Abstract
Cell membranes possess a complex three-dimensional architecture, including nonrandom lipid lateral organization within the plane of a bilayer leaflet, and compositional asymmetry between the two leaflets. As a result, delineating the membrane structure–function relationship has been a highly challenging task. Even in simplified model systems, the interactions between bilayer leaflets are poorly understood, due in part to the difficulty of preparing asymmetric model membranes that are free from the effects of residual organic solvent or osmotic stress. To address these problems, we have modified a technique for preparing asymmetric large unilamellar vesicles (aLUVs) via cyclodextrin-mediated lipid exchange in order to produce tensionless, solvent-free aLUVs suitable for a range of biophysical studies. Leaflet composition and structure were characterized using isotopic labeling strategies, which allowed us to avoid the use of bulky labels. NMR and gas chromatography provided precise quantification of the extent of lipid exchange and bilayer asymmetry, while small-angle neutron scattering (SANS) was used to resolve bilayer structural features with subnanometer resolution. Isotopically asymmetric POPC vesicles were found to have the same bilayer thickness and area per lipid as symmetric POPC vesicles, demonstrating that the modified exchange protocol preserves native bilayer structure. Partial exchange of DPPC into the outer leaflet of POPC vesicles produced chemically asymmetric vesicles with a gel/fluid phase-separated outer leaflet and a uniform, POPC-rich inner leaflet. SANS was able to separately resolve the thicknesses and areas per lipid of coexisting domains, revealing reduced lipid packing density of the outer leaflet DPPC-rich phase compared to typical gel phases. Our finding that a disordered inner leaflet can partially fluidize ordered outer leaflet domains indicates some degree of interleaflet coupling, and invites speculation on a role for bilayer asymmetry in modulating membrane lateral organization.
Introduction
Cells have evolved to produce a great diversity of lipids, providing cellular membranes with remarkable functionality. Within the plasma membrane (PM) alone, hundreds of distinct lipid species serve both as a barrier to the external environment, as well as a solvent for the membrane’s protein machinery. It is increasingly clear that the three-dimensional spatial organization of these lipid molecules can have profound effects on protein function.1−3
Synthetic liposomes (vesicles) have proven to be highly successful model systems for elucidating membrane properties that are otherwise difficult to study in vivo. In part, their utility stems from the fact that model membranes, compared to their natural counterparts, are chemically simpler and well-defined, and thereby enable stringent experimental control and detailed theoretical calculations at the molecular level. Nevertheless, the use of model systems requires a careful balancing act, where experimental tractability is weighed against biological relevance. The inherent tension between these conflicting goals is exemplified by transbilayer compositional asymmetry. While the existence of asymmetry in natural cell membranes has been known since the early 1970s,4 the vast majority of model membrane studies still employ symmetric vesicles, despite concerns about their ability to capture crucial aspects of lipid–lipid and lipid–protein interactions present in vivo.2,5 Progress toward more realistic models of cell membranes has been hindered by the lack of a robust method for preparing asymmetric vesicles, including tools for precisely quantifying their composition and degree of asymmetry. Consequently, experimental data from asymmetric bilayers are scarce, and the effects of asymmetry on membrane structure and physical properties remain poorly understood.
Recent years have seen renewed effort toward the production of asymmetric membranes in a wide range of sample geometries, including supported6−8 and unsupported9 planar bilayers, and freely floating vesicles of various sizes.10−15 Of special utility are large unilamellar vesicles (LUVs) with diameters on the order of 100 nm. Because they are amenable to study by diverse nanoanalytical techniques, LUVs have become workhorse model systems for protein–membrane interaction studies, and structural characterization using small-angle scattering, fluorescence and NMR. While protocols for asymmetric LUV (aLUV) preparation have been developed, they suffer from drawbacks that can compromise the membrane environment or hinder structural characterization. For example, inverted emulsion techniques10,11,15 can provide excellent control of leaflet composition and achieve nearly complete asymmetry, but can trap organic solvents in the bilayer. aLUVs produced by catalyzed lipid exchange13 are solvent-free, but have relied on a dense sucrose vesicle core for LUV purification. Entrapped sucrose presents an additional and potentially undesirable source of structural perturbation arising from sucrose-lipid interactions16 and/or membrane tension from the resulting osmotic gradient.17
Here, we present a novel experimental strategy for determining asymmetric bilayer composition and structure based on differential isotopic labeling of aLUVs. A key aspect of our approach is the use of sucrose-free LUVs to minimize structural perturbations from membrane tension, which required modification of existing protocols for aLUV preparation. We have therefore organized the paper as follows.
First, we describe a modification of catalyzed lipid exchange that eliminates osmotic gradients, enabling the production of large amounts of solvent- and stress-free aLUVs with a well-defined size distribution. We use isotopic labeling to avoid fluorescent or spin-labeled lipids that can potentially perturb bilayer structure. Differentially deuterated donor and acceptor lipids enable the use of NMR and gas chromatography to precisely quantify the compositions of both leaflets following lipid exchange, while small-angle neutron scattering (SANS) is used to determine the bilayer structure with subnanometer resolution.
Next, in order to identify any structural perturbations caused by experimental conditions, we characterized aLUVs with different isotopic variants of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) in the inner and outer leaflets. Within measurement uncertainty, these aLUVs were identical to symmetric POPC LUVs with respect to bilayer thickness and area per lipid.
Finally, we undertook a detailed structural characterization of aLUVs having a mixture of POPC and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) in the outer leaflet, and POPC in the inner leaflet. SANS analysis revealed both outer leaflet phase separation and interleaflet coupling, with the latter manifested as reduced lipid packing density in the outer leaflet ordered phase. We discuss the implications of this finding for lateral organization in cellular membranes.
Experimental Section
Preparation of Asymmetric LUVs
Solvent-free exchange protocols achieve an asymmetric lipid distribution through cyclodextrin (CD)-mediated lipid exchange between donor and acceptor vesicles.12−14 The acceptor vesicles thus provide lipids for the aLUV inner leaflet, while the donor vesicles provide different lipids for the outer leaflet through catalyzed exchange. This process requires coincubation and eventual separation of donors and acceptors, most easily accomplished by taking advantage of density or size differences between the two vesicle populations.12,13 Established protocols make use of density differences by trapping a concentrated sucrose solution in the acceptor LUV core, allowing aLUVs to be purified from larger, lower-density donor multilamellar vesicles (MLVs) and residual CD by ultracentrifugation.13 Our modified protocol reversed this scheme, instead trapping sucrose between the donor lamellae (Figure 1 and Supporting Information Figure S2). This important change both eliminated sucrose from the aLUV core, and enhanced the difference in sedimentation velocities between donor and acceptor vesicles, due to the larger size and density of the sucrose-loaded donor MLVs. As a result, low-speed centrifugation (20 000g) was sufficient to achieve >95% purification of aLUVs by mass. Subsequent dilution/concentration cycles with centrifugal ultrafiltration devices (100 kDa molecular weight cutoff, 5000g) allowed for the efficient removal of residual sucrose and CD, as well as the ability to exchange H2O with D2O for small-angle neutron scattering (SANS) and 1H NMR experiments. Importantly, the modified protocol yielded large amounts of aLUVs suitable for sample-intensive techniques. Details of the protocol and assessment of sample purity are found in the Supporting Information.
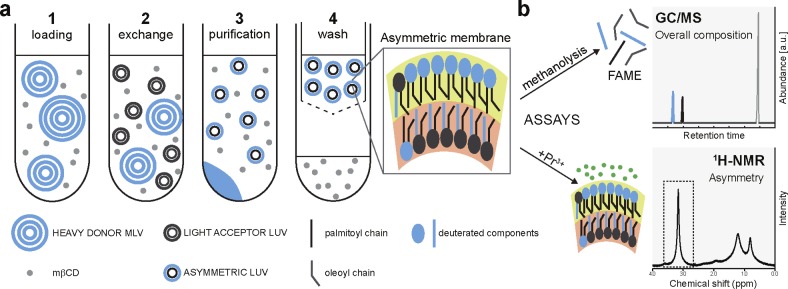
Figure 1.
(a) Illustration of aLUV preparation: (1) methyl-β-cyclodextrin (mβCD) is loaded with lipid from donor MLVs entrapped with sucrose; (2) mβCD catalyzes lipid exchange between donor MLVs and acceptor LUVs; (3) large, heavy donor MLVs are removed by centrifugation; (4) mβCD is removed with a centrifugal concentrator, and the aLUV sample is recovered from the retentate. (b) GC is used to quantify the overall composition of the aLUVs following derivatization of acyl chains to fatty acid methyl esters (FAMEs), and 1H NMR is used to quantify lipid asymmetry in a lanthanide shift experiment. For a detailed description of the preparation and assays, see the Supporting Information.
Characterization of aLUV Composition and Structure
Asymmetric LUVs prepared by the new protocol were characterized for donor exchange efficiency, degree of asymmetry and nanostructure using assays designed to avoid large labels that can potentially affect membrane properties. In particular, we exploited the chemical similarity of hydrogen and deuterium, and the ability of gas chromatography (GC), NMR spectroscopy, and SANS to distinguish between protiated lipids and their deuterated counterparts.
The composition of the asymmetric vesicles’ two leaflets was determined by combining two assays, each of which relies on isotopically labeled lipids. First, donor exchange efficiency was evaluated for aLUVs having differentially chain-labeled donor and acceptor. Exchange efficiency is directly given by the ratio of labeled to unlabeled lipids, and was readily quantified because the sn-1 fatty acid methyl ester (FAME) derivatives (i.e., methyl palmitate and methyl palmitate-d31) were fully resolved by capillary gas chromatography (Figure S3). Second, the degree of asymmetry—i.e., the distribution of lipids between the two leaflets—was evaluated for aLUVs having differentially head-labeled donor and acceptor (i.e., choline vs choline-d13). The choline distribution was determined using solution 1H NMR, which detects choline but not choline-d13, by quantifying the shift of the outer leaflet choline methyl resonances in the presence of externally added paramagnetic lanthanide ion Pr3+ (Figures S4–S6).18 Pr3+ does not permeate into the LUV core on the time scale of days, and the interaction is therefore specific for outer leaflet headgroups.19 Because bilayer defects at the boundaries between gel and fluid domains may facilitate passive ion transport,20 we also verified that phase-separated vesicles were impermeable to Pr3+ during the time required to measure the NMR spectrum (Supporting Information Figure S8).
We used SANS to characterize bilayer structure on the subnanometer length scale. The unique scattering signatures of deuterium and hydrogen generate strong interleaflet contrast for isotopically asymmetric bilayers. Differential donor/acceptor labeling schemes allowed us to independently determine both inner and outer leaflet structural properties, including thickness, headgroup water content, and lateral area per lipid, which is related to lipid packing density (Figures 3 and S9).
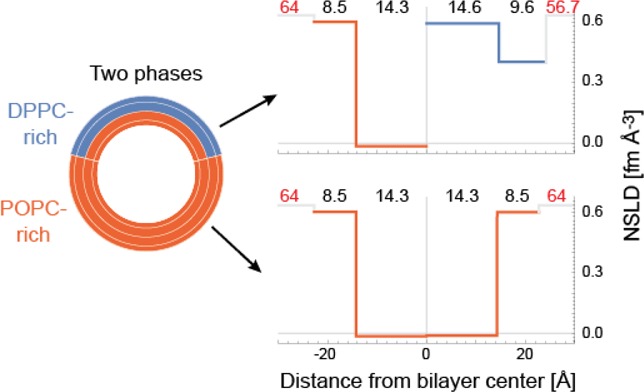
Figure 3.
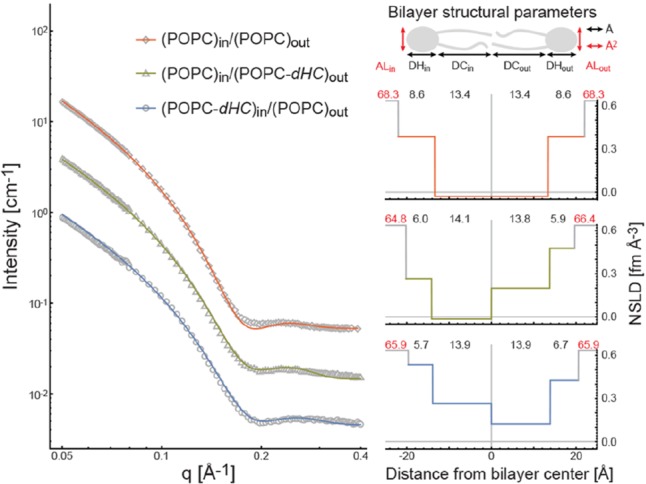
Structure of isotopically asymmetric POPC aLUVs at 20 °C determined by SANS. Left: SANS data (open symbols) and fits to the data (solid colored lines) for POPC aLUVs with different isotopic labeling of the inner and outer leaflets (Tables S2–S3). Upper right: schematic cartoon of the bilayer unit cell used to model SANS data; structural parameters obtained from the analysis include headgroup and hydrocarbon thicknesses (DH and DC, respectively) and area per lipid (AL) for inner and outer leaflets. Lower right: best-fit neutron scattering length density (NSLD) profiles color coded to SANS curves. Recovered structural parameters from left to right: inner leaflet AL, DH, and DC; outer leaflet DC, DH, and AL. Structural parameters are listed in Table S6.
SANS data were analyzed using a four-slab bilayer model for the neutron scattering length density (NSLD) profile. The model was constrained with the compositional information obtained from GC and NMR experiments, and lipid headgroup and acyl chain volumes obtained from literature.21,22 The addition of independent composition and volume constraints allowed us to reduce the number of fitting parameters. Full details of the model and data analysis are found in the Supporting Information.
Results
Isotopically Asymmetric POPC aLUVs
Because aLUV preparation involves subjecting lipid vesicles to cyclodextrin and centrifugal filtration, it is important to assess structural changes caused by experimental conditions. To this end, we prepared aLUVs composed of POPC and its chain (-dC), headgroup (-dH), and fully labeled (-dHC) isotopic variants (Figure S1), and compared them to symmetric POPC LUVs prepared by conventional techniques.
We first examined a combination of labeled donor and acceptor lipids (POPC and POPC-dHC) that is amenable to both GC and 1H NMR analysis. This allowed us to characterize the asymmetric vesicles’ inner and outer leaflet composition (details are found in the Supporting Information). Isotopically asymmetric POPC aLUVs prepared from an initial 2:1 donor/acceptor molar ratio exhibited ∼75% exchange efficiency and a high degree of asymmetry (Figure 2a and Tables S2–S3). Scrambled LUVs prepared with lipid extracted from aLUVs were found to have a symmetric transbilayer choline distribution (Supporting Information Figure S7).
Figure 2.
Characterization of aLUV composition. (a) Upper: 1H NMR spectra reveal the outer leaflet (yellow) and inner leaflet (red) population of protiated headgroup lipid, after external addition of the shift reagent Pr3+. The black curve is the sum of fitted peaks including trace glycerol and residual mβCD (purple). Lower: the composition of three aLUV samples determined by joint GC, 1H NMR, and SANS analysis (see also Tables S2–S4 and Figures S4–S6). (b) The stability of POPC aLUVs is demonstrated by the inner/outer distribution of POPC donor exchanged into POPC-dH acceptor vesicles, shown immediately following aLUV preparation (0 h, 22 °C) and after 24 h of incubation at room temperature (24 h, 22 °C). A gradual loss of asymmetry is observed over 4 days of incubation at 50 °C.
To assess the stability of lipid asymmetry, we exchanged POPC donor into POPC-dH acceptor vesicles, and monitored POPC asymmetry with 1H NMR. No appreciable flip-flop was observed over 24 h (Figure 2b), consistent with previous reports for POPC (t1/2 > 1000 h at 37 °C).23 After heating the sample to 50 °C, a gradual loss of asymmetry was observed over the course of 4 days.
Comparing different POPC aLUVs (Figure 2), small differences in exchange efficiency were observed. Because the amount of exchange depends on several factors including the ratio of donor to acceptor lipid, and the temperature and duration of the exchange step, some sample-to-sample variation in exchange efficiency is not surprising, and further underscores the importance of precise assays for aLUV composition like those described here.
Structural parameters for isotopically asymmetric POPC aLUVs determined by SANS (Figure 3, Table S6) were (within error) identical to those of symmetric POPC LUVs (Table S5, Figure S8), and compared well with literature values,21,24 indicating that sample preparation conditions did not alter the bilayer structure. In contrast, comparison of the membrane thickness of symmetric LUVs with and without a 25 wt% sucrose core indicated significant bilayer thinning in the presence of a sucrose core, consistent with osmotically generated membrane tension (Figure S11). This finding underscores the importance of stress-free vesicles for characterizing bilayer structure. Centrifugal filtration did not affect vesicle integrity or significantly alter the vesicle size distribution as judged by dynamic light scattering (Supporting Information Table S8).
Donor contamination in the form of MLVs and small unilamellar vesicles (SUVs) was not detected by SANS and NMR, respectively (Figures S12–S15), in asymmetric preparations. However, a small quantity of SUVs was found in donor-only control samples, an indication that minor contamination (<5% by mass) from donor-derived or mixed donor/acceptor SUVs may intrude in some cases. In particular, a small SUV population in POPC outer/POPC-dHC inner samples is inferred from the slightly larger amount of POPC found by NMR in the vesicles’ inner leaflet (Figure 2a). A greater propensity for contamination is expected for lower density donor lipids, due to their reduced separation efficiency from acceptor vesicles. If complete removal of SUVs is necessary, sucrose density gradients can be employed.13
Chemically Asymmetric DPPC/POPC aLUVs
We next examined chemically asymmetric aLUVs composed of POPC and DPPC. Exchanging the high-melting donor lipid DPPC (TM 41 °C) into low-melting POPC acceptor vesicles (TM −2 °C) should increase the outer leaflet order relative to the inner leaflet. For ease of discussion, we will refer to such vesicles as “OO/DI”, an abbreviation for “ordered outer/disordered inner”.
Our sample preparation conditions employed a 3:1 ratio of DPPC donors to POPC acceptors, with exchange performed at room temperature. Asymmetric vesicles analyzed by GC, 1H NMR, and SANS were found to contain 34 mol % DPPC in the outer leaflet and 2 mol % DPPC in the inner leaflet (Figure 2a, Table S4). At room temperature, symmetric bilayers with this outer leaflet composition (i.e., DPPC:POPC = 34:66 mol %) are immiscible and contain coexisting gel and fluid phases, enriched in DPPC and POPC respectively.25 However, different interleaflet interactions present in asymmetric bilayers may alter the compositions or properties of the coexisting phases, or abolish phase separation entirely.9
We found that SANS data could not be adequately modeled with a single asymmetric form factor, implying the presence of coexisting phases (Figure 4). Indeed, the data were well fitted by a weighted sum of two bilayer form factors, corresponding to coexisting POPC- and DPPC-enriched phases in the outer leaflet and a uniform POPC phase in the inner leaflet (Figure 4 and Table S7). The possibility of two distinct vesicle populations with different compositions (which could arise from budding of phase domains) was ruled out by DLS measurements, which indicated a single LUV size distribution centered at ∼130 nm both before and after lipid exchange (Supporting Information Table S8).
Figure 4.
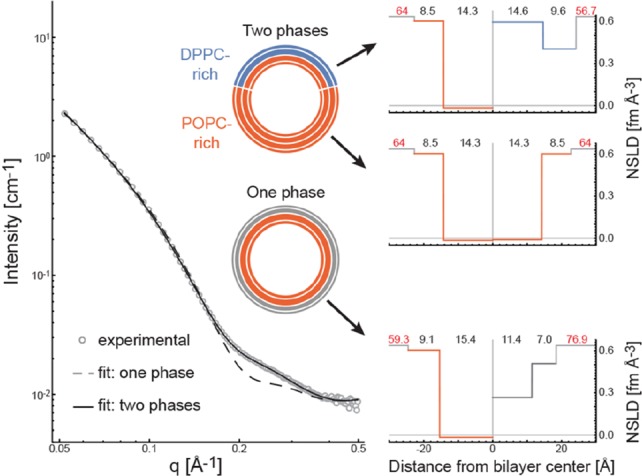
Structure of chemically asymmetric DPPC/POPC aLUVs at 20 °C determined by SANS. Left: SANS data (open circles) for aLUVs composed of DPPC-dC and POPC-dH, containing 34 mol % DPPC-dC in the outer leaflet and 98 mol % POPC-dH in the inner leaflet (Table S4). Experimental data were modeled assuming either a single outer leaflet phase (dashed line) or two outer leaflet phases (solid line) as indicated by schematic vesicles. Right: best-fit NSLD profiles for one phase (lower) and two phase (upper) models, with recovered structural parameters as in Figure 3. Structural parameters are listed in Table S7.
Importantly, best fit values of the area per lipid (AL) indicate a marked reduction in lipid packing density of the DPPC-rich ordered phase (56.7 Å2/molecule, Table S7), compared to typical gel phase packing (47.2 Å2 for DPPC at 20 °C).26 The presence of 18 mol % POPC in the DPPC-rich phase is unlikely to account for this difference: assuming that the areas of individual species are additive, and using a value of 62.7 Å2 for POPC at 20 °C21 yields an AL of 50.2 Å2 for the DPPC/POPC mixture. In fact, our best fit AL for the outer leaflet DPPC-rich phase lies between this value, and that of fluid phase DPPC (63.1 Å2 at 50 °C).21
Discussion
Interleaflet Coupling in Asymmetric, Phase-Separated Bilayers
Our observations for DPPC/POPC aLUVs suggest some degree of interleaflet coupling, whereby a leaflet senses and responds to the physical state of the apposing leaflet. Strong coupling of lipid dynamics has been observed in asymmetric giant unilamellar vesicles (GUVs) having a similar composition to our OO/DI LUVs: when the outer leaflet of POPC acceptor vesicles was partially replaced with brain or milk sphingomyelin (SM), lipid diffusion was reduced in both leaflets.14 Interestingly, for these asymmetric OO/DI GUVs, inner leaflet structural order was almost completely decoupled from outer leaflet order. This finding is consistent with our structural modeling of asymmetric OO/DI LUVs, in which we observe no change in inner leaflet POPC packing density after partial outer leaflet exchange with the more ordered DPPC. However, we did find substantially altered properties of the outer leaflet ordered domains, which appear to be partially fluidized by coupling to a POPC inner leaflet. In contrast, for OO/DI LUVs in which the outer leaflet was almost completely replaced with SM, little or no decrease in outer leaflet order or transition temperature (relative to pure SM vesicles) was observed.13 The lower levels of exchange in our DPPC/POPC aLUVs result in an outer leaflet DPPC-rich phase occupying a total area fraction of 0.37; however, we do not know the size and number of DPPC-rich domains, and therefore cannot exclude the possibility that boundary effects and interfacial phenomena are contributing to the observed order. It is likely that both composition (e.g., DPPC vs SM, as well as the extent of outer leaflet exchange) and temperature are important parameters for determining coupling strength in asymmetric bilayers. Establishing the rules that govern interleaflet coupling will be facilitated by the experimental strategy outlined here, which enables direct measurement of inner and outer leaflet structural properties in probe-free bilayers.
Our finding that interleaflet coupling can alter the packing of ordered domains may have implications for cell membrane organization. For example, a unifying theme of raft functionality is the selectivity of raft phases for specific proteins.1,27 However, ordered phases in symmetric model membranes exclude most transmembrane proteins, including those thought to partition favorably into membrane rafts.28,29 The discrepancies between protein partitioning in vitro and in vivo may be related to the relative packing density of the coexisting environments: significantly greater differences in hydrocarbon chain order are observed for coexisting liquid-disordered (Ld) and liquid-ordered (Lo) phases in model membranes, compared to coexisting phases in PM-derived vesicles.30 Our early results suggest a previously unconsidered mechanism that can influence the relative packing density of coexisting phases, namely, bilayer asymmetry.
Limitations of SANS Modeling
SANS data are relatively featureless, and the possibility of a nonunique fit (especially for models having multiple free parameters) must always be considered. We chose to use a four-slab NSLD model, arguably the simplest reasonable model for asymmetric bilayer structure, in order to minimize the number of fit parameters. We also constrained as many parameters as possible, by incorporating lipid volumetric data obtained from the literature (Table S1 and eqs 15–16 in the Supporting Information), in addition to leaflet composition information from GC and NMR measurements.
Given the simplicity of the model and the number of constraints that were used, it is not surprising that fits to the SANS data are imperfect, for example near the first scattering minimum at q ∼ 0.2 Å–1 of POPCo/POPCi aLUVs (Figure 3, red curve). The form factor of a perfectly symmetric, flat bilayer should decay to zero at the first minimum, but experimental data obtained from spherical vesicles often show nonzero intensity, even in nominally symmetric bilayers.31 Consequently, flat bilayer form factors, like those used here, often result in poor fits at minima between scattering lobes for samples having low interleaflet contrast. Still, the fact that reasonably good fits are achieved despite the imposition of many external constraints, gives us confidence that the reported bilayer structure is essentially correct at the resolution of the slab model (i.e., ∼1 nm). The probability of a unique fit can be increased through the joint refinement of data from differently contrasted samples that share a common structure, for example DPPC/POPC aLUVs having different isotopic variants, but similar levels of exchange.
Outlook
We combined SANS measurements with sensitive analytical tools for quantifying asymmetric bilayer composition, resulting in the first detailed structural characterization of an asymmetric model membrane. In aLUVs having a mixture of ordered and disordered lipids in the outer leaflet and a disordered lipid in the inner leaflet, we found distinct differences in structural properties as compared to symmetric LUVs, notably reduced gel phase packing density. Future studies of synthetic asymmetric bilayers mimicking raft phases (i.e., by incorporating cholesterol) will likely result in Ld and Lo phases with more biologically faithful properties and partitioning behaviors. More broadly, the procedures outlined here will pave the way for controlled studies of asymmetric bilayer structure and interleaflet coupling, and of the interplay between transmembrane proteins and biologically relevant membranes.
Acknowledgments
We thank Lance Gill, Edward Hagaman, Thad Harroun, Qingqing Lin, Gerald Rechberger, Mijin Son, Chris Stanley, and Klaus Zangger for technical assistance and helpful discussions. This work acknowledges support from the Austrian Science Fund (FWF) project P27083 (to G.P.); U.S. National Science Foundation Grant DMR 1404985 (to E.L.); U.S. National Institutes of Health Grant GM105684 (to G.W.F.); the U.S. Department of Energy (DOE) Office of Basic Energy Sciences (BES) through the EPSCoR Grant DE-FG02-08ER46528 (to J.D.N.); the University of Tennessee-Oak Ridge National Laboratory (ORNL) Joint Institute for Biological Sciences (to F.A.H.); the Laboratory Directed Research and Development Program of ORNL (to J.K., R.F.S., J.D.N., and F.A.H.), managed by UT-Battelle, LLC, for the DOE; and from the Scientific User Facilities Division of the DOE BES, for the EQ-SANS instrument at the ORNL Spallation Neutron Source, managed by UT-Battelle, LLC under US DOE Contract No. DE-AC05-00OR22725.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.langmuir.5b04562.
Detailed experimental procedures, and theory; eight data tables [Tables S1–S8]; three figures relating to sample preparation [Figures SS1-S3]; five figures relating to NMR measurement [Figures S4–S8]; three figures relating to SANS measurements [Figures S9–S11]; four figures relating to contamination assessment [Figures S12–S15]; materials and methods (PDF)
Author Present Address
◊ Infineon Technologies Austria AG, Development Center Graz, Babenbergerstr. 10, A-8010 Graz, Austria
Author Contributions
+ F.A.H., D.M., M.D., and B.G. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Lingwood D.; Simons K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Simons K.; Gerl M. J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Frewein M.; Kollmitzer B.; Heftberger P.; Pabst G. Lateral Pressure-Mediated Protein Partitioning into Liquid-Ordered/Liquid-Disordered Domains. Soft Matter 2016, 12, 3189. 10.1039/C6SM00042H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane Structure: Some General Principles. Science 1973, 181, 622–629. 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- van Meer G.; Voelker D. R.; Feigenson G. W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J. M.; Kiessling V.; Tamm L. K. Measuring Lipid Asymmetry in Planar Supported Bilayers by Fluorescence Interference Contrast Microscopy. Langmuir 2005, 21, 1377–1388. 10.1021/la047654w. [DOI] [PubMed] [Google Scholar]
- Kiessling V.; Crane J. M.; Tamm L. K. Transbilayer Effects of Raft-Like Lipid Domains in Asymmetric Planar Bilayers Measured by Single Molecule Tracking. Biophys. J. 2006, 91, 3313–3326. 10.1529/biophysj.106.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C.; Kiessling V.; Tamm L. K. Coupling of cholesterol-rich lipid phases in asymmetric bilayers. Biochemistry 2008, 47, 2190–2198. 10.1021/bi7021552. [DOI] [PubMed] [Google Scholar]
- Collins M. D.; Keller S. L. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 124–128. 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautot S.; Frisken B. J.; Weitz D. A. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 10718–10721. 10.1073/pnas.1931005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T.; Miura Y.; Komatsu Y.; Kishimoto Y.; Vestergaard M.; Takagi M. Construction of Asymmetric Cell-Sized Lipid Vesicles from Lipid-Coated Water-in-Oil Microdroplets. J. Phys. Chem. B 2008, 112, 14678–14671. 10.1021/jp807784j. [DOI] [PubMed] [Google Scholar]
- Cheng H. T.; Megha; London E. Preparation and Properties of Asymmetric Vesicles That Mimic Cell Membranes: Effect Upon Lipid Raft Formation and Transmembrane Helix Orientation. J. Biol. Chem. 2009, 284, 6079–6092. 10.1074/jbc.M806077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. T.; London E. Preparation and properties of asymmetric large unilamellar vesicles: interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature. Biophys. J. 2011, 100, 2671–2678. 10.1016/j.bpj.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiantia S.; London E. Acyl chain length and saturation modulate interleaflet coupling in asymmetric bilayers: effects on dynamics and structural order. Biophys. J. 2012, 103, 2311–2319. 10.1016/j.bpj.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elani Y.; Purushothaman S.; Booth P. J.; Seddon J. M.; Brooks N. J.; Law R. V.; Ces O. Measurements of the effect of membrane asymmetry on the mechanical properties of lipid bilayers. Chem. Commun. 2015, 51, 6976–6979. 10.1039/C5CC00712G. [DOI] [PubMed] [Google Scholar]
- Andersen H. D.; Wang C.; Arleth L.; Peters G. H.; Westh P. Reconciliation of opposing views on membrane-sugar interactions. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 1874–1878. 10.1073/pnas.1012516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuyan A. G.; Cohen F. S. Raft composition at physiological temperature and pH in the absence of detergents. Biophys. J. 2008, 94, 2654–2666. 10.1529/biophysj.107.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. B.; Faller J. W.; Gilliam J. M.; Barrnett R. J. Lanthanide Ion-Induced Isotropic Shifts and Broadening for Nuclear Magnetic Resonance Structural Analysis of Model Membranes. Proc. Natl. Acad. Sci. U. S. A. 1973, 70, 1814–1818. 10.1073/pnas.70.6.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G. R. A. Kinetics of ionophore-mediated transport of Pr3+ ions through phoshpolipid membranes using 1H NMR spectroscopy. FEBS Lett. 1975, 58, 194–196. 10.1016/0014-5793(75)80257-2. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D.; Jacobson K.; Nir S.; Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta, Biomembr. 1973, 311, 330–348. 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Kučerka N.; Nieh M.-P.; Katsaras J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta, Biomembr. 2011, 1808, 2761–2771. 10.1016/j.bbamem.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Tristram-Nagle S.; Liu Y.; Legleiter J.; Nagle J. F. Structure of Gel Phase DMPC Determined by X-Ray Diffraction. Biophys. J. 2002, 83, 3324–3335. 10.1016/S0006-3495(02)75333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M.; Fukuda M.; Kudo T.; Matsuzaki N.; Azuma T.; Sekine K.; Endo H.; Handa T. Flip-Flop of Phospholipids in Vesicles: Kinetic Analysis with Time-Resolved Small-Angle Neutron Scattering. J. Phys. Chem. B 2009, 113, 6745–6748. 10.1021/jp900913w. [DOI] [PubMed] [Google Scholar]
- Fogarty J. C.; Arjunwadkar M.; Pandit S. A.; Pan J. Atomically detailed lipid bilayer models for the interpretation of small angle neutron and X-ray scattering data. Biochim. Biophys. Acta, Biomembr. 2015, 1848, 662–672. 10.1016/j.bbamem.2014.10.041. [DOI] [PubMed] [Google Scholar]
- Svetlovics J. A.; Wheaten S. A.; Almeida P. F. Phase separation and fluctuations in mixtures of a saturated and an unsaturated phospholipid. Biophys. J. 2012, 102, 2526–2535. 10.1016/j.bpj.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W. J.; Tristram-Nagle S.; Suter R. M.; Nagle J. F. Structure of gel phase saturated lecithin bilayers: temperature and chain length dependence. Biophys. J. 1996, 71, 885–891. 10.1016/S0006-3495(96)79290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorent J. H.; Levental I. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem. Phys. Lipids 2015, 192, 23. 10.1016/j.chemphyslip.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Bacia K.; Schuette C. G.; Kahya N.; Jahn R.; Schwille P. SNAREs prefer liquid-disordered over ”raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J. Biol. Chem. 2004, 279, 37951–37955. 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- Kahya N.; Brown D. A.; Schwille P. Raft partitioning and dynamic behavior of human placental alkaline phosphatase in giant unilamellar vesicles. Biochemistry 2005, 44, 7479–7489. 10.1021/bi047429d. [DOI] [PubMed] [Google Scholar]
- Kaiser H. J.; Lingwood D.; Levental I.; Sampaio J. L.; Kalvodova L.; Rajendran L.; Simons K. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 16645–16650. 10.1073/pnas.0908987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S.; Heller W. T. Peptide-Induced Asymmetric Distribution of Charged Lipids in a Vesicle Bilayer Revealed by Small-Angle Neutron Scattering. J. Phys. Chem. B 2011, 115, 9831–9837. 10.1021/jp204045t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.