozaffarian, 2013 (United States) | CHS | 2692 M/F aged ≥65 yr | 1625 | Q5 vs. Q1 | EPA: 0.83 (0.71–0.98) DHA: 0.80 (0.67–0.94) | Gas chromatography | Age, sex, BMI, WC, physical activity, race, education, enrollment site, fatty acids measurement batch, smoking, prevalent diabetes, AF, and drug- treated hypertension, and intakes of alcohol, tuna or other broiled or baked fish, fried fish, red meat, fruit, vegetables, and dietary fiber. | 7 |
| Marklund, 2015 (Sweden) | Residents living in Stockholm County, 14.5 yr | 4232M/F aged 60 yr | 356 | Q4 vs. Q1d | EPA: 0.81 (0.72–0.91) DHA: 0.75 (0.68–0.84) | Gas chromatography | Sex, BMI, smoking, physical activity, education, alcohol intake, diabetes mellitus, drug-treated hypertension, and drug-treated hypercholesterolemia. | 8 |
AF, atrial fibrillation; BMI, body mass index; CHS, Cardiovascular Health Study; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; F, females; FFQ, Food frequency questionnaire; HDL-C, high-density lipoprotein cholesterol; IWHS, Iowa Women’s Health Study; LDL-C, low-density lipoprotein cholesterol; M, males; MUFA, monounsaturated fatty acid; NSAIDs, non-aspirin nonsteroidal anti-inflammatory drugs; PUFA, polyunsaturated fatty acid; Q, quartile/quintile; SCCS, Southern Community Cohort Study; SBP, systolic blood pressure; SFA, saturated fatty acids; SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study; ULSAM, The Uppsala Longitudinal Study of Adult Men; WC, waist circumference; WHR, waist/hip ratio; yr, years.
aRisk estimates for total CVD mortality.
bRisk estimates for total non-CVD mortality.
cThe estimated SD was 0.52% for EPA and 0.19% for DHA, respectively.
dThis study also reported risk estimates for per SD (1% for EPA and 0.2% for DHA, respectively) increase in circulating long-chain n-3 fatty acids, and presented sex-specific results.
N-3 LCPUFA intake and all-cause mortality
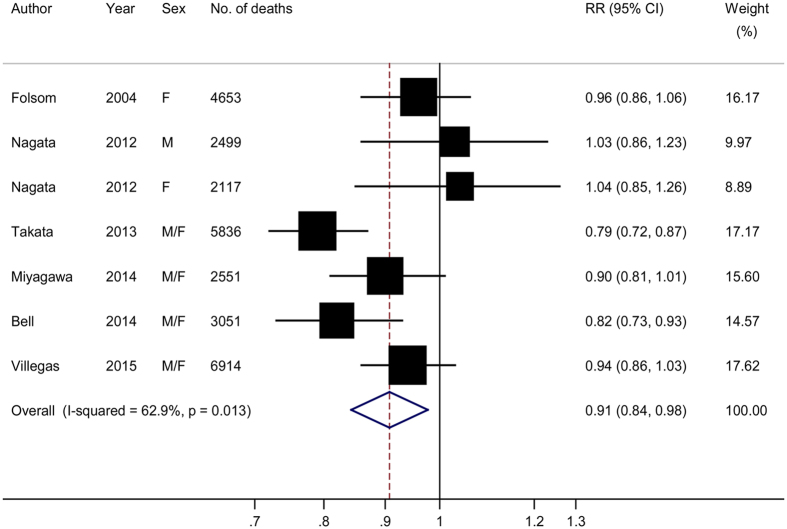
A meta-analysis of the 7 prospective studies suggested a summary RR of 0.91 (95% CI: 0.84–0.98) for the highest compared with lowest categories of n-3 LCPUFA intake, with moderate heterogeneity (P-heterogeneity = 0.01, I2 = 62.9%) (Fig. 2). There was no evidence of publication bias (P values for Egger and Begg tests ≥0.30). Meta-regression analysis showed that the observed heterogeneity was not explained by pre-defined study and population characteristics (Table 2, P values for difference ≥0.14). A sensitivity analysis conducted by omitting one study at each turn showed a RR range of 0.88 (95% CI: 0.81–0.95) to 0.93 (95% CI: 0.87–0.99), and the overall I2 reduced from 62.9% to 32.3% when the Chinese cohorts by Takata et al.17 were omitted. Three independent studies (2 publications13,17) reported separate results for EPA and DHA intake in relation to all-cause mortality, and the summary high-versus-low RR was 0.83 (95% CI: 0.75–0.92, I2 = 51.5%) for EPA and 0.81 (95% CI: 0.74–0.95, I2 = 38.5%) for DHA, respectively.
Figure 2. Risk estimates of all-cause mortality for the highest compared with lowest intake of long-chain n-3 polyunsaturated fatty acids in individual studies and all combined.
F, female; M, male.
Table 2. Subgroup analysis for the association of n-3 LCPUFA intake (high vs. low) and risk of all-cause mortality.
| N | RR (95% CI) | P heterogeneity | I2(%) | Pdifference | |
|---|---|---|---|---|---|
| Overall | 7 | 0.90 (0.83–0.98) | 0.005 | 70.0 | |
| Area | |||||
| Asian | 4 | 0.90 (0.77–1.05) | 0.003 | 82.6 | 0.91 |
| USA | 3 | 0.91 (0.83–0.99) | 0.12 | 53.4 | |
| Duration | |||||
| ≥10 years | 5 | 0.91 (0.81–1.03) | 0.004 | 77.8 | 0.74 |
| <10 years | 2 | 0.88 (0.77–1.01) | 0.08 | 68.2 | |
| Sex | |||||
| Male | 1 | 1.03 (0.86–1.23) | – | – | Ref. |
| Female | 2 | 0.98 (0.90–1.07) | 0.45 | 0 | 0.72 |
| Both | 5 | 0.86 (0.79–0.94) | 0.05 | 62.7 | 0.24 |
| Mean/median age at baseline | |||||
| ≥55 years | 2 | 0.89 (0.76–1.04) | 0.05 | 73.2 | 0.75 |
| <55 years | 5 | 0.92 (0.83–1.01) | 0.01 | 67.8 | |
| Range of intakea | |||||
| ≥0.30 g/d | 4 | 0.91 (0.85–0.97) | 0.22 | 31.4 | 0.14 |
| <0.30 g/d | 2 | 0.79 (0.72–0.87) | –b | –b | |
| Quality scores | |||||
| ≥8 | 5 | 0.91 (0.82–1.01) | 0.005 | 76.8 | 0.84 |
| <8 | 2 | 0.89 (0.76–1.04) | 0.05 | 73.2 | |
| Subtypes | |||||
| EPA | 3 | 0.83 (0.75–0.92) | 0.15 | 51.5 | 0.83 |
| DPA | 3 | 0.81 (0.74–0.90) | 0.20 | 38.5 | |
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LCPUFA, long-chain polyunsaturated fatty acids.
aThe mean/median intakes in the highest categories minus those in the lowest categories was the range of intake. This analysis excluded the study by Nagata et al. in which the intake levels for each category were not reported.
bOnly two cohorts from one publication were included in this stratum, and so no result for heterogeneity test were reported here.
Circulating EPA and DHA and all-cause mortality
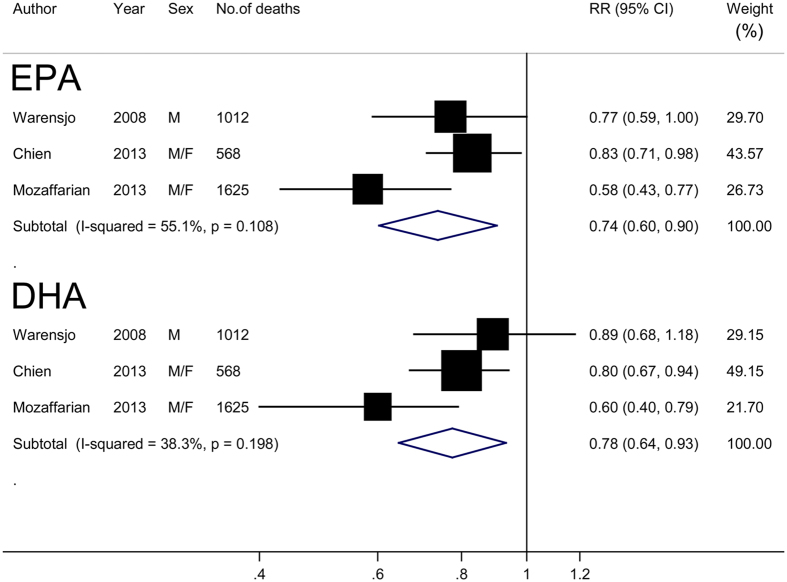
Three of the 4 studies reported high-versus-low circulating EPA and DHA and risk of all-cause mortality. The summary RR was 0.74 (95% CI: 0.60–0.90) for EPA and 0.78 (95% CI: 0.64–0.93) for DHA, with moderate heterogeneity (I2 = 55.1% and 38.3%, respectively) (Fig. 3). There was no evidence of publication bias (all P values ≥ 0.30).
Figure 3. Risk estimates of all-cause mortality for the highest compared with lowest proportions of circulating eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to total fatty acids in blood for individual studies and all combined.
F, female; M, male.
Dose-response analysis
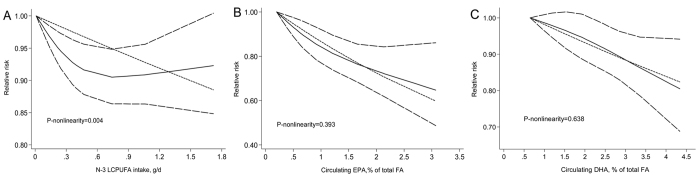
One study16 on dietary n-3 LCPUFA intake was not included in these analyses because the levels of the intake for each category were not available. Pooling the remaining 6 studies showed a summary RR of 0.94 (95% CI: 0.89–0.99) for an increment in n-3 LCPUFA intake of 0.3 g/d, with moderate heterogeneity (P-heterogeneity = 0.01, I2 = 70.2%) (Supplementary Fig. S1). There was evidence of a nonlinear association (P-nonlinearity = 0.004) (Fig. 4A), with a tendency to plateau at high intakes (>0.6 g/d). However, this observation should be treated with caution because all data for high intakes were from one Japanese cohort15.
Figure 4.
Risk estimates of all-cause mortality associated with dietary long-chain n-3 polyunsaturated fatty acids (panel A) and circulating eicosapentaenoic acid (panel B) and docosahexaenoic acid (panel C) in a restricted cubic spline random-effects meta-analysis. FA, fatty acids; LCPUFA, long-chain polyunsaturated fatty acids.
All four studies were included in the dose-response analysis of circulating EPA and DHA and all-cause mortality. The summary RR was 0.80 (95% CI: 0.65–0.98) and 0.79 (95% CI: 0.63–0.99) for each 1% increment in the proportions of EPA and DHA in total circulating fatty acids, with moderate to high heterogeneity (I2 = 74.5% and 79.3%, respectively) (Supplementary Fig. S2). There was no evidence of a nonlinear assocation between circulating EPA or DHA and all-cause mortality (P values for nonlinearity >0.30) (Fig. 4B,C). There was no evidence of publication bias across the dose-response analyses (all P values ≥ 0.09).
Discussion
Major findings
In this meta-analysis involving over 30 thousand deaths events from 11 prospective studies, both dietary and circulating n-3 LCPUFA are shown to be significantly associated with reduced risk of all-cause mortality, and the associations are similar for EPA and DHA.
Other results from observational studies and clinical trials
In a recent meta-analysis of 12 prospective studies, Zhao et al.36 report a moderate reduction in all-cause mortality associated with intake of fish, a major dietary source of n-3 LCPUFA. Significantly inverse associations of dietary fish and dietary and circulating n-3 LCPUFA with risk of CHD have been consistently shown in prospective observational studies2,10. Conversely, clinical evidence regarding the health benefits of n-3 LCPUFA has been continuously inconsistent. Mozaffarian and Wu5 review the cumulative observational and clinical evidence published before 2011 regarding the effects of n-3 LCPUFA on CVD and related risk factors, and conclude that there is strong evidence supporting a protective effect of n-3 LCPUFA on cardiac death. Nonetheless, more recent meta-analyses fail to show any significant protection either on CVD or on all-cause mortality. For instance, Rizos et al.11 and Chowdhury et al.10 each pooled data from 17 RCTs and report no significant effect of n-3 LCPUFA supplementation on all-cause mortality, with a summary RR of 0.96 (95% CI, 0.91–1.02) and 0.94 (95% CI: 0.86–1.03), respectively. Using a cumulative meta-analysis, Rizos et al.11 further find that a protection of n-3 LCPUFA on all-cause mortality is restricted to RCTs that are published before 2007.
The reasons for the disparate findings between observational and clinical studies are not fully understood, but several possibilities merit consideration. First, most of the RCTs, as argued by Mozaffarian and Wu5, are small in sample size and short in duration, and therefore may be of a low statistical power to detect a moderate-to-weak effect associated with long-term uses. Second, participants in the trials are mostly those subjects who are at high risk for, or already suffering from CVD rather than the general populations. In such a condition, the benefits of n-3 LCPUFA may be diminished or offset by disease status or corresponding dietary modifications and medical treatments. A recent review37 summarize that post-hoc analyses of RCTs support beneficial effects of n-3 LCPUFA on CVD prevention among statins non-users, and conclude that emerging uses of statins and less deficiencies of n-3 LCPUFA among participants of recent trials may explain why especially early RCTs, but not recent ones find the health benefits of n-3 LCPUFA. Given that n-3 LCPUFA and statins share several mechanisms whereby they may exert health effects (e.g., improving endothelial function, reducing inflammation, and slowing atherosclerotic progresses5,38), statins may mask the action of n-3 LCPUFA in a competitive fashion.
Third, the association of n-3 LCPUFA with health outcomes may be nonlinear. A 2006 pooled analysis39 of prospective studies and randomized trials indicate that the most protections of EPA and DHA intake on CHD deaths could be achieved when the intake is up to 0.25 g/d. Furthermore, the effects on antiarrhythmia, antithrombosis, and reducing heart rate and blood pressure (but not triglycerides) also appear nonlinear, with tendencies to plateau at the intakes of 0.5–0.75 g/d39. A recent report from the Cardiovascular Health Study21 demonstrate that EPA and DHA in plasma increase linearly and sharply with increasing dietary EPA and DHA lower than 0.5 g/d, whereas there are limited subsequent change in plasma concentrations despite larger increases in dietary intake. These observations suggest that diet and endogenous metabolism may jointly determine to what degree n-3 LCPUFA intake may exert their benefits to human body. If the benefits of n-3 LCPUFA indeed tend to be saturable at low-to-moderate levels, the null effects observed in the trials without taking into account diet background of participants are not surprising.
Fourth, it is possible that other nutrients (e.g., vitamins, minerals, and proteins) in fish or other foods rather than n-3 LCPUFA are beneficial. It is difficult, perhaps not possible for observational studies to accurately distinguish the effects between n-3 LCPUFA and these nutrients. Finally, even if n-3 LCPUFA are one of the causal components in fish, their consumption as part of a matrix of other nutrients in foods may be essential for the benefits. We consider the fourth possibility less likely because of multiple lines of evidence from experimental research, prospective observational studies, as well as human intervention trials supporting the potential cardiovascular benefits of n-3 LCPUFA.
Strengths and limitations of the current study
Major strengths of this meta-analysis include the prospective design of original studies and the large number of events involved in the analyses. However, several limitations of this study should also be acknowledged. Since this meta-analysis is based on observational studies, potential influences of residual or unmeasured confounders on our findings cannot be fully excluded. Dietary information was mostly collected with self-reported FFQs in the original studies, which may introduce measurement error and lead some participants to be misclassified. Such misclassification would likely be nondifferential in cohort studies and to attenuate any true association. This may partly explain the observation that the associations between circulating n-3 LCPUFA and all-cause mortality were stronger than those between dietary intakes and all-cause mortality. Furthermore, the common methods we used to detect potential publication bias may be of a limited power when the number of studies is relatively small. Thus, potential impacts of publication bias on our results cannot be completely excluded.
Conclusions
In summary, this meta-analysis of prospective observational studies suggests that both dietary and circulating n-3 LCPUFA are significantly inversely associated with risk of all-cause mortality. More large prospective studies conducted among individuals with high intakes are needed to address whether there is a nonlinear association. Future well designed primary prevention trials that account for nutrition status, health conditions, and medication usages of participants are also warranted to confirm our findings and those from others.
Methods
Literature search
This study was planned, conducted, and reported in adherence to the guidelines of the ‘Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group’40. A literature search was performed on PubMed (January 1, 1966 to November 30, 2015) and EMBASE (January 1, 1980 to November 30, 2015) databases using the search strategy as follows: (n-3 fatty acids OR omega-3 fatty acids OR marine fatty acids OR n-3 polyunsaturated fatty acids OR n-3 PUFA OR docosahexaenoic acid OR eicosapentaenoic acid OR DHA OR EPA) AND (mortality OR death) AND (cohort OR prospective OR nested). Bibliographies in the retrieved full articles were also carefully hand searched for additional studies. Attempts were also made to contact relevant authors for additional information.
Study selection
Studies that met the following criteria were considered: 1) the study design was prospective; 2) the exposure of interest was dietary or circulating n-3 LCPUFA; 3) the outcome of interest was all-cause mortality 4) relative risks (RRs) with corresponding 95% confidence intervals (CIs) were reported or could be estimated. When multiple publications from the same study were available, the one with the largest number of events was selected.
Data extraction and quality assessment
Using a standardized data-collection form, the following data were extracted from each included study: the first author’s last name, publication year, country of origin, source of populations, study duration, age and sex of participants, number of events and participants, categories of n-3 LCPUFA, the maximally adjusted RRs with 95% CIs, methods for exposure assessments, and potential confounders accounted for in the statistical model. The study quality was evaluated with the 9-star Newcastle-Ottawa Scale (NOS)41. Literature selection, data extraction and quality assessment were conducted independently by two authors (G-CC and L-QQ), with any disagreement resolved by consensus.
Statistical analysis
In a Japanese cohort15, mortality risks associated with n-3 LCPUFA intake were reported by causes of deaths (CVD and non-CVD). We combined results from the sub-cohorts with a fix-effects model, and included the overall estimates in the meta-analysis. A DerSimonian and Laird random-effects model42, which considers both within-and between-study variation was assigned to calculate the summary risk estimates. Heterogeneity test was performed using Q and I2 statistics43. For the Q statistic, P < 0.1 was considered as statistically significant; and for the I2 statistic, the following cut-off points were used: <30% (little or no heterogeneity), 30–75% (moderate heterogeneity) and >75% (high heterogeneity). Potential publication bias was investigated with both Egger regression and Begg correlation tests44,45. Meta-regression analyses were performed to explore potential sources of heterogeneity according to geographic area, duration of follow-up, sex and age of participants, range of the exposure, and quality score of included studies. We also separately evaluated mortality risks associated with dietary/circulating EPA and DHA.
Given the distinct cut-off points across studies, dose-response analyses were performed with the method proposed by Greenland and Longnecker46 and Orsini et al.47. The method requires the number of cases and person-years and the risk estimates with their variance for at least 3 quantitative exposure categories. For studies that did not provide the number of cases/person-years in each exposure category, the data were estimated from total number of cases/person-years. For each study, the median/mean level of exposure for each category was assigned to each corresponding risk estimate. When the median/mean level per category was not provided, the midpoint of the upper and lower boundaries in each category was assigned as an average level. If the highest category was open-ended, the width of the interval was assumed to be the same as in the second highest category. For 1 study19 where the categorized levels of circulating EPA and DHA were not provided, we contacted the corresponding author and obtained the data. For 2 studies20,22 where results for circulating EPA and DHA were reported as a continuous variable (1-SD increase), we rescaled the RR to a 1% increase in circulating EPA and DHA. One of the 2 studies did not report SD values, and the values were estimated using reported inter-quartile ranges according to the methods developed by Hozo et al.48. The results of linear dose-response analyses were presented for a 0.3 g/day increment in dietary n-3 LCPUFA (approximates 1 serving/week of fatty fish intake), and for a 1% increment in circulating EPA/DHA. We further examined a potential nonlinear relationship between dietary/circulating n-3 LCPUFA and all-cause mortality by modeling exposure levels using restricted cubic splines with 3 knots at percentiles 10%, 50% and 95% of the distribution49,50. A P value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to zero. All statistical analyses were performed using STATA software, version 11.0 (STATA Corp., College Station, TX, USA).
Additional Information
How to cite this article: Chen, G.-C. et al. N-3 long-chain polyunsaturated fatty acids and risk of all-cause mortality among general populations: a meta-analysis. Sci. Rep. 6, 28165; doi: 10.1038/srep28165 (2016).
Supplementary Material
Acknowledgments
We sincerely thank the authors who kindly provided us with original unpublished data, and Norman Salem Jr at DSM Nutrition Products, Nutritional Lipids, Columbia, MA, USA for his comments that improved our manuscript.
Footnotes
Author Contributions G.-C.C. and L.-Q.Q. designed the study, completed the literature search and data extraction. G.-C.C. developed search strategies and drafted the manuscript; G.-C.C. and J.Y. performed the statistical analyses. M.E. and W.Z. critically reviewed the manuscript and contributed to the discussion. All authors assisted in the interpretation of the analyses and the revision of the manuscript.
References
- Bang H. O., Dyerberg J. & Nielsen A. B. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1, 1143–5 (1971). [DOI] [PubMed] [Google Scholar]
- He K. et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation 109, 2705–11 (2004). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis 221, 536–43 (2012). [DOI] [PubMed] [Google Scholar]
- Miller P. E., Van Elswyk M. & Alexander D. D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens 27, 885–96 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D. & Wu J. H. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58, 2047–67 (2011). [DOI] [PubMed] [Google Scholar]
- Leon H. et al. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ 337, a2931 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton P. M., Harris W. S. & Appel L. J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106, 2747–57 (2002). [DOI] [PubMed] [Google Scholar]
- Kromhout D., Giltay E. J. & Geleijnse J. M. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 363, 2015–26 (2010). [DOI] [PubMed] [Google Scholar]
- Galan P. et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 341, c6273 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R. et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 160, 398–406 (2014). [DOI] [PubMed] [Google Scholar]
- Rizos E. C., Ntzani E. E., Bika E., Kostapanos M. S. & Elisaf M. S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 308, 1024–33 (2012). [DOI] [PubMed] [Google Scholar]
- Silva V., Barazzoni R. & Singer P. Biomarkers of fish oil omega-3 polyunsaturated fatty acids intake in humans. Nutr Clin Pract 29, 63–72 (2014). [DOI] [PubMed] [Google Scholar]
- Bell G. A. et al. Intake of long-chain omega-3 fatty acids from diet and supplements in relation to mortality. Am J Epidemiol 179, 710–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom A. R. & Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol 160, 1005–10 (2004). [DOI] [PubMed] [Google Scholar]
- Miyagawa N. et al. Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: a 24-year follow-up of NIPPON DATA80. Atherosclerosis 232, 384–9 (2014). [DOI] [PubMed] [Google Scholar]
- Nagata C. et al. Total fat intake is associated with decreased mortality in Japanese men but not in women. J Nutr 142, 1713–9 (2012). [DOI] [PubMed] [Google Scholar]
- Takata Y. et al. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am J Epidemiol 178, 46–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas R., Takata Y., Murff H. & Blot W. J. Fish, omega-3 long-chain fatty acids, and all-cause mortality in a low-income US population: Results from the Southern Community Cohort Study. Nutr Metab Cardiovasc Dis 25, 651–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. L. et al. Comparison of predictive performance of various fatty acids for the risk of cardiovascular disease events and all-cause deaths in a community-based cohort. Atherosclerosis 230, 140–7 (2013). [DOI] [PubMed] [Google Scholar]
- Marklund M. et al. Polyunsaturated Fat Intake Estimated by Circulating Biomarkers and Risk of Cardiovascular Disease and All-Cause Mortality in a Population-Based Cohort of 60-Year-Old Men and Women. Circulation 132, 586–94 (2015). [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. et al. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med 158, 515–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warensjo E., Sundstrom J., Vessby B., Cederholm T. & Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 88, 203–9 (2008). [DOI] [PubMed] [Google Scholar]
- Hu F. B., Cho E., Rexrode K. M., Albert C. M. & Manson J. E. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 107, 1852–7 (2003). [DOI] [PubMed] [Google Scholar]
- Lindberg M., Asberg A., Midthjell K. & Bjerve K. S. Plasma phospholipid EPA and DHA are divergently associated with overall mortality in newly diagnosed diabetic patients: results from a follow-up of the Nord-Trondelag Health (HUNT) Study, Norway. J Nutr Sci 2, e35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M. et al. Low levels of serum n-3 polyunsaturated fatty acids are associated with worse heart failure-free survival in patients after acute myocardial infarction. Circ J 77, 153–62 (2013). [DOI] [PubMed] [Google Scholar]
- Lee S. H. et al. Blood eicosapentaenoic acid and docosahexaenoic acid as predictors of all-cause mortality in patients with acute myocardial infarction–data from Infarction Prognosis Study (IPS) Registry. Circ J 73, 2250–7 (2009). [DOI] [PubMed] [Google Scholar]
- Poole C. D. et al. Omega-3 Fatty acids and mortality outcome in patients with and without type 2 diabetes after myocardial infarction: a retrospective, matched-cohort study. Clin Ther 35, 40–51 (2013). [DOI] [PubMed] [Google Scholar]
- Pottala J. V., Garg S., Cohen B. E., Whooley M. A. & Harris W. S. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: the Heart and Soul study. Circ Cardiovasc Qual Outcomes 3, 406–12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin-Ramirez E. et al. Dietary fatty acids intake and mortality in patients with heart failure. Nutrition 30, 1366–71 (2014). [DOI] [PubMed] [Google Scholar]
- Jiang W. et al. Plasma omega-3 polyunsaturated fatty acids and survival in patients with chronic heart failure and major depressive disorder. J Cardiovasc Transl Res 5, 92–9 (2012). [DOI] [PubMed] [Google Scholar]
- Eide I. A. et al. The association between marine n-3 polyunsaturated fatty acid levels and survival after renal transplantation. Clin J Am Soc Nephrol 10, 1246–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki K. et al. Docosahexaenoic acid is an independent predictor of all-cause mortality in hemodialysis patients. Am J Nephrol 33, 105–10 (2011). [DOI] [PubMed] [Google Scholar]
- Terashima Y. et al. Inverse association between docosahexaenoic acid and mortality in patients on hemodialysis during over 10 years. Hemodial Int 18, 625–31 (2014). [DOI] [PubMed] [Google Scholar]
- Dalmeijer G. W. et al. Dairy intake and coronary heart disease or stroke–a population-based cohort study. Int J Cardiol 167, 925–9 (2013). [DOI] [PubMed] [Google Scholar]
- Praagman J. et al. The relationship between fermented food intake and mortality risk in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Br J Nutr 113, 498–506 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao L. G. et al. Fish consumption and all-cause mortality: a meta-analysis of cohort studies. Eur J Clin Nutr (2015). [DOI] [PubMed] [Google Scholar]
- de Lorgeril M., Salen P., Defaye P. & Rabaeus M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: do statins inhibit omega-3? BMC Med 11, 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. & Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 203, 325–30 (2009). [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. & Rimm E. B. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296, 1885–99 (2006). [DOI] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–12 (2000). [DOI] [PubMed] [Google Scholar]
- Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994). [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–9 (1992). [DOI] [PubMed] [Google Scholar]
- Orsini N., Bellocco R. & Greenland S. Generalized least squares for trend estimation of summarized dose-respose data. Stata J 6, 40–57 (2006). [Google Scholar]
- Hozo S. P., Djulbegovic B. & Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5, 13 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349, g4490 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.