Abstract
Use of smokeless tobacco products (STPs) is associated with oral cavity cancer and other health risks. Comprehensive analysis for chemical composition and toxicity is needed to compare conventional and newer STPs with lower tobacco-specific nitrosamines (TSNAs) yields. Seven conventional and 12 low-TSNA moist snuff products purchased in the U.S., Sweden, and South Africa were analyzed for 19 chemical constituents (International Agency for Research on Cancer classified carcinogens), pH, nicotine, and free nicotine. Chemicals were compared in each product using Wilcoxon rank-sum test and principle component analysis (PCA). Conventional compared to low-TSNA moist snuff products had higher ammonia, benzo[a]pyrene, cadmium, nickel, nicotine, nitrate, and TSNAs and had lower arsenic in dry weight content and per mg nicotine. Lead and chromium were significantly higher in low-TSNA moist snuff products. PCA showed a clear difference for constituents between conventional and low-TSNA moist snuff products. Differences among products were reduced when considered on a per mg nicotine basis. As one way to contextualize differences in constituent levels, probabilistic lifetime cancer risk was estimated for chemicals included in The University of California's Carcinogenic Potency Database (CPDB). Estimated probabilistic cancer risks were 3.77-fold or 3-fold higher in conventional compared to low-TSNA moist snuff products under dry weight or under per mg nicotine content, respectively. In vitro testing for the STPs indicated low level toxicity and no substantial differences. The comprehensive chemical characterization of both conventional and low-TSNA moist snuff products from this study provides a broader assessment of understanding differences in carcinogenic potential of the products. In addition, the high levels and probabilistic cancer risk estimates for certain chemical constituents of smokeless tobacco products will further inform regulatory decision makers and aid them in their efforts to reduce carcinogen exposure in smokeless tobacco products.
Keywords: cancer risk, carcinogens, smokeless tobacco, moist snuff, TSNAs
Graphical Abstract
1. INTRODUCTION
Smokeless tobacco products (STPs) have been increasingly promoted in recent years by major tobacco companies as an alternative to smoking (Hatsukami et al., 2007), and the global market has grown considerably (Delnevo et al., 2014). STPs includes chewing tobacco (loose leaf, plug, or twist), snuff (moist and dry), and dissolvable (lozenges, sticks, strips, and orbs) (IARC, 2007). On the U.S. market, conventional and low-TSNA moist snuff products are most popular forms of STPs (Stepanov et al., 2008a; Xue et al., 2014). Moist snuff products are sold as small pouches or powder and are held in the mouth between the lip or cheek and gum or sniffed up the nose, rather than smoking (Stepanov et al., 2008a). Tobacco companies are marketing these products with colorful packages and sweet flavors, which make them more attractive to youth, young adults, and women as a substitute for smoking (Adkison et al., 2014).
Low-TSNA moist snuff products sold in the recent within Sweden, the US, and elsewhere are distinguished from conventional moist snuff products because they are produced using different curing methods that greatly reduce TSNAs, namely air- and sun-cured in contrast to conventional moist snuff STPs that tent to include blends in fire-cured tobacco (Foulds et al., 2003; Rutqvist et al., 2011). In addition to these differences, low-TSNA moist snuff products contain pasteurized tobacco and generally refrigerated immediately after production to help minimize of the risk of the formation of TSNAs and other toxicants in the product during storage, whereas conventional moist snuff products are mostly fermented, allowing continued formation of TSNAs (Foulds and Furberg, 2008; Osterdahl and Slorach, 1983; Twombly, 2010).
Although the use of the STPs is considered less harmful than smoking for exclusive users because STPs do not yield combustion products when used, they still contain a large number of chemicals and carcinogens (IARC, 2007). By The Food and Drug Administration (FDA) under The Family Smoking Prevention and Tobacco Control Act of 2009 (Tobacco Control Act or TCA), a tobacco product including the STPs standard, what is put into STPs, has been established (Section 907)
(http://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm263053.htm). Moreover, The FDA Center for Tobacco Products current Research Priorities include the study of smokeless tobacco toxicity (https://prevention.nih.gov/tobacco-regulatory-science-program/research-priorities), and has established a list of harmful and potentially harmful constituents (HPHCs) in tobacco including STPs (http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297981.pdf). Nine constituents in STPs are currently in enforcement discretion to require reporting for industry (http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297981.pdf).
Scientific evidences have shown that health risks associated with snus use are lower than those associated with cigarette smoking (Lewin et al., 1998; Schildt et al., 1998; Ye et al., 1999), but it should be noted that there are some population studies for adverse effects of STPs use (Hecht et al., 2007; Luo et al., 2007; Martin et al., 1999; Zhou et al., 2013). A large study found that exposure levels of 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in STPs users (n=182) were comparable to those in cigarette smokers (n=420) (Hecht et al., 2007). Clinical outcomes for oral leukoplakia showed association with use of U.S. conventional moist snuff products including Copenhagen, Skoal, and Kodiak (Martin et al., 1999). In a case-control study, individuals who reported 10 or more years of STPs use had a significantly increased risk of head and neck squamous cell carcinoma (HNSCC), suggesting adverse effects of long-term use of STPs(Zhou et al., 2013). Another study showed that use of Swedish snus could heighten a user's risk for pancreatic cancer (Luo et al., 2007)
According to The International Agency for Research on Cancer (IARC), approximately 30 known or probable human carcinogens in STPs are identified (IARC, 2007). A number of studies have reported chemical composition of STPs worldwide, but the majority of the studies have limited on dry snuff, chewing tobacco, plug, tobacco pellets, conventional moist snuff, or Swedish snus (Hoffmann and Djordjevic, 1997; IARC, 2007; Lawler et al., 2013; Rodu and Jansson, 2004). Recently, some studies have reported large variation in the levels of some toxicants in low-TSNA moist snuff products; however, these studies have mainly focused on a limited range of toxicants such as polycyclic aromatic hydrocarbons (PAHs) (Stepanov et al., 2008a; Stepanov et al., 2010) and TSNAs (Lawler et al., 2013; Stepanov et al., 2008a).
Smokeless tobacco is classified as a known human carcinogen by IARC, and various constituents are similarly classified (http://monographs.iarc.fr/). Currently, there is no way to relate a particular level of a chemical constituent in STPs to cancer risk. The University of California's Carcinogenic Potency Database (CPDB) provides a carcinogenic potency from systematic and unifying analysis of chronic and long-term animal cancer tests in literature through 2001 and by the National Cancer Institute/National Toxicology Program through 2004, which can be used to contextualize the magnitude for differences among risk by individual constituents . This approach has been done for cigarette smoke by Fowles and Dybing (Fowles and Dybing, 2003) using the US Environmental Protection Agency (EPA)'s cancer potency values, analogous to what is available from the CPDB (USEPA, 1991), and probabilistic lifetime cancer risk for selected Swedish snus and moist snuff products were estimated by Ayo-Yusuf and Connolly using CPDB's carcinogen potency values (Ayo-Yusuf and Connolly, 2011). These databases somewhat assign cancer risks based on experimental animal studies and safety quotients to account for uncertainty. Thus, while they are a means to compare exposures, it is unknown if the risk estimates provided from these methods reflect actual differences in risk to humans.
Due to several advantages of in vitro studies(i.e., relatively inexpensive and rapid screening of many samples) (Johnson et al., 2009) to assess the toxicological impact of tobacco products, in vitro toxicology tests including Ames testing, Neutral Red cytotoxicity, and micronucleus assay have been wildly used in STPs, but it has been limited on the number of conventional moist snuff, chewing STPs, or Swedish STPs (Johnson et al., 2009; Rickert et al., 2009).
Here, we aimed to comprehensively determine the wide range of chemical constituents including FDA's HPHCs in most popular moist snuff tobacco products used in the U.S., Sweden, and South Africa. We further compared chemical compounds and provide some context for risk related to differences of chemical levels by a classification of the products into two groups, conventional and low-TSNA moist snuff products.
2. MATERIALS AND METHODS
2.1. Smokeless tobacco products
We used 19 most popular moist snuff products including 7 conventional (Husky Straight Long-cut, Grizzly Fine Cut Wintergreen Fine cut, Timber Wolf Fine Cut Natural Fine cut, Copenhagen Mid-Cut Black Bourbon Flavored, Copenhagen Pouches, Copenhagen Snuff, Skoal Bandits Mint) and 12 low-TSNA moist snuff tobacco products. The latter includes 7 U.S. products (Camel Frost Snus, Camel Spice Snus, Camel Original Snus, Marlboro Mild Snus, Marlboro Mint Snus, Marlboro Rich Snus, and Marlboro Spice Snus), 3 Swedish products (Ettan Lossnus, General Mini Portion, and Skruf Stark Portion), and 2 South Africa products (Taxi Super Snuff Gwayi and Peter Stuyvesant Coffee Snus). These products were purchased in local retail outlets in USA (Washington, D.C. and Connersville, IN), Sweden, and South Africa between 2007 and 2009.
The Kentucky reference materials 2S1 loose-leaf chewing tobacco, 1S2 dry snuff, 2S3 moist snuff, and 2R4F cigarette also were analyzed (Supporting Table 1) (Johnson et al., 2009).
2.2. Smokeless tobacco chemical analysis
The chemical constituents were analyzed by Arista Laboratories (Richmond, VA, USA); a commercial laboratory experienced in tobacco analysis. Each product was analyzed in triplicate for chemical analysis, and results are reported as the mean of these values. We analyzed pH, nicotine, free nicotine, and 19 chemical constituents including 4 TSNAs (N′-Nitrosoanabasine [NAB], N′-Nitrosoanatabine [NAT], 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone [NNK], N′-Nitrosonornicotine [NNN]), 6 metals (arsenic, cadmium, chromium, lead, nickel, and selenium), 3 humectants (propylene glycol, glycerol, and triethylene glycol), and 6 others (ammonia, benzo[a]pyrene, formaldehyde, N-nitrosodimethylamine (NDMA), and nitrate). The results presented were on a dry weight and per mg of nicotine content as indicated. The detailed chemicals and analytical method is provided in Supporting Table 2. The chemical levels were normalized to uniform tobacco content by correcting for moisture through drying to a constant weight. Results were reported on a dry weight content which was calculated from wet weight content. The per mg of nicotine content was then calculated by dividing the dry weight content concentration by a nicotine level.
2.3. In vitro toxicity
All products were extracted with dimethyl sulfoxide (DMSO) using an ultrasonic homogenizer, filtered, and stored at −80 °C prior to assays. The assays performed were the Ames Salmonella reverse mutation assay, Neutral Red Uptake (NRU) Cytotoxicity assay, and the micronucleus (MN) assay using the Health Canada Official Method T-501, T-502, and T-503 (Canada, 2004a, b, c), respectively by Labstat International Inc. (Kitchener, Ontario). Each product was assayed in triplicates from 3 separate extractions. The detailed information for a strain or cell line, treated doses, and controls is provided in Supporting Table 2. The absorbance readings of the NRU assay were expressed relative to negative control absorbance reading in the result. In the in vitro MN assay, 1,000 randomly selected cells were scored for the presence of micronuclei.
2.4. IARC Group Carcinogens
Smokeless tobacco is declared as a known human carcinogen by the IARC Monographs Working Group (IARC, 2007). IARC classifies carcinogens into five groups; Group 1 (carcinogenic to humans), Group 2A (probably carcinogenic to humans), Group 2B (possibly carcinogenic to humans), Group 3 (unclassifiable as to carcinogenicity in humans), and Group 4 (probably not carcinogenic to humans). Here, we assessed all chemicals previously identified in conventional and low-TSNA moist snuff tobacco products within Groups 1-3 for which we could develop a CPDB potency factor.
2.5. CPDB Carcinogenic Potency Factor
Probabilistic cancer risk estimates were calculated using the method applied by Ayo-Yusuf and Connolly for STPs using CPDB's carcinogenic potency factor (CPF) (Ayo-Yusuf and Connolly, 2011), based on the toxicological risk assessment principles to cigarette smoke chemical constituents developed by Fowles and Dybing (Fowles and Dybing, 2003). The risk calculations were limited to selected constituents for which the CPF is available in the CPDB. The CPF is based on the daily dose rate in mg/kg-bodyweight/day to induce tumors in half of test animals that would have remained tumor-free at zero dose. For the purposes of this study, it was arbitrarily assumed that 10 grams dry weight product per day was consumed by a 70 kg adult STPs user over a 30 year period during a 70-year lifetime (Ayo-Yusuf and Connolly, 2011; Prabhakar et al., 2013; Zakiullah et al., 2012). The equation used was: incremental lifetime cancer risk=ADE lifetime × CPF (where ADElifetime=lifetime average daily oral exposure (mg/kg bodyweight/day) and CPF=carcinogenic potency factor ((mg/kg bodyweight/day)−1)). The formula for estimating the lifetime ADE was: ADElifetime = ADE × number of years snuffing/average lifetime. The probabilistic lifetime cancer risk estimates for per mg nicotine content was also estimated based on levels of chemicals on the per mg nicotine. The probabilistic lifetime cancer risk for potential toxicity was then estimated based on the reported bioavailability of each carcinogen (transferrable to body, the amounts that can be absorbed by body) (Ayo-Yusuf and Connolly, 2011; Jarup et al., 1998; Stepanov et al., 2008b); 85% transfer for NNN, NNK, and NAB; 36% transfer for formaldehyde and NDMA; 6% transfer for benzo[a]pyrene, cadmium, and lead.
2.6. Statistical analysis
To determine similarity and differences in features of chemicals in the conventional and low-TSNA moist tobacco products, we used unsupervised clustering analysis, Principal Components Analysis (PCA), using JMP® v10 (Cary, NC). PCA is a procedure for finding hypothetical variables called components which account for as much of the variance in multidimensional data as possible (Abdi, 2010). It produced linear combinations of the variables (chemicals) to generate the axes known as principal components, or PCs. In this study, PC1 and PC2 were shown in the results. Factor analysis was performed using Varimax as a rotation method. The most variable chemical in PC1 and PC2 was given in a PCA rotated factor loading. To determine the significant difference on the chemical levels between conventional and low-TSNA moist snuff tobacco products, the Wilcoxon rank-sum test was used. Chemical concentrations below the limit of detection (LOD) or limit of quantification (LOQ) were substituted for one half of LOD or LOQ for statistical analysis, respectively (Musharraf et al., 2012; Navas-Acien et al., 2004). The Wilcoxon rank-sum test was also used to evaluate differences in in vitro toxicity among products. JMP® v10 was used for all statistical analyses. All statistical tests were two-sided and P values <0.05 were considered statistically significant.
3. RESULTS
3.1. pH, Nicotine, and Free nicotine
The nicotine levels in low-TSNA moist snuff products (mean 18.77 mg/g ± SD 4.89) were significantly lower than conventional products (mean 24.48 mg/g ± SD 3.39) at p<0.05 (Supporting Table 1). One of the low-TSNA moist snuff tobacco products (Skruf Stark Portion, Sweden) showed the highest nicotine level (31.6 mg/g), followed by the conventional products (Supporting Table 1). There were no statistical differences for pH (mean 7.83 in conventional vs. 7.59 in low-TSNA moist snuff products) and free nicotine (mean 9.27 mg/g in conventional vs. 6.15 mg/g in low-TSNA moist snuff products) between the two groups. The four U.S. Marlboro low-TSNA moist snuff products (Mild, Mint, Rich, and Spice) had the lowest pH (6.53-6.84) and free nicotine (0.49-0.99 mg/g) (Supporting Table 1).
3.2. Application of Principal Component Analysis to chemical constituents
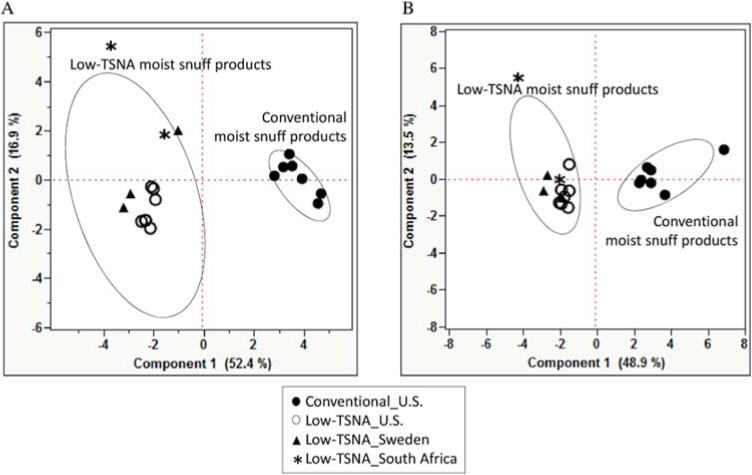
To explore the variance of chemical composition of 19 products, we performed Principal Component Analysis (PCA) using all chemicals analyzed in order to describe the group of the products by different country, as shown in Figure 1. Each closed dot, open dot, triangle, or star indicates each product. Products were grouped into two groups, conventional and low-TSNA moist snuff products. PCA showed that two principal components (PC1 and PC2) explained about 70% of variance in the data under both dry weight (Figure 1A) and per mg nicotine content (Figure 1B). NNN and lead was the most variable chemical under both dry weight and per mg nicotine content for PC1 and for PC2, respectively (Supporting Table 3) The result showed the clear separation between conventional and low-TSNA moist snuff products at PC 1, indicating the variations on chemical levels between groups under both dry weight and per mg nicotine content (Figure 1). A larger variation in the levels of chemicals was observed within low-TSNA moist snuff products compared to conventional products at PC2. The PCA under per mg nicotine (Figure 1B) showed a tighter clustering (less variation) among the products. Only Grizzly Fine Cut Wintergreen, a conventional moist snuff product, showed a different PC1 value from the other conventional moist snuff products (Figure 1B). Additionally, one of the South African products, Taxi Super Snuff Gwayi, behaved differently from other products under both dry weight and per mg nicotine content (Figure 1). U.S. low-TSNA moist snuff products were shown to have similar chemical levels by brands (Camel Snus or Marlboro Snus) regardless of flavors under dry weight (Figure 1A). Each chemical constituent for conventional and low-TSNA moist snuff products according to both dry weight and per mg nicotine content on average along with the standard deviation (SD) is presented in Table 1. The detailed chemical levels on dry weight and per mg nicotine content for the each products analyzed are provided in Supporting Table 1.
Figure 1. Principle Component Analysis (PCA).
Two dimensional PCA of 19 chemical levels on dry weight basis (A) and per mg nicotine basis (B). x axis, first principal component 1; y axis, second principal component 2. The ellipses are 95% confidence limits of each group.
Table 1.
Chemical levels for conventional and low-TSNA moist snuff products based on dry weight and per mg nicotine.
| Dry weight basis | per mg nicotine basis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional moist snuff products (N=7) | Low-TSNA moist snuff products (N=12) | Conventional moist snuff products (N=7) | Low-TSNA moist snuff products (N=12) | |||||||
| Chemical | Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | p-value |
| Arsenic (μg/g)1,a | 0.27 | 0.01 | 0.73 | 0.39 | * | 0.01 | 0.002 | 0.04 | 0.02 | * |
| Benzo[a]pyrene (ng/g)1,a | 77.75 | 24.90 | 1.47 | 1.38 | **** | 3.29 | 1.39 | 0.08 | 0.07 | **** |
| Cadmium (μg/g)1,a | 1.28 | 0.14 | 0.72 | 0.12 | **** | 0.05 | 0.01 | 0.04 | 0.01 | ** |
| Formaldehyde (μg/g)1,a | 0.32 | 0.01 | 0.63 | 0.62 | NS | 0.01 | 0.00 | 0.03 | 0.03 | NS |
| NNK (μg/g)1,a | 1.55 | 0.73 | 0.31 | 0.12 | **** | 0.07 | 0.05 | 0.02 | 0.005 | **** |
| NNN (μg/g)1,a | 4.47 | 0.95 | 1.13 | 0.47 | **** | 0.19 | 0.07 | 0.06 | 0.01 | **** |
| NDMA (μg/g)2A | 0.69 | 1.22 | 0.21 | 0.26 | NS | 0.03 | 0.05 | 0.01 | 0.01 | NS |
| Nitrate (mg/g)2A | 27.76 | 4.13 | 9.72 | 2.75 | **** | 1.15 | 0.20 | 0.51 | 0.14 | **** |
| Lead (μg/g)2B | 0.46 | 0.19 | 0.65 | 0.38 | NS | 0.02 | 0.01 | 0.04 | 0.02 | ** |
| Nickel (μg/g)2B | 3.24 | 0.46 | 1.75 | 0.71 | ** | 0.13 | 0.02 | 0.09 | 0.03 | ** |
| Chromium (μg/g)3 | 1.34 | 0.31 | 2.25 | 1.42 | NS | 0.06 | 0.01 | 0.13 | 0.07 | ** |
| NAB (μg/g)3 | 0.33 | 0.10 | 0.05 | 0.02 | **** | 0.01 | 0.005 | 0.003 | 0.001 | **** |
| NAT (μg/g)3 | 4.99 | 2.01 | 0.71 | 0.25 | **** | 0.21 | 0.10 | 0.04 | 0.01 | **** |
| Selenium (μg/g)3 | 0.27 | 0.01 | 0.28 | 0.14 | NS | 0.01 | 0.002 | 0.01 | 0.01 | NS |
| Ammonia (mg/g) | 8.92 | 3.25 | 1.34 | 0.59 | **** | 0.37 | 0.14 | 0.08 | 0.03 | **** |
| Glycerol (mg/g) | 0.58 | 0.89 | 8.56 | 17.46 | NS | 0.02 | 0.03 | 0.54 | 1.06 | NS |
| Propylene Glycol (mg/g) | 4.14 | 9.71 | 53.20 | 17.43 | **** | 0.16 | 0.36 | 3.04 | 1.24 | **** |
IARC Group1
IARC Group2A
IARC Group 2B
IARC Group 3
Harmful and Potentially Harmful Constituents established in smokeless tobacco by FDA
p<0.0001
*** 0.0001 to 0.001
0.001 to 0.01
0.01 to 0.05, NS not Significant
3.3. IARC Group 1 Carcinogens
Six of the investigated chemicals are classified as Group 1 known carcinogens (Table 1 and Supporting Table 1) and all chemicals have identified as HPHCs by FDA in STPs. Under dry weight content, benzo[a]pyrene, cadmium, NNK, and NNN, on average, higher in conventional than low-TSNA moist snuff products (p=0.00004); whereas arsenic was significantly higher (2.74-fold) in low-TSNA moist snuff than conventional products (p=0.017). The significant differences of these chemicals were also found under per mg nicotine (Table 1).
Formaldehyde did not differ significantly between the groups under both dry weight and per mg nicotine content. The greatest difference between conventional and low-TSNA moist snuff products were found with benzo[a]pyrene by 52.9-fold under dry weight and 43-fold under per mg nicotine content (Table 1).
3.4. IARC Group 2 Carcinogen
Two of IARC Group 2A probable human carcinogens (NDMA and nitrate) and 2 of IARC Group 2B carcinogens (lead and nickel) were measured in this study (Table 1 and Supporting Table 1). Under both dry weight and per mg nicotine content, a wide range of NDMA levels was found in both conventional and low-TSNA moist snuff products (Table 1), but no significant difference was observed (p>0.05). The concentration of nitrate was significantly higher (2.86-fold) in conventional compared to low-TSNA moist snuff products under dry weight (p=0.00004) and a slightly less difference (2.26-fold) was found under per mg nicotine content (p=0.0001). There was no difference of lead under dry weight content in low-TSNA compared to conventional moist snuff products (p=0. 39), but significant higher (1.90-fold) in low-TSNA moist snuff products under per mg nicotine content (p=0.001). We also observed a significantly higher level of nickel under both dry weight and per mg nicotine content in conventional than low-TSNA moist snuff products (p<0.05) (Table 1).
3.5. IARC Group 3 Carcinogen
Chromium, NAB, NAT, and selenium were in IARC Group 3. Chromium concentrations varied widely within low-TSNA moist snuff products in the range of 0.82-6.09 μg/g, while its levels were very similar among conventional moist snuff products (1.10-1.80 μg/g) under dry weight content (Supporting Table 1). There was no statistical difference for the level of chromium between conventional and low-TSNA moist snuff products under dry weight content (p=0.08), but on average 2.3-fold higher in low-TSNA moist snuff products under per mg nicotine content (p=0.0037). Unlike chromium, the levels of NAB and NAT varied among conventional moist snuff products, being more homogeneous among low-TSNA moist snuff products under both dry weight and per mg nicotine content (Supporting Table 1). The levels of NAB and NAT were significantly higher in conventional compared to low-TSNA moist snuff products (p=0.00004 for both NAB and NAT under both dry weight and per mg nicotine content) (Table 1). Selenium in low-TSNA moist snuff products varied in the range of 0.14-0.49 μg/g under dry weight and 0.01-0.03 μg/g under per mg nicotine, but no statistical different was found compared to conventional moist snuff products in both dry weight and per mg nicotine content (Supporting Table 1).
3.6. Other chemicals
Other potential toxicants, but not classified by IARC as known, probably or possible human carcinogens were determined (Table 1). Ammonia and three humectants including glycerol, propylene glycol, and triethylene glycol were analyzed (Supporting Table 1). Ammonia was present approximately 7-fold higher in conventional (mean: 8.92 mg/g ± SD 3.25) compared to low-TSNA moist snuff products (mean: 1.34 mg/g ± SD 0.59) under dry weight, but less difference was found under per mg nicotine content (Table 1). Ammonia levels were significantly different under both bases (p=0.00004). Glycerol was lower (below 0.25 mg/g) in all conventional moist snuff products except Copenhagen Mid-Cut Black (U.S., 2.6 mg/g), but higher in low-TSNA moist snuff products with a wide range of concentrations (0.05-62.90 mg/g) under dry weight (Supporting Table 1). Propylene glycol was highly present in all 12 low-TSNA moist snuff products while it was only detected in 2 out of 9 conventional moist snuff products (Supporting Table 1). Triethylene glycol had a lower than LOQ in all products analyzed in this study (Data not shown).
3.7. Probabilistic Cancer Risk Estimate Yield Comparisons
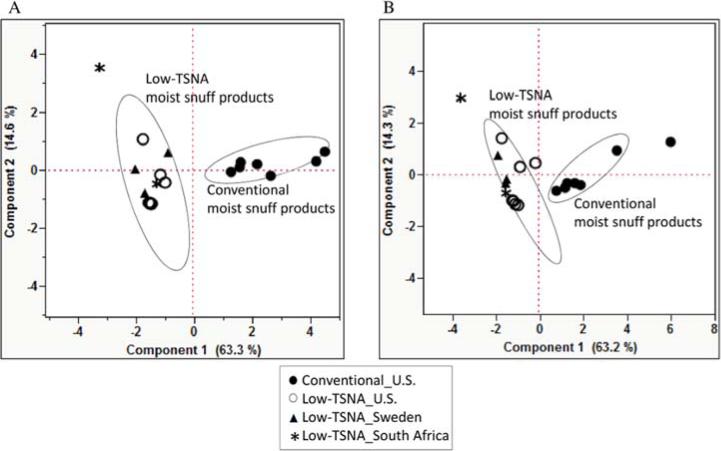
The calculated probabilistic cancer risk estimates for conventional and low-TSNA moist snuff products analyzed, using the CPDB methodology and under both dry weight and per mg nicotine base are shown in Table 2. Probabilistic cancer risk estimates of eight chemicals for which CPF data are available in CPDB were calculated. It included five of IARC Group1 (benzo[a]pyrene, cadmium, formaldehyde, NNK, and NNN), two of Group2 (lead and NDMA), and one of Group 3 (NAB). In PCA, the less variation (closer together) on probabilistic cancer risk estimates for 8 chemicals between conventional and low-TSNA moist snuff products was observed in per mg nicotine (Figure 2A) compared to dry weight content (Figure 2B). Although probabilistic cancer risk for conventional products was different from low-TSNA moist snuff products by clear separation, two conventional moist snuff products (Grizzly Fine Cut Wintergreen and Timber Wolf Fine Cut Natural) behaved differently from other conventional products under both dry weight and per mg nicotine content (Figure 2). Under both dry weight and per mg nicotine content, the probabilistic cancer risk estimate of NAB and cadmium was the most variable based on PC1 and PC2 (Supporting Table 4), respectively.
Table 2.
Probabilistic cancer risk estimates for conventional and low-TSNA moist snuff products based on dry weight and per mg nicotine.
| Dry weight basis | per mg nicotine basis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional moist snuff products (N=7) | Low-TSNA moist snuff products (N=12) | Conventional moist snuff products (N=7) | Low-TSNA moist snuff products (N=12) | |||||||
| Chemical | Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | p-value |
| Benzo[a]pyrene1,a | 2.95E-07 | 9.44E-08 | 5.57E-09 | 5.25E-09 | **** | 1.25E-08 | 5.26E-09 | 2.82E-10 | 2.46E-10 | **** |
| Cadmium1,a | 2.17E-04 | 2.29E-05 | 1.23E-04 | 1.99E-05 | **** | 8.99E-06 | 1.19E-06 | 6.77E-06 | 1.51E-06 | ** |
| Formaldehyde1,a | 5.20E-06 | 1.47E-07 | 1.02E-05 | 1.02E-05 | NS | 2.16E-07 | 3.70E-08 | 5.40E-07 | 5.21E-07 | NS |
| NNK1,a | 8.06E-04 | 3.79E-04 | 1.60E-04 | 6.01E-05 | **** | 3.53E-05 | 2.43E-05 | 8.62E-06 | 2.78E-06 | **** |
| NNN1,a | 2.43E-03 | 5.17E-04 | 6.17E-04 | 2.55E-04 | **** | 1.03E-04 | 3.55E-05 | 3.25E-05 | 7.12E-06 | **** |
| NDMA2A | 1.58E-04 | 2.80E-04 | 4.90E-05 | 5.87E-05 | NS | 6.60E-06 | 1.09E-05 | 2.68E-06 | 3.67E-06 | NS |
| Lead2B | 3.35E-08 | 1.41E-08 | 4.76E-08 | 2.78E-08 | NS | 1.38E-09 | 5.57E-10 | 2.55E-09 | 1.36E-09 | ** |
| NAB3 | 1.36E-06 | 4.11E-07 | 2.20E-07 | 9.26E-08 | **** | 5.68E-08 | 2.08E-08 | 1.17E-08 | 4.06E-09 | **** |
IARC Group1
IARC Group2A
IARC Group 2B
IARC Group 3
Harmful and Potentially Harmful Constituents established in smokeless tobacco by FDA
p<0.0001
***0.0001 to 0.001
0.001 to 0.01
* 0.01 to 0.05, NS not Significant
Figure 2. Principle Component Analysis (PCA) of probabilistic cancer risk estimates under dry weight (A) and per mg nicotine basis (B).
Two dimentional PCA of probabilistic cancer risk estimate from eight carcinogens (benzo[a]pyrene, cadmium, Formaldehyde, NDMA, NNN, NNK, NAB, and lead). x axis, first principal component 1; y axis, second principal component 2. The ellipses are 95% confidence limits of each group.
Conventional moist snuff products had statistically significantly higher probabilistic cancer risks for five chemicals compared to low-TSNA moist snuff products analyzed (0.01<p<0.0001); benzo[a]pyrene (52.98-fold and 44.33-fold), NAB (6.16-fold and 4.09-fold), NNK (5.04-fold and 4.09-fold), NNN (3.94-fold and 3.15-fold), and cadmium (1.77-fold and 1.33-fold) under both dry weight and per mg nicotine content, respectively. Under dry weight, low-TSNA moist snuff products had a relatively higher probabilistic cancer risks for formaldehyde (1.97-fold), and lead (1.42-fold) compared to conventional products, but were not statistically different (Table 2). Under per mg nicotine content, low-TSNA moist snuff products had significantly higher probabilistic cancer risk estimate for lead (1.85-fold) (p=0.009) compared to conventional products in addition to the five chemicals that had different probabilistic cancer risks under dry weight content between groups (above). Among low-TSNA moist snuff products, U.S. products had a significantly lower level of benzo[a]pyrene compared to non-U.S. products under both dry weight (4.12-fold and per mg nicotine content (3.2-fold) (p=0.0025 for both). Probabilistic cancer risk for cadmium was only statistically higher (1.45-fold) based on per mg nicotine (p=0.005) in U.S. low-TSNA than non-U.S. low-TSNA moist snuff products. Overall combined estimated probabilistic cancer risks were 3.77-fold or 3-fold higher in conventional compared to low-TSNA moist snuff products under dry weight and per mg nicotine content, respectively (Table 2).
Total probabilistic cancer risk estimates showed the large difference among the products under both dry weight and per mg nicotine content, up to about 9-fold when comparing the lowest probabilistic cancer risk estimate (Taxi Super Snuff Gwayi) to the highest probabilistic cancer risk (Grizzly Fine Cut Wintergreen).
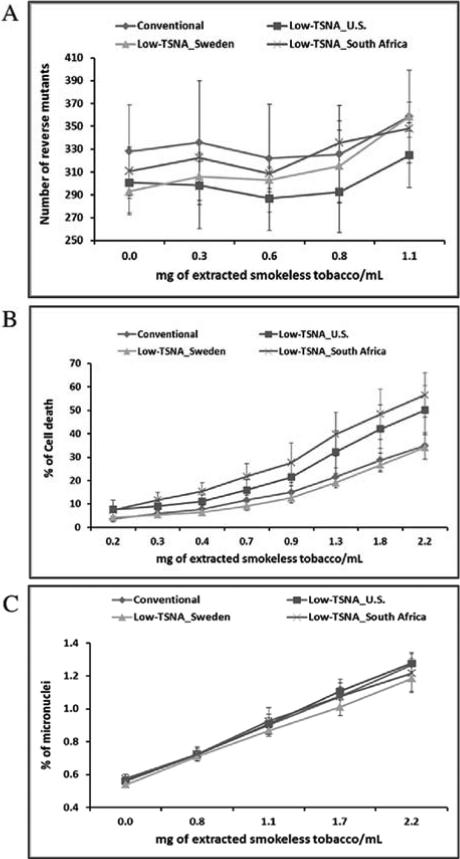
3.8. In vitro toxicity of STPs
Ames test showed a dose-related positive increase in the number of mutation revertants per plate. The largest increase (average 22%) in mutagenicity was observed in Swedish low-TSNA moist snuff products with the highest addition of the extracted products (1.1 mg/mL) compared to its absence (p=0.049). The number of reverse mutants was shown to be slightly increased as the dose increased in the other products as well (8-12%), but no significant increase in the number of reverse mutants with S9 activation was found (Figure 3A). Loss of cell viability was observed by NRU Cytotoxicity test in CHO cells with exposure of 2.2 mg/ mL of extracts in all products (Figure 3B). Statistically significant difference of cytotoxicity was observed between conventional and low-TSNA moist snuff products (p=0.045) although Swedish low-TSNA moist snuff products showed the similarly lower cytotoxicity to conventional moist snuff products. The statistically significantly higher cytotoxicity was observed in South Africa and U.S. low-TSNA moist snuff products compared to conventional products (p=0.04) and the mean of % of cell death was 56.5%, 50%, and 34.8 %, respectively.
Figure 3. In vitro toxicity results.
(A) Ames Salmonella reverse mutation assay performed using Salmonella typhimurium TA102 strain. (B) Cytotoxicity evaluated by Neutral Red Uptake Cytotoxicity assay. (C) Genotoxic test carried out by In Vitro Micronucleus assay. All experiments were performed in triplicates. Data is the means± standard deviation of measurements from triplicates.
The genotoxicity test of the moist snuff products, in vitro MN, showed that the mean of % of micronuclei observed was statistically significantly increased (122%-127%) (p=1.47×10−7) with treatment of the products in all products compared to controls (Figure 3C), but no differences were observed among the products.
4. DISCUSSION
The study presented herein characterized a variety of chemicals contained in 7 popular conventional moist snuff products and 12 popular low-TSNA moist snuff products manufactured in the U.S., Sweden and South Africa. The results show that chemical levels are clearly different between conventional and low-TSNA moist snuff products under dry weight content, but there is less variation when considering a per mg nicotine content. Moreover, a larger variation among low-TSNA moist snuff products compared to conventional products for chemical levels were observed. Relative probabilistic cancer risk estimates were 3.77-fold higher under dry weight and 3-fold higher under per mg nicotine content in conventional than low-TSNA moist snuff products. Furthermore, we observed that all analyzed moist snuff products presented some low level of in vitro toxicity through cell death, genetic damage, and chromosome damage, but no substantial differences across the products.
PCA analysis showed that the chemical levels of the tested products were very similar by brands as they were clustered in the group of Camel snus or Marlboro snus, and flavors apparently has little overall effect. Moreover, U.S. low-TSNA moist snuff products were similar to Swedish low-TSNA moist snuff products as they were closely located in PCA. One of the South African low-TSNA moist snuff products in this study, Taxi Super Snuff Gwayi, is manufactured by Swedish Match and has lower TSNAs levels. However, this product proved to be an outlier in PCA analysis, showing different characteristics from other products analyzed.
Some previous studies have reported that conventional STPs have higher pH than snus products, which in turn leads to higher levels of free nicotine delivery (Pappas et al., 2008). In this study, there was a large variation in the level of free nicotine across the brands, and this is similar to a prior study in selected U.S. STPs (Stepanov et al., 2008a). This is due to varying ammonia levels that are known to affect pH by converting nicotine to free nicotine (Domino, 1998); substantially higher amounts of ammonia was found in conventional compared to low-TSNA moist snuff products in this study. The variation for free nicotine presumably affects the addictive potential and the risk of nicotine-related adverse effects, where U.S. low-TSNA moist snuff products are designed to be lower.
The most abundant and strongest carcinogens in STPs are TSNAs (IARC, 2007), and they are considered important for developing oral cancer in STP users (Hoffmann et al., 1991). Nitrate content is the main factor contributing to an increase and accumulation of TSNAs (Fischer et al., 1989). Overall, the results herein showed a relatively lower amount of nitrates and TSNAs in low-TSNA moist snuff products compared to in conventional products, due to reduced TSNAs formation during tobacco processing. To contextualize the difference in TSNAs levels among the moist snuff products, the probabilistic cancer risk estimates for TSNAs varied as much as approximately 10-fold. This indicates that there can be further industry changes in STP production to minimize differences among the various high-containing TSNA products. Importantly, per mg nicotine based chemical constituents and estimated probabilistic cancer risk closes the gap between the STPs, indicating the importance of consideration as raw data and the best direct comparison of STPs.
Although the World Health Organization study stresses the importance for heavy metals and the regulation of tobacco products (World Health Organization Study Group on Tobacco Product, 2012), there have been few reports and limited comparisons for heavy metals between conventional and low-TSNA moist snuff products (Borgerding et al., 2012; Rickert et al., 2009). The concentrations of metals in this study are comparable to popular brand of moist snuff products from the Canadian market reported in a previous study (World Health Organization Study Group on Tobacco Product, 2012), but their levels were generally higher in our study. It may be because metal contents in the products widely vary by geographic regions.
The STPs tested here had minimal in vitro toxicity, and the observed different levels through increasing mutagenicity, less cell viability, and genetic damage across the moist snuff products were small. While overall a linear increase of cell viability and genetic damage by treatments of STP extracts was observed, a relatively large variation was found in a mutagenicity test. A similar dose dependent in vitro toxicity results were also previously observed in low activities of extracts of moist snuff products sold on Canadian market (Rickert et al., 2009). Although we compared the toxicity results across STPs, the results were not overall greatly different, indicating that STPs may not be toxic, carcinogenic, or mutagenic enough to be detected using this method or these assays have limited or no usefulness to comparing toxicity of different STPs in vitro.
This study had several strengths. This is the first, to our knowledge, to apply multidimensional data analysis to determine the similarity and differences in features of chemicals and probabilistic cancer risks among the most popular moist snuff products worldwide. In order to compare the products, we analyzed a wider range of toxic and carcinogenic elements including all except two Harmful and Potentially Harmful Constituents established by FDA. In addition to the chemical analysis, we provide some insight to compare potential toxicity in terms of in vitro toxicity of moist snuff tobacco products worldwide. Additionally, we were able to assess the relative differences among the products in terms of probabilistic cancer risk.
It is important to note some limitations of this study. This study did not include all, or many of the commercial brands of moist snuff STPs, so this study may not be representative of all brands. Another limitation is a choice of brands of moist snuff products worldwide. The amount of metals in tobacco products could widely vary depending on the environmental conditions and geographic regions where the tobacco is grown. Also, it is known that products with a given name can be formulated differently over time. Another limitation of this study is the use of the CPDB's carcinogenic potency factors to provide a context to the different levels of chemical constituents among the products analyzed because the relationship to actual human cancer risk is unknown, and the CPDB values are only intended to provide regulatory guidance for allowable environmental exposure and not intended to provide quantitative differences in risk among various exposures. Since use of STP involves an exposure to a complex mixture of chemical constituents, it is difficult to understand how a difference in a particular level of a constituent would affect differences in cancer risks for the complex mixture. Finally, although in vitro toxicology tests allowed us to gain insights into the adverse effects of moist snuff products on in vitro setting, it is difficult to apply these data to human risk because the cell culture conditions do not exist in humans.
In conclusion, the comprehensive chemical and toxicological analysis of moist snuff products as done here provides a broader assessment of understanding differences in carcinogenic potential of conventional and low-TSNA moist snuff products. Potentially, this study would help for informing regulatory decision makers in their efforts.
Supplementary Material
Research highlights.
The first study to apply multidimensional data analysis to determine the similarity and differences in features of chemicals and cancer risks among the most popular moist snuff products worldwide.
Identification of clearly different toxicant constituents through multidimensional data approach for a wide range of chemicals.
Differences in toxicant levels when expressed on a dry weight basis, but less variation when results are normalized for nicotine content.
Higher cancer risk estimates for dry weight determinations than nicotine normalized determination.
ACKNOWLEDGEMENT
Research reported in this publication was supported by grant number P50CA180908 from the National Cancer Institute of the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA. Transdisciplinary Tobacco Use Research Center grants P50 84718 from the National Cancer Institute and the National Institute on Drug Abuse, and NCI N01-PC-64402 by the National Cancer Institute under contract, Laboratory Assessment of Tobacco Use Behavior and Exposure to Toxins. The authors are grateful to Drs. Cecilia Magnusson and Lekan Ayo-Yusuf for purchasing the moist snuff products from Sweden and South Africa. We also would like to acknowledge Drs. Richard Higby and William Rickert for contribution to the chemical analysis and the in vitro toxicity assays, respectively.
ABBREVIATIONS
- CPDB
California's Carcinogenic Potency Database
- CPF
carcinogenic potency factor
- DMSO
dimethyl sulfoxide
- EPA
Environmental Protection Agency
- FDA
Food and Drug Administration
- HNSCC
head and neck squamous cell carcinoma
- HPHCs
harmful and potentially harmful constituents
- IARC
International Agency for Research on Cancer
- LOD
limit of detection
- LOQ
limit of quantification
- MN
micronucleus
- NAB
N′-Nitrosoanabasine
- NAT
N′-Nitrosoanatabine
- NDMA
N′-nitrosodimethylamine
- NNK
nicotine-derived nitrosamine ketone
- NNK
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N′-Nitrosonornicotine
- NRU
Neutral Red Uptake
- PAHs
polycyclic aromatic hydrocarbons
- PCA
principle component analysis
- SD
standard deviation
- STPs
smokeless tobacco products
- TSNAs
tobacco-specific N-nitrosamines
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Shields serves as an expert witness in tobacco company litigation on behalf of plaintiffs.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
REFERENCES
- University of California; Berkeley: http://potency.berkeley.edu/pdfs/ChemicalTable.pdf Carcinogenic Potency Database (CPDB) [Google Scholar]
- Abdi H.a.W., L. J. Principal component analysis. WIREs Comp Stat. 2010;2:433–459. [Google Scholar]
- Adkison SE, Bansal-Travers M, Smith DM, O'Connor RJ, Hyland AJ. Impact of smokeless tobacco packaging on perceptions and beliefs among youth, young adults, and adults in the U.S: findings from an internet-based cross-sectional survey. Harm reduction journal. 2014;11:2. doi: 10.1186/1477-7517-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayo-Yusuf OA, Connolly GN. Applying toxicological risk assessment principles to constituents of smokeless tobacco products: implications for product regulation. Tobacco control. 2011;20:53–57. doi: 10.1136/tc.2010.037135. [DOI] [PubMed] [Google Scholar]
- Borgerding MF, Bodnar JA, Curtin GM, Swauger JE. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regulatory toxicology and pharmacology : RTP. 2012;64:367–387. doi: 10.1016/j.yrtph.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Canada H. Official method T-501. Bacterial Reverse Mutation Assay for Mainstream Tobacco Smoke. Official method T-501 Version 1.1. 2004a:1–19. [Google Scholar]
- Canada H. Official Method T-502. Neutral Red Uptake Assay for Mainstream Tobacco Smoke. 2004b [Google Scholar]
- Canada H. Official Method T - 503. In Vitro Micronucleus Assay for Mainstream Tobacco Smoke. 2004c [Google Scholar]
- Delnevo CD, Wackowski OA, Giovenco DP, Manderski MT, Hrywna M, Ling PM. Examining market trends in the United States smokeless tobacco use: 2005-2011. Tobacco control. 2014;23:107–112. doi: 10.1136/tobaccocontrol-2012-050739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF. Tobacco smoking and nicotine neuropsychopharmacology: some future research directions. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1998;18:456–468. doi: 10.1016/S0893-133X(97)00193-0. [DOI] [PubMed] [Google Scholar]
- Fischer S, Spiegelhalder B, Preussmann R. Preformed tobacco-specific nitrosamines in tobacco--role of nitrate and influence of tobacco type. Carcinogenesis. 1989;10:1511–1517. doi: 10.1093/carcin/10.8.1511. [DOI] [PubMed] [Google Scholar]
- Foulds J, Furberg H. Is low-nicotine Marlboro snus really snus? Harm reduction journal. 2008;5:9. doi: 10.1186/1477-7517-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Ramstrom L, Burke M, Fagerstrom K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tobacco control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products new tobacco-delivery systems. American journal of preventive medicine. 2007;33:S368–378. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Murphy SE, Riley WT, Le C, Luo X, Mooney M, Hatsukami DK. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1567–1572. doi: 10.1158/1055-9965.EPI-07-0227. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Djordjevic MV. Chemical composition and carcinogenicity of smokeless tobacco. Advances in dental research. 1997;11:322–329. doi: 10.1177/08959374970110030301. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Rivenson A, Chung FL, Hecht SS. Nicotine-derived N-nitrosamines (TSNA) and their relevance in tobacco carcinogenesis. Critical reviews in toxicology. 1991;21:305–311. doi: 10.3109/10408449109017917. http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297981.pdf. https://prevention.nih.gov/tobacco-regulatory-science-program/research-priorities. [DOI] [PubMed] [Google Scholar]
- IARC . IARC monographs on the evaluation of carcinogenic risk to humans. Vol. 89. IARC Press; Lyon, France: 2007. pp. 55–60. [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scandinavian journal of work, environment & health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- Johnson MD, Schilz J, Djordjevic MV, Rice JR, Shields PG. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:3263–3304. doi: 10.1158/1055-9965.EPI-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler TS, Stanfill SB, Zhang L, Ashley DL, Watson CH. Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;57:380–386. doi: 10.1016/j.fct.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, Biorklund A, Rutqvist LE. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer. 1998;82:1367–1375. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Luo J, Ye W, Zendehdel K, Adami J, Adami HO, Boffetta P, Nyren O. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet. 2007;369:2015–2020. doi: 10.1016/S0140-6736(07)60678-3. [DOI] [PubMed] [Google Scholar]
- Martin GC, Brown JP, Eifler CW, Houston GD. Oral leukoplakia status six weeks after cessation of smokeless tobacco use. Journal of the American Dental Association. 1999;130:945–954. doi: 10.14219/jada.archive.1999.0335. [DOI] [PubMed] [Google Scholar]
- Musharraf SG, Shoaib M, Siddiqui AJ, Najam-Ul-Haq M, Ahmed A. Quantitative analysis of some important metals and metalloids in tobacco products by inductively coupled plasma-mass spectrometry (ICP-MS). Chemistry Central journal. 2012;6:56. doi: 10.1186/1752-153X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Peruga A, Breysse P, Zavaleta A, Blanco-Marquizo A, Pitarque R, Acuna M, Jimenez-Reyes K, Colombo VL, Gamarra G, Stillman FA, Samet J. Secondhand tobacco smoke in public places in Latin America, 2002-2003. Jama. 2004;291:2741–2745. doi: 10.1001/jama.291.22.2741. [DOI] [PubMed] [Google Scholar]
- Osterdahl BG, Slorach SA. Volatile N-nitrosamines in snuff and chewing tobacco on the Swedish market. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1983;21:759–762. doi: 10.1016/0278-6915(83)90210-7. [DOI] [PubMed] [Google Scholar]
- Pappas RS, Stanfill SB, Watson CH, Ashley DL. Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. Journal of analytical toxicology. 2008;32:281–291. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- Prabhakar V, Jayakrishnan G, Nair SV, Ranganathan B. Determination of Trace Metals, Moisture, pH and Assessment of Potential Toxicity of Selected Smokeless Tobacco Products. Indian journal of pharmaceutical sciences. 2013;75:262–269. doi: 10.4103/0250-474X.117398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert WS, Joza PJ, Trivedi AH, Momin RA, Wagstaff WG, Lauterbach JH. Chemical and toxicological characterization of commercial smokeless tobacco products available on the Canadian market. Regulatory toxicology and pharmacology : RTP. 2009;53:121–133. doi: 10.1016/j.yrtph.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Rodu B, Jansson C. Smokeless tobacco and oral cancer: a review of the risks and determinants. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2004;15:252–263. doi: 10.1177/154411130401500502. [DOI] [PubMed] [Google Scholar]
- Rutqvist LE, Curvall M, Hassler T, Ringberger T, Wahlberg I. Swedish snus and the GothiaTek(R) standard. Harm reduction journal. 2011;8:11. doi: 10.1186/1477-7517-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildt EB, Eriksson M, Hardell L, Magnuson A. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. International journal of cancer Journal international du cancer. 1998;77:341–346. doi: 10.1002/(sici)1097-0215(19980729)77:3<341::aid-ijc6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008a;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Upadhyaya P, Carmella SG, Feuer R, Jensen J, Hatsukami DK, Hecht SS. Extensive metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008b;17:1764–1773. doi: 10.1158/1055-9965.EPI-07-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Villalta PW, Knezevich A, Jensen J, Hatsukami D, Hecht SS. Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chemical research in toxicology. 2010;23:66–73. doi: 10.1021/tx900281u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twombly R. Snus use in the U.S.: reducing harm or creating it? Journal of the National Cancer Institute. 2010;102:1454–1456. doi: 10.1093/jnci/djq404. [DOI] [PubMed] [Google Scholar]
- USEPA Risk Assessment Guidance for Superfund Volume I.. Development of Risk-Based Preliminary Remediation Goals (Part B) EPA 540/R-92/003. 1991 [Google Scholar]
- World Health Organization Study Group on Tobacco Product, R. WHO Study Group on Tobacco Product Regulation. Report on the scientific basic of tobacco product regulation: fourth report of a WHO study group. World Health Organization technical report series. 2012:1–83. 81 p following 83. [PubMed] [Google Scholar]
- Xue J, Yang S, Seng S. Mechanisms of Cancer Induction by Tobacco-Specific NNK and NNN. Cancers. 2014;6:1138–1156. doi: 10.3390/cancers6021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Ekstrom AM, Hansson LE, Bergstrom R, Nyren O. Tobacco, alcohol and the risk of gastric cancer by sub-site and histologic type. International journal of cancer Journal international du cancer. 1999;83:223–229. doi: 10.1002/(sici)1097-0215(19991008)83:2<223::aid-ijc13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Zakiullah Saeed, M., Muhammad N, Khan SA, Gul F, Khuda F, Humayun M, Khan H. Assessment of potential toxicity of a smokeless tobacco product (naswar) available on the Pakistani market. Tobacco control. 2012;21:396–401. doi: 10.1136/tc.2010.042630. [DOI] [PubMed] [Google Scholar]
- Zhou J, Michaud DS, Langevin SM, McClean MD, Eliot M, Kelsey KT. Smokeless tobacco and risk of head and neck cancer: evidence from a case-control study in New England. International journal of cancer Journal international du cancer. 2013;132:1911–1917. doi: 10.1002/ijc.27839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.