Abstract
Cancer cachexia is defined as a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass with or without loss of fat mass. The syndrome cannot be fully reversed by conventional nutritional support, and despite an increased number of studies related to cancer cachexia, the underlying mechanisms are still poorly defined and therapeutic options are limited. This review focuses on recent studies investigating mechanisms and pathways in cancer cachexia. The role of molecular and functional imaging in identifying cachexia at an earlier stage, in identifying potential metabolic targets and pathways, and in assessing treatment efficacy is also reviewed.
Introduction
Cancer cachexia is defined as a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass, with or without loss of fat mass that cannot be fully reversed by conventional nutritional support. The syndrome leads to progressive functional impairment [1]. The pathophysiology of the syndrome is characterized by a negative protein and energy balance that is driven by a variable combination of reduced food intake, or anorexia, and abnormal metabolism [1]. Cachexia combined with anorexia negatively impinges on the quality of life, decreases tolerance to treatments, and reduces overall survival of cancer patients. Cachexia is encountered not only in cancer, but also in other life-threatening diseases, such as acquired immunodeficiency syndrome, rheumatoid arthritis, chronic obstructive pulmonary disease, and organ failure [2]. In cancer patients, the cachectic syndrome is a major cause of morbidity and mortality. Progressive cachexia indicates poor prognosis with a shorter survival time, and accounts for nearly 20% of all cancer deaths [3, 4]. Presence of cachexia is identified from a weight loss of 10% or more within 6 months. The rate and amount of weight loss are directly related to survival in cancer patients [5].
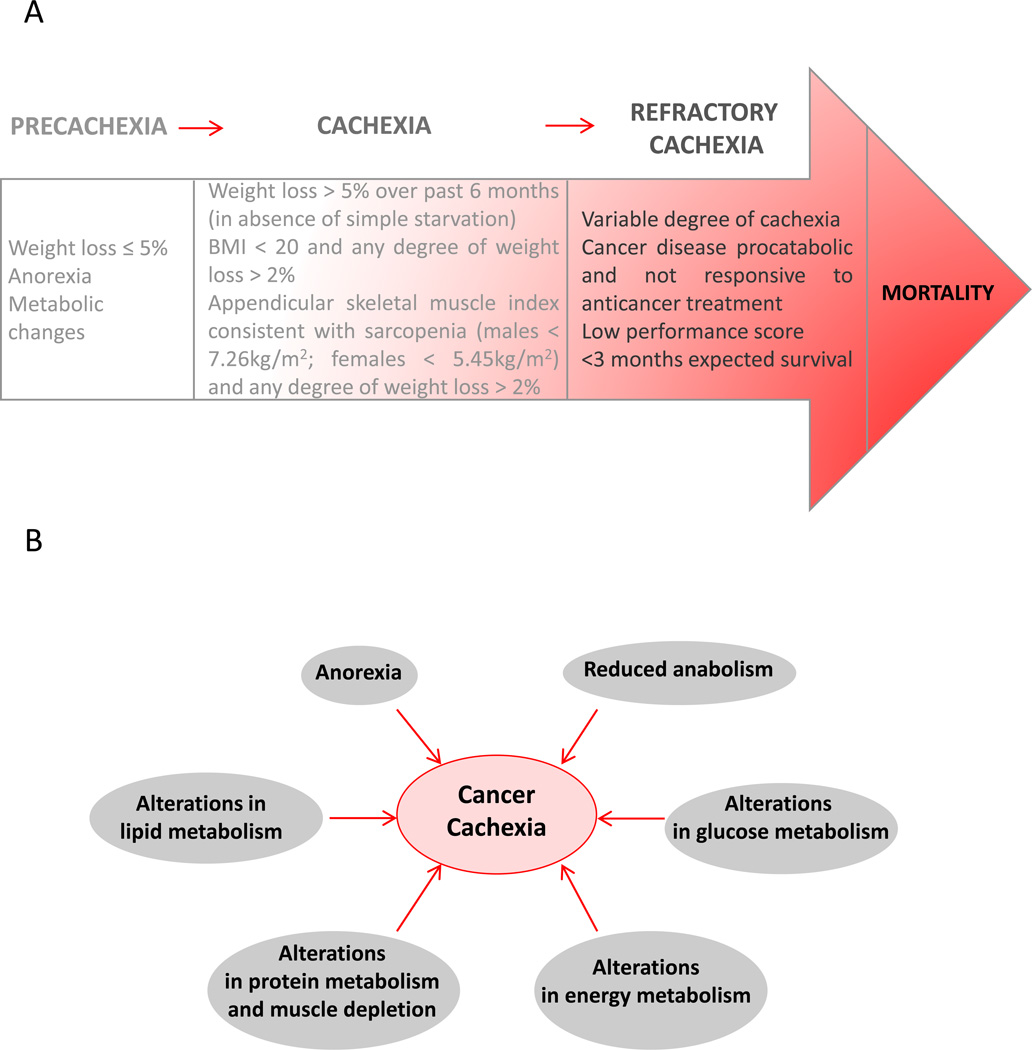
Fearon et al., have described three stages of the cachectic syndrome, precachexia, cachexia, and refractory cachexia (Figure 1A) [1]. The precachectic stage is characterized by early clinical and metabolic signs, such as weight loss (less than 5%), anorexia, and impaired glucose tolerance. The progression to cachexia depends upon multiple factors such as systemic inflammation, cancer type and stage, low food intake, and response to treatment. The diagnostic criteria associated with these stages are listed in Figure 1A. Finally, in refractory cachexia, active management of weight loss is no longer possible due to active catabolism or presence of cachectic factors. Patients with severe muscle wasting are unlikely to benefit from treatments intended to result in gain of lean tissue and function. At this stage, the goal of therapy is palliation of symptoms and reduction of distress. It is therefore critically important to identify early onset of cachexia as well as develop molecular interventions to reduce or delay its onset.
Figure 1.
(A) Three stages of the cachectic syndrome and corresponding diagnostic criteria. Adapted with permission from [1]. (B) Pathogenesis of cancer cachexia.
I. MOLECULAR MECHANISMS
As described in Figure 1B, cachexia is characterized by a combination of events. There is a negative protein and energy balance driven by a combination of reduced food intake and abnormal metabolism. Anorexia often occurs in the course of the disease, along with i) alterations in lipid metabolism such as hyperlipidemia and reduced circulating levels of HDL, reduced activity of lipoprotein lipase, ii) alterations in glucose metabolism such as impaired glucose tolerance, insulin resistance, increase in gluconeogenesis from amino acids and from lactate, iii) alterations in protein metabolism and muscle depletion such as increased protein degradation combined with reduced protein anabolism, and iv) alterations in energy metabolism such as increased resting energy expenditure. Reduced anabolism with decreased expression of positive regulators of muscle mass (MyoD), or overexpression of negative regulators (myostatin), or changes in other pathways such as insulin-like growth factor I (IGF-1) are also frequently observed in cachectic patients. The cachectic syndrome arises also from a pro-inflammatory response from the host and the production of catabolic factors and cytokines by the tumor, such as interleukin (IL)-1, 6 and TNF-α [6]. Inflammation has been described as a key factor in cancer cachexia but studies targeting inflammatory cytokines have shown limited effects [7]. Several studies have focused on the cytokines TNF-α and IL6, however, neutralizing them has not suppressed cachexia occurrence [8], and an imperfect correlation exists between their level and the occurrence of the syndrome [7].
More recently, the role of brown adipose tissue (BAT) in cachexia has attracted significant attention. The generation of brown adipose cells in white adipose tissue (WAT) seems to be an early change occurring before the appearance of clinical signs in mice [9]. Brown adipose cells express uncoupling protein 1 (UCP1), a mitochondrial protein that switches mitochondrial respiration from adenosine triphosphate (ATP) generation to thermogenesis. The presence of UCP1 in BAT mediates proton leakage across the inner mitochondrial membrane, decreasing the level of coupling of respiration to adenosine diphosphate (ADP) phosphorylation. Heat is then produced instead of energy stored as ATP [10]. BAT activity correlates with total and resting energy expenditure. A phenotypic switch from WAT to brown fat occurs before the initial stage of cancer cachexia and prior to skeletal muscle atrophy [9]. Browning of adipose tissue was observed in cancer cachexia patients, along with an association between adipocyte atrophy and UCP1 expression [9]. Browning of WAT was also observed in pre-cachectic mice and increased as cachexia developed, together with an increase of UCP1. WAT browning, together with enhanced thermogenesis in BAT, contributed to increased systemic energy expenditure. The pro-inflammatory cytokine IL6 can increase UCP1 expression in WAT, and treatment targeting IL6 was shown to reduce WAT browning and cachexia in C26 colon cancer cachectic mice [9]. In another set of experiments, treatment of genetically engineered cachectic skin cancer K5-SOS mice with a selective β3-adrenergic receptor (β3-AR) antagonist ameliorated cachexia and decreased UCP1 levels in subcutaneous WAT [9]. These data suggested that inhibition of WAT browning represents a promising approach to ameliorate the severity of cachexia in cancer patients [9].
Cachexia has been associated with increased resting energy expenditure level that has been linked to greater thermogenesis by brown fat. In a Lewis lung carcinoma model of cancer cachexia, tumor-derived parathyroid-hormone-related protein (PTHrP) was observed to play a critical role in cachexia by driving the expression of genes involved in thermogenesis in adipose tissues [11]. PTHrP increased UCP1 protein levels and raised oxygen consumption. Inhibition of PTHrP signaling resulted in reduction of brown adipose tissue, and a reduction of muscle mass loss, and strength loss [11]. PTHrP was detected in the plasma of tumor bearing mice, and an injection of anti-PTHrP protected the mice against weight loss. The treatment blocked both adipose tissue and muscle mass loss without affecting tumor progression. These results were supported by a clinical investigation that revealed an association between higher PTHrP concentrations, a greater degree of lean tissue wasting, and elevated energy expenditure in a cohort of cancer patients [11].
In addition to cancer cells, stromal cells play a critical role in the establishment of cachexia [12. Depletion of fibroblast activation protein-α positive (FAP+) stromal cells in a transgenic mouse model, that permits both the bioluminescence imaging of cells expressing FAP and their conditional ablation, caused cachexia that was characterized by muscle atrophy rather than loss of myocytes [12]. One of the consequences of the FAP+ stromal cell depletion was a transient increase of atrogin-1 and MuRF-1. Interestingly, no increase was observed in IL6, TNF-α, and corticosterone levels. Inoculation of mice with the pro-cachectic cell line C26 induced similar changes, i.e., decrease of FAP+ stromal cells, increase in atrogin-1 and MuRF-1 levels, and reduction of muscle mass and weight loss [12]. These results were reproducible in the KPC (KrasG12D/+; Tp53R172H/+; Pdx-1 Cre) pancreatic tumor model [12] and demonstrate the role played by stromal cells in cachexia.
II. TREATMENT OPTIONS
Since the pathogenesis of cachexia is multifactorial, treatments should incorporate multiple-target strategies. Multiple therapeutic approaches with limited success have been tested in preclinical and clinical investigations in recent years.
A daily treatment with a Jak2 inhibitor resulted in reduction of cachexia without affecting tumor growth in transgenic Pdx1-cre;LSL-KrasG12D;INK4a/arffl/fl mice that develop systematically spontaneous pancreatic adenocarcinoma with acute global cachexia [13]. Activation of Jak2 leads to a strong expression of IL6. Reduction of Jak2 activity decreases IL6 expression. Jak2 is also involved in the expression of other potential activators of cachexia, such as IL11, leukemia inhibitory factor (LIF), oncostatin M (OM), and ciliary neurotrophic factor (CNTF) that may explain the efficacy of targeting Jak2 in reducing cancer cachexia [13].
Some therapeutic approaches involve the use of pharmaconutrients with anti- inflammatory properties, such as omega-3 fatty acids (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)). As substrates of cyclooxygenase (COX) enzyme, omega-3 fatty acids, and especially EPA, counteract the pro-inflammatory response of thromboxanes and prostaglandins derived from arachidonic acid, through competition for COX [14]. The anabolic effects of omega-3 fatty acids are independent of their action on inflammation, but the exact mechanisms of the anabolic response are not completely understood [14].
Ghrelin is a peptide hormone involved in anabolic and homeostatic functions that has shown promising effects in cancer cachexia, although the mechanisms of action are not completely understood [15]. Therapeutic administration of ghrelin or one of its analogs has been shown to increase energy intake [16], and counteract loss of body mass and function [15, 17]. In animal studies, ghrelin administration improved food intake, body weight and lean body mass retention [18–20]. Acylated ghrelin binds to the growth hormone secretagogue receptor 1α (GHSR-1α) that is widely expressed in the hypothalamic and pituitary regions. It mediates growth hormone release, enhancing appetite and increasing adipose tissue deposition. Ghrelin influences not only hormonal release, energy homeostasis, and appetite modulation, but also metabolic and anti- inflammatory responses. It can affect the inflammatory aspect of cachexia by inducing the release of IL10, an anti-inflammatory cytokine that can in return reduce IL1-β, IL6 and TNF-α. Grehlin suppresses NFκB that is involved in the ubiquitin-proteasome pathway, thereby inhibiting muscle and protein catabolism. Atrogin-1 and MuRF-1 have been shown to be significantly reduced after ghrelin administration [18]. Additional studies are required to determine the treatment schedule and dosage and to assess any side-effects and complications that could include a potential stimulation of tumor growth. Anamorelin, a new potent selective ghrelin receptor agonist mimicking the N-terminal active core of ghrelin [17], is currently in phase III clinical trials for the treatment of cachexia in non-small cell lung cancer [20].
ActRIIB is a high affinity activin type 2 receptor that mediates signaling through a subset of TGF-β family ligands, including activin and myostatin. These ligands play a critical role in regulating muscle mass [21]. Pharmacological inhibition of the ActRIIB pathway was shown to not only prevent further muscle wasting but also completely reverse loss of skeletal muscle and cancer-induced cardiac atrophy in several models of cancer cachexia [21]. The treatment abolished activation of the ubiquitin-proteasome system and the induction of atrophy-specific ubiquitin ligases in muscles. It also markedly stimulated muscle stem cell growth. Interestingly, the treatment prolonged animal survival despite an absence of effect on tumor growth, fat loss, and pro-inflammatory cytokines (IL6, IL1-β, TNF-α) production [21]. While cachexia is characterized by a loss of muscle and adipose tissue, the wasting of these tissues can be regulated by different pathways. In cachectic C26 colon tumor bearing mice, ActRIIB antagonism dramatically prolonged survival by preventing muscle wasting and inducing net muscle growth, without altering fat mass. In this study, only the maintenance of muscle mass correlated with enhanced survival in cancer bearing mice [21].
It is evident that nutritional and metabolic interventions are essential to improve outcomes for cancer patients [22]. The “parallel pathway” was recently described as a preventive and therapeutic management strategy for cancer cachexia [22]. This multiprofessional and multimodal approach would ensure an early appropriate and continuous nutritional and metabolic support to cancer patients. It would involve an oncological management path in parallel with a nutritional and metabolic one. Oncological management would include disease staging, elaboration of a therapeutic plan, first-line therapy, follow-up, periodical re-evaluations and second line treatment if necessary. Nutritional and metabolic management would include nutritional screening and assessment, elaboration of a nutritional plan with a first-level nutritional intervention, and then follow-up and periodical re-evaluations that could lead to a higher level of nutritional and metabolic strategies. Nutritional support can reduce the number of complications and shorten the recovery phase, it can ameliorate the rate of infections with a better control of cancer-related symptoms such as fatigue, and improve tolerance to anticancer treatments.
III. IMAGING CACHEXIA
Current state-of-the-art molecular and functional imaging can be applied to noninvasively characterize tumors that induce cachexia, to develop noninvasive biomarkers to identify tumors likely to induce cachexia, and to identify changes in normal tissues that are typical of the induction of cachexia. This ability to detect the onset or presence of cachexia noninvasively could be expanded to include detecting response to treatment and to the development of new therapies.
Most clinical imaging studies describe muscle mass assessment, using techniques such as cross-sectional imaging (computed tomography (CT) or magnetic resonance imaging (MRI)), and dual energy x-ray imaging (DEXA) [23]. CT imaging provides an accurate assessment of muscle mass and body composition in patients with cancer, and can potentially predict prognosis and possibly chemotherapy toxicity [24–26]. Lumbar CT images, for example, can be used to measure total skeletal muscle cross-sectional area and to estimate total body fat-free mass [27]. Imaging can be useful, especially in the case of “hidden cachexia”, when muscle and fat loss can be masked by weight gain due to ascites or peripheral edema, or in the case of sarcopenic obesity (obesity with depleted muscle mass) [6, 27]. In addition to skeletal muscle and adipose tissue, organ size can be quantified with CT as shown in a study performed on patients during colorectal cancer cachexia progression [28]. A viscerally driven cachexia syndrome, with loss of muscle mass and adipose tissue, was associated with an increase in liver and spleen size [28].
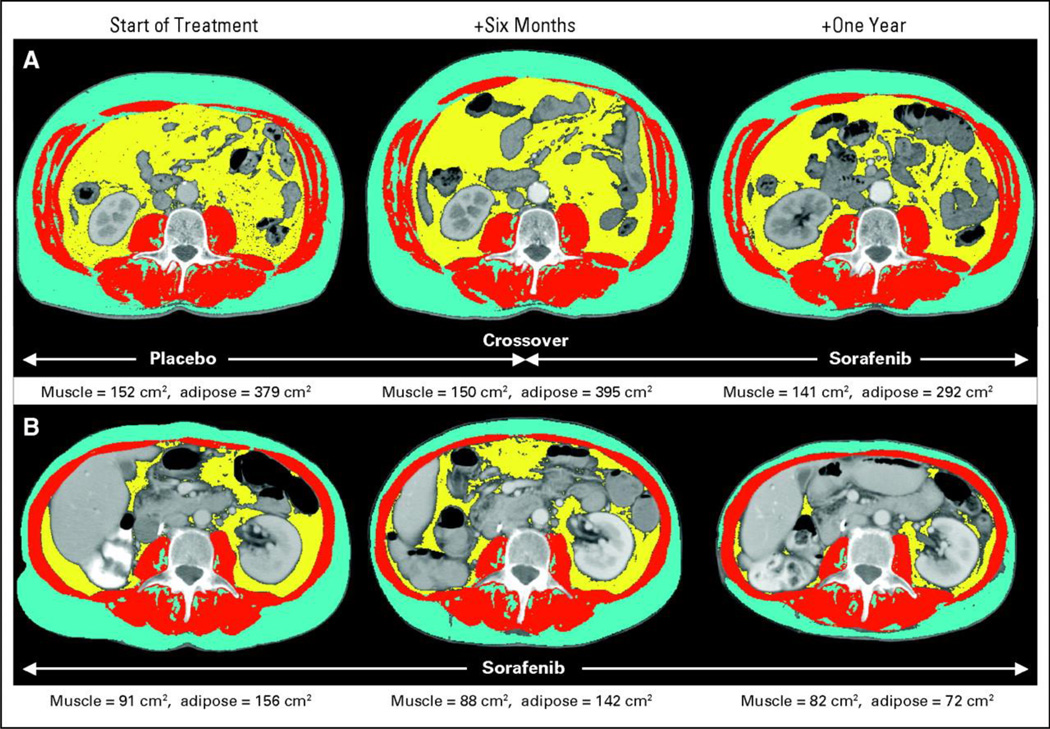
CT imaging has also been applied to assess anti-tumor treatment effects on muscle mass. Treatment of a metastatic renal cell cancer with Sorafenib, a multikinase inhibitor with anti-angiogenic properties, was shown to exacerbate muscle loss in patients (Figure 2) [29]. Another study explored the impact of insulin-like growth factor receptor (IGFR-1) inhibition on muscle mass. IGF-1 plays a role in the growth of multiple tumor types, including pancreatic cancer, but also serves as a growth factor for muscle; the impact of IGF-1 therapeutic targeting on muscle mass was unknown. The study showed that patients treated with anti-IGF-1 therapy (MK-0646) did not lose significantly more muscle than the non-MK-treated patients, though there was a trend in this direction [30].
Figure 2.
Representative CT images and tissue areas: (A) placebo crossed over to Sorafenib. (B) Sorafenib at 6 months and 1 year, showing loss of muscle (red) after initiation of Sorafenib and loss of subcutaneous (blue) and visceral (yellow) adipose tissue in the long-term Sorafenib-treated patient. Adapted with permission from [29].
CT is not the only imaging technique that can be used to study cachexia in the clinical setting. MRI can also be applied to measure muscle cross-sectional area and volume, and can specifically distinguish between different tissue types, such as muscle and subcutaneous fat [31]. Fatty infiltration within muscle can also be quantified. Using MRI it was shown that muscles of cachectic patients were not only smaller but also less homogeneous than muscles from healthy control [31]. Multimodal MR techniques can be performed to assess skeletal muscle morphology, metabolism, and microcirculation, and evaluate the effect of cachexia on these parameters [32]. While morphologic parameters, body mass index, cross-sectional area and total fiber size were described as lower in cachectic patients compared to healthy volunteers, microcirculation and muscular energy metabolites, pH, and trimethyl-ammonium-containing compounds were comparable in both groups [32]. MRI was used to assess the size of the quadriceps in a phase I/II trial that tested the safety, tolerability and efficacy of a novel combination of an anabolic β2-agonist and an appetite stimulant in patients with cancer cachexia [33]. Hand-grip strength, lower limb extensor power, physical activity and quality of life were the other criteria used in the study to assess the efficacy of the treatment. Muscle mass and/or function were improved in most patients completing the course, and further investigation in larger, randomized trials is necessary to confirm those preliminary results [33].
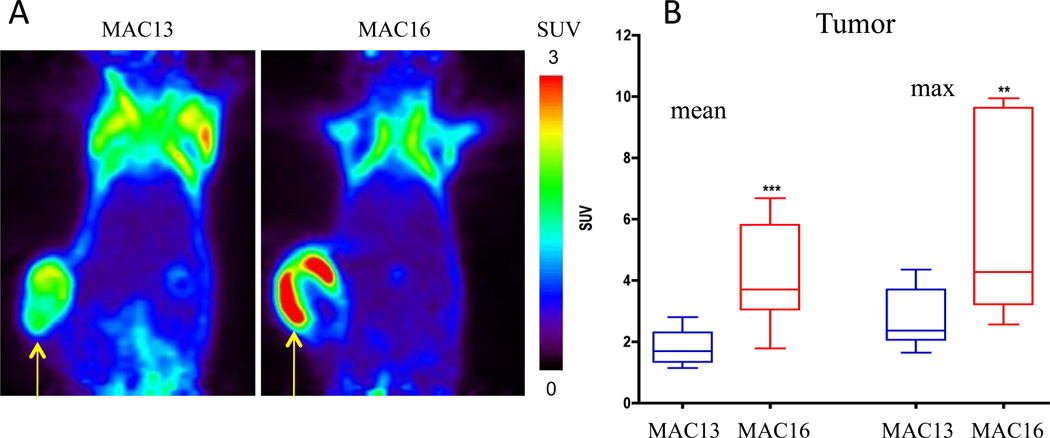
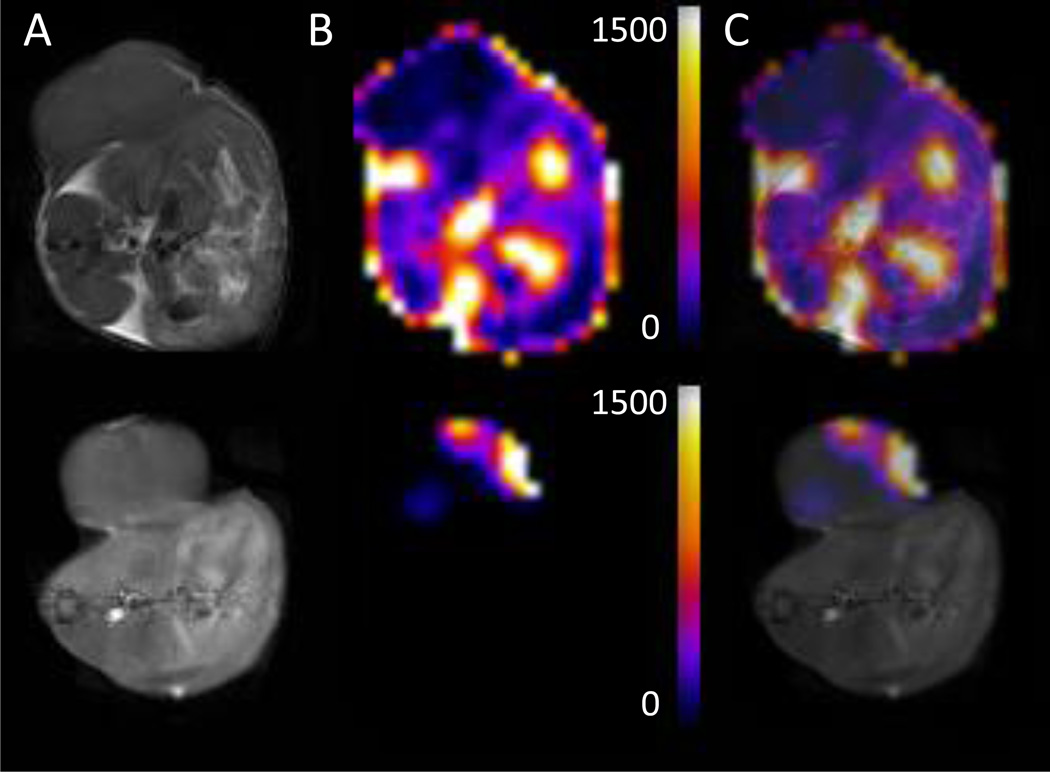
Imaging can be used in preclinical studies to better understand the cachectic syndrome, and to identify metabolic signatures indicative of a cachexia-inducing tumor that could be used as biomarkers before the occurrence of weight loss. In 1H magnetic resonance spectroscopic imaging (MRSI) of a murine colon adenocarcinoma (MAC) model, cachectic MAC16 tumors were characterized by increased total choline (tCho) compared to non-cachectic MAC13 tumors [34]. Lactate+lipids maps showed that although the lactate+lipids content was not different between MAC13 and MAC16 tumors, the peripheral signal, most likely originating from subcutaneous lipids, was lower around the MAC16 tumor. MRI studies were complemented by 18FDG PET analysis that revealed an increased uptake of 18FDG in the cachectic tumors compared to non-cachectic MAC13 tumors (Figure 3) [34]. These metabolic patterns may represent new noninvasive biomarkers and targets in the detection, management, and treatment of cachexia. Imaging of normal tissues can also provide indices of cachexia. As shown in Figure 4, representative T1-weighted images of MAC13 and MAC16 tumor bearing mice and representative lactate+lipids maps obtained from the same cross-sectional slice, demonstrated profound depletion of the signal in normal tissue in MAC16 (cachectic) tumor-bearing mice compared to MAC13 (non-cachectic) tumor-bearing mice [34].
Figure 3.
(A) Representative PET images of 18FDG uptake in MAC13 and MAC16 tumor bearing mice (SUV: standardized uptake value). (B) Quantification of the uptake in the tumors. Values represent Mean ± SEM (MAC13, n = 8, MAC16, n = 5; * P < 0.005). Adapted with permission from [34].
Figure 4.
(A) Cross-sectional T1-weighted images, (B) cross-sectional lactate+lipids maps, and (C) merged images from (A) and (B) of MAC13 (upper panel) and MAC16 (lower panel) tumor-bearing mice. T1-weighted images were acquired from the corresponding 4 mm slice used for MRSI using a spin-echo sequence with an echo time of 10 ms, a repetition time of 500 ms, and an in-plane spatial resolution of 125 µm. Lipid maps were generated from MRSI data and normalized to the water signal. Volumes were comparable for the MAC13 (540 mm3) and MAC16 (545 mm3) tumors. Adapted with permission from [34].
It is essential to diagnose patients at the pre-cachexia stage, since once the syndrome is well-established treatment is much more difficult, and advanced cachexia is refractory to treatment. Experimental studies have demonstrated the early occurrence of browning of WAT in the cachectic syndrome. Human studies have demonstrated that 18FDG uptake in BAT is directly proportional to the expression of UCP1, and that 18FDG imaging could be used as a surrogate marker to non-invasively measure BAT activity in vivo 10]. While in a post-mortem study, there was a high prevalence of BAT in cachectic cancer patients compared to age-matched non-cancer subjects [35], this correlation has not been found so far in clinical studies [10]. Applying 18FDG PET imaging could be useful in cachexia diagnosis and merits further investigation [10].
CONCLUSION
Despite a significant increase in research on cancer cachexia, limited therapeutic options are available and the underlying mechanisms are still poorly defined. Recent preclinical studies have revealed novel therapeutic targets but these have to be investigated in human applications. Current state-of-the-art multi-modality molecular and functional imaging approaches are ideally suited to better understand the cachectic syndrome and the effect of these novel targets. Better treatments of cachexia are urgently required and would have a significant impact on improving the quality of life for patients and on increasing their life expectancy. New potential targets, such as ActRIIB and PTHrP, have shown promising results in preclinical studies and need to be further explored in preclinical and clinical settings. Inhibition of WAT browning represents a promising target in cancer cachexia, and the availability of BAT imaging agents could help in the early diagnosis, and in the assessment of treatment efficacy. Despite new promising discoveries, cancer cachexia is still a major cause of morbidity and mortality, and better early diagnostic tools and better treatment options are critical to minimizing the impact of this complex syndrome.
Acknowledgments
We gratefully acknowledge support from P50 CA103175, P30 CA006973, R01 CA73850, R01 CA82337, R01 CA136576, R01 CA138515 and The Tina Brozman Foundation.
Abbreviations
- BAT
brown adipose tissue
- COX
cyclooxygenase
- CT
computed tomography
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FDG
Fluorodeoxyglucose
- IGF-1
insulin-like growth factor
- IGFR-1
insulin-like growth factor receptor
- IL
interleukin
- MAC
murine adenocarcinoma
- MRI
magnetic resonance imaging
- MRS
magnetic resonance sprectroscopy
- MRSI
magnetic resonance sprectroscopic imaging
- PET
positron emission tomography
- PTHrP
parathyroid-hormone-related protein
- SUV
standardized uptake value
- tCho
total choline
- TNF
tumor necrosis factor
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
REFERENCES
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Tisdale MJ. Cancer cachexia. Langenbecks Arch Surg. 2004;389(4):299–305. doi: 10.1007/s00423-004-0486-7. [DOI] [PubMed] [Google Scholar]
- 3.Tisdale MJ, Dhesi JK. Inhibition of weight loss by omega-3 fatty acids in an experimental cachexia model. Cancer Res. 1990;50(16):5022–5026. [PubMed] [Google Scholar]
- 4.Loberg RD, Bradley DA, Tomlins SA, Chinnaiyan AM, Pienta KJ. The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J Clin. 2007;57(4):225–241. doi: 10.3322/canjclin.57.4.225. [DOI] [PubMed] [Google Scholar]
- 5.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 6.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 7.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, Mattar BI, Loprinzi CL. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9) Lung Cancer. 2010;68(2):234–239. doi: 10.1016/j.lungcan.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, Brans B. Molecular imaging of brown adipose tissue in health and disease. European journal of nuclear medicine and molecular imaging. 2014;41(4):776–791. doi: 10.1007/s00259-013-2611-8. [DOI] [PubMed] [Google Scholar]
- 11.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJB, Zhao Q, Caballero OL, Larder R, Coll AP, O'Rahilly S, Brindle KM, Teichmann SA, Tuveson DA, Fearon DT. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. Journal of Experimental Medicine. 2013;210(6):1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilabert M, Calvo E, Airoldi A, Hamidi T, Moutardier V, Turrini O, Iovanna J. Pancreatic cancer-induced cachexia is Jak2-dependent in mice. J Cell Physiol. 2014;229(10):1437–1443. doi: 10.1002/jcp.24580. [DOI] [PubMed] [Google Scholar]
- 14.Di Girolamo FG, Situlin R, Mazzucco S, Valentini R, Toigo G, Biolo G. Omega-3 fatty acids and protein metabolism: enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17(2):145–150. doi: 10.1097/MCO.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 15.Molfino A, Formiconi A, Rossi Fanelli F, Muscaritoli M. Ghrelin: from discovery to cancer cachexia therapy. Curr Opin Clin Nutr Metab Care. 2014;17(5):471–476. doi: 10.1097/MCO.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 16.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(6):2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21(1):129–137. doi: 10.1007/s00520-012-1500-1. [DOI] [PubMed] [Google Scholar]
- 18.Porporato PE, Filigheddu N, Reano S, Ferrara M, Angelino E, Gnocchi VF, Prodam F, Ronchi G, Fagoonee S, Fornaro M, Chianale F, Baldanzi G, Surico N, Sinigaglia F, Perroteau I, Smith RG, Sun Y, Geuna S, Graziani A. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J Clin Invest. 2013;123(2):611–622. doi: 10.1172/JCI39920. PMCID: 3561827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molfino A, Gioia G, Muscaritoli M. The hunger hormone ghrelin in cachexia. Expert Opin Biol Ther. 2013;13(4):465–468. doi: 10.1517/14712598.2013.748031. [DOI] [PubMed] [Google Scholar]
- 20.Pietra C, Takeda Y, Tazawa-Ogata N, Minami M, Yuanfeng X, Duus EM, Northrup R. Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: preclinical profile. J Cachexia Sarcopenia Muscle. 2014 doi: 10.1007/s13539-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Muscaritoli M, Molfino A, Gioia G, Laviano A, Rossi Fanelli F. The "parallel pathway": a novel nutritional and metabolic approach to cancer patients. Intern Emerg Med. 2011;6(2):105–112. doi: 10.1007/s11739-010-0426-1. [DOI] [PubMed] [Google Scholar]
- 23.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 24.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(22):6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 25.Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 26.Del Fabbro E. More is better: a multimodality approach to cancer cachexia. Oncologist. 2010;15(2):119–121. doi: 10.1634/theoncologist.2010-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. The Lancet Oncology. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 28.Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89(4):1173–1179. doi: 10.3945/ajcn.2008.27273. PMCID: 2667460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 2010;28(6):1054–1060. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 30.Fogelman DR, Holmes H, Mohammed K, Katz MH, Prado CM, Lieffers J, Garg N, Varadhachary GR, Shroff R, Overman MJ, Garrett C, Wolff RA, Javle M. Does IGFR1 inhibition result in increased muscle mass loss in patients undergoing treatment for pancreatic cancer? J Cachexia Sarcopenia Muscle. 2014 doi: 10.1007/s13539-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray C, MacGillivray TJ, Eeley C, Stephens NA, Beggs I, Fearon KC, Greig CA. Magnetic resonance imaging with k-means clustering objectively measures whole muscle volume compartments in sarcopenia/cancer cachexia. Clin Nutr. 2011;30(1):106–111. doi: 10.1016/j.clnu.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Weber MA, Krakowski-Roosen H, Schroder L, Kinscherf R, Krix M, Kopp-Schneider A, Essig M, Bachert P, Kauczor HU, Hildebrandt W. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol. 2009;48(1):116–124. doi: 10.1080/02841860802130001. [DOI] [PubMed] [Google Scholar]
- 33.Greig CA, Johns N, Gray C, MacDonald A, Stephens NA, Skipworth RJ, Fallon M, Wall L, Fox GM, Fearon KC. Phase I/II trial of formoterol fumarate combined with megestrol acetate in cachectic patients with advanced malignancy. Support Care Cancer. 2014;22(5):1269–1275. doi: 10.1007/s00520-013-2081-3. [DOI] [PubMed] [Google Scholar]
- 34.Penet MF, Gadiya MM, Krishnamachary B, Nimmagadda S, Pomper MG, Artemov D, Bhujwalla ZM. Metabolic signatures imaged in cancer-induced cachexia. Cancer research. 2011;71(22):6948–6956. doi: 10.1158/0008-5472.CAN-11-1095. PMCID: 3217079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shellock FG, Riedinger MS, Fishbein MC. Brown adipose tissue in cancer patients: possible cause of cancer-induced cachexia. J Cancer Res Clin Oncol. 1986;111(1):82–85. doi: 10.1007/BF00402783. [DOI] [PubMed] [Google Scholar]