Abstract
Aim
The evidence needed for tropical countries to take informed decisions on influenza vaccination is scarce. This article reviews policy, availability, use and effectiveness of seasonal influenza vaccine in tropical and subtropical countries.
Method
Global health databases were searched in three thematic areas – policy, availability and protective benefits in the context of human seasonal influenza vaccine in the tropics and subtropics. We excluded studies on monovalent pandemic influenza vaccine, vaccine safety, immunogenicity and uptake, and disease burden.
Results
Seventy‐four countries in the tropics and subtropics representing 60% of the world's population did not have a national vaccination policy against seasonal influenza. Thirty‐eight countries used the Northern Hemisphere and 21 countries the Southern Hemisphere formulation. Forty‐six countries targeted children and 57 targeted the elderly; though, the age cut‐offs varied. Influenza vaccine supply increased twofold in recent years. However, coverage remained lower than five per 1000 population. Vaccine protection against laboratory‐confirmed influenza in the tropics ranged from 0% to 42% in the elderly, 20–77% in children and 50–59% in healthy adults. Vaccinating pregnant women against seasonal influenza prevented laboratory‐confirmed influenza in both mothers (50%) and their infants <6 months (49–63%).
Conclusion
Guidelines on vaccine composition, priority risk groups and vaccine availability varied widely. The evidence on vaccine protection was scarce. Countries in the tropics and subtropics need to strengthen and expand their evidence‐base required for making informed decisions on influenza vaccine introduction and expansion, and how much benefit to expect.
Keywords: Influenza vaccines, policy, treatment effectiveness
Introduction
Influenza may affect up to 10% of the world's population every year 1 with substantial morbidity and mortality.2, 3, 4, 5 With immunization being one of the most powerful and cost effective interventions against infectious diseases,6 vaccination remains the main strategy to protect populations against influenza‐associated morbidity and mortality. The World Health Assembly (WHA 56.19) resolved in 2003 to increase the use of seasonal influenza vaccines to protect individuals at high risk for influenza and related complications,7 and in 2005, it mandated the WHO (WHA58.5) to work with international and national partners to increase access to influenza vaccines.8 The WHO Global Action Plan for Influenza Vaccines launched in 2006 aims to promote evidence‐based use of seasonal influenza vaccine as a strategy towards pandemic preparedness.9, 10 The immediate goal is to increase by 2016 the global vaccine production capacity to produce enough vaccine to equitably immunize 70% of the world population with a pandemic vaccine that gives an adequate protection within 6 months of vaccine seed transfer to manufacturers in the event of a pandemic.11
Over the last decade, an increasing number of countries in the tropics and subtropics have introduced seasonal influenza vaccination in their national immunization policies and/or expanded their policies to include maternal influenza immunization.12, 13 This is critical given the WHO Strategic Advisory Committee of Experts on Immunization recommendation in 2012 that countries with seasonal influenza vaccine programmes prioritize persons at high risk of severe influenza virus infection, including pregnant women at any stage of their pregnancy; children aged 6 months to 5 years, the elderly and individuals with underlying health conditions such as HIV/AIDS, asthma and chronic heart or lung diseases.14 Unlike in the temperate regions, influenza seasonality in the tropics is less distinct with multiple and less pronounced peaks with often year‐round transmission.15, 16 In the absence of clear guidance on which vaccine formulation to use and when to vaccinate in the tropics and subtropics, countries often lack evidence to make informed decisions. As one of the early steps of a larger WHO effort to improve seasonal influenza vaccine use and pandemic preparedness in the tropics and subtropics, this article reviews the current status of (i) national policy and guidelines, (ii) coverage and use and (iii) efficacy and effectiveness, of seasonal influenza vaccine in the context of the tropics and subtropics.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) statement was used to guide the process and reporting of our review (Appendix A).17The outcomes of interest were to review the current status of national policy for seasonal influenza vaccination, national guidelines for priority population groups and vaccine composition, vaccine use and effectiveness. Vaccination coverage was estimated as the number of vaccine doses distributed expressed per 1000 population. Vaccine effectiveness estimates were reviewed separately for laboratory‐confirmed influenza, for different risk groups, further stratified by type of vaccine and antigenic match wherever possible.
We searched multiple databases (United States National Library of Medicine, Cochrane Library, WHO Library Information System, Latin American and Caribbean Health Sciences Literature, National Databases of Indian Medical Journals) using different combinations of search terms (with synonyms and closely related words) such as ‘seasonal influenza’, ‘influenza vaccine’, ‘tropics’, ‘effectiveness’, ‘efficacy’, ‘policy’, ‘guidelines’, ‘timing’, ‘composition’, ‘coverage’, ‘availability’, ‘uptake’, ‘production’, ‘manufacturing’ and ‘campaign’. An example of a search query is shown in Appendix B. We screened the title and abstract of all articles to determine eligibility. We also screened other articles that showed up as related during the search. We retrieved the full text of all eligible articles and further assessed for inclusion. We screened articles that were newly identified in the references cited in the full‐text articles. We reviewed all the past systematic reviews and meta‐analyses on vaccine efficacy and effectiveness as well as all the individual studies that had been included in these reviews. Duplicates were removed and the title and abstract were screened for eligibility by two reviewers independently. Articles were included based on consensus between the two reviewers. For multiple articles referring to the same study, we included the article with the most recent findings. To access grey literature, we first contacted institutions, networks and individuals known to be involved in influenza research, used snow‐balling technique to further identify other influenza researchers and searched conference proceedings and agency reports to identify ongoing or unpublished studies. Researchers were requested to share preliminary summaries of unpublished studies to assess their eligibility for inclusion in the review. We plotted funnel plots of estimates of vaccine effectiveness and efficacy for published and unpublished studies separately for elderly, children and healthy adults to assess the extent of publication bias if any. We also reviewed four global databases on seasonal influenza vaccine use administered by the WHO and UNICEF or maintained by vaccine manufacturers. We triangulated information regarding influenza policy, guidelines and coverage from the different global databases – in case of discordant findings, we considered the information from the most recent database as valid.
We limited our review to 138 countries and territories (excluding Australia) representing 79% of the world's population, situated partly or wholly between the north and south 38th lateral (Appendix C) and restricted our inclusions to articles in the English language or any other language provided an abstract was available in English. We included articles related to policies and guidelines for seasonal human influenza vaccine use in tropical and subtropical countries, articles that referred to seasonal influenza vaccine composition, timing of vaccination, vaccine production, availability and coverage, and vaccine efficacy and effectiveness. We excluded studies that focused on avian or pandemic influenza vaccine or pandemic preparedness. Studies on safety and immunogenicity of influenza vaccines, determinants of influenza vaccine uptake, licensing and regulatory aspects of influenza vaccine were also excluded. Studies that focused solely on influenza seasonality, disease burden, antigenic and genetic characteristics were beyond the scope of this review. Data was extracted directly from the full‐text articles into structured tables containing all the relevant variables of interest such as the status of influenza vaccination policy, year in which vaccine policy was introduced, vaccine formulation used, targeted high‐risk population groups, whether available in the private or public sector, vaccine coverage, etc. For vaccine effectiveness and efficacy studies, we extracted information on the study year, study design, study population (size and risk group types), vaccine type (live attenuated or inactive), formulation used (Northern or Southern Hemisphere), ascertainment of exposure (i.e. vaccination), outcome (e.g. influenza‐like illness, pneumonia, hospitalization, laboratory‐confirmed influenza), efficacy, effectiveness and other potential confounding factors such as antigenic match of vaccine with circulating influenza virus. Simple proportions with 95% confidence intervals, the range of proportions across different studies, stratified by relevant variables such as age were used to describe the outcome variables. We did not repeat a meta‐analysis for vaccine efficacy and vaccine effectiveness, as more than 20 such efforts (including 11 Cochrane Collaboration reviews) have been carried out in the past. Moreover, we did not identify any new published studies from the tropics and subtropics since the last meta‐analysis of studies from low‐ and middle‐income countries.18 The risk of bias in the selection and ascertainment of exposure and outcome for cohort and case–control vaccine effectiveness studies was assessed independently by two reviewers (SH and JS) using the Newcastle–Ottawa bias assessment scale.19 We similarly assessed the methodological quality for the vaccine efficacy trials based on randomization, allocation, blinding and follow‐up using the Cochrane risk of bias assessment tool.20 Wherever appropriate, we triangulated information from the global databases to validate the information extracted from the literature.
Results
Of the 3637 articles and 34 unpublished papers identified as of 30 September 2014, 3247 were deemed ineligible based on the screening of the title and abstract. A further 178 articles were excluded after a full‐text appraisal. A total of 215 published (vaccine policy and guidelines – 27, timing and composition – 49, supply, availability and coverage – 37 and vaccine efficacy – 102) and 31 unpublished articles (vaccine policy and guidelines – 8, timing and composition – 4, supply, availability and coverage – 8 and vaccine efficacy – 11) and four global databases on seasonal influenza vaccine use were included in the final review (Figure 1). The risk of bias in selection of the vaccinated cohort, comparability of the vaccinated and non‐vaccinated participants, outcome ascertainment or differential loss to follow‐up was high in most cohort studies (Appendix D). The risk of bias in selection of cases or dissimilar non‐response rate in cases and controls was high in most case–control studies (Appendix E). The risk of bias was lower (except in three non‐randomized controlled trials) in the vaccine efficacy trials (Appendix F). Most of the published systematic reviews and meta‐analysis (especially the reviews published after 2000) referred to the Cochrane Manual for systematic reviews; though, only one review explicitly stated that it adhered to the PRISMA statement. There was no evidence to suggest publication bias in the context of vaccine effectiveness studies (results not shown). The review covers 138 countries and territories representing about 79% of the world's population situated in the tropics and subtropics.
Figure 1.
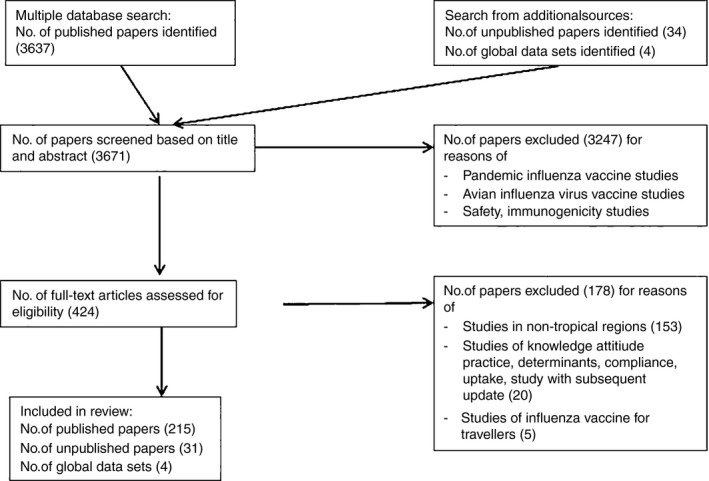
PRISMA flowchart of selection and exclusion of articles.
Seasonal influenza immunization policy
Sixty‐four of 138 tropical and subtropical countries representing 20% of the world's population had a national vaccination policy against seasonal influenza (Figure 2).21 Countries such as Bangladesh, China, India, Pakistan and Sri Lanka in Asia representing about 45% of the world's population did not have a national vaccination policy against influenza. All of Central and South America except Guyana, Haiti, Saint Kitts & Nevis and Saint Vincent and the Grenadines had a seasonal influenza immunization policy.22 On the other hand, only six countries (Côte d'Ivoire, Egypt, Libya, Mauritius, Tunisia and South Africa) in Africa had a national immunization policy or guideline against seasonal influenza.
Figure 2.

Countries in the tropics and subtropics with a national influenza immunization policy.
National policy guidance on vaccine composition was available for 63 countries – 38 and 21 countries recommended the use of the Northern and Southern Hemisphere formulation respectively, whereas three countries (Brunei Darussalam, Marshall Islands and Singapore) recommended both formulations. Peru experimented with both formulations in 2012 but subsequently reverted to one formulation. Five countries situated in the Southern Hemisphere tropics used the Northern Hemisphere formulation, whereas eight situated in the Northern Hemisphere tropics used the Southern Hemisphere formulation. Moreover, four countries (Costa Rica, El Salvador, Guatemala and the Philippines) situated in the Northern Hemisphere tropics had switched from a Northern to a Southern Hemisphere formulation in recent years (Figure 3).
Figure 3.

National guidelines on seasonal influenza vaccine formulation for countries in the tropics and subtropics. NH, Northern Hemisphere; SH, Southern Hemisphere.
Countries with a national policy for influenza vaccine followed the WHO recommendations for targeting one or more high‐risk groups – the elderly, children, individuals with underlying health conditions such as HIV/AIDS, asthma and chronic heart or lung diseases and healthcare professionals.14 The age range of children and the elderly targeted for influenza immunization varied among the countries.23 Forty‐six countries targeted children of 6 months to 5 years, whereas 57 countries targeted the elderly either above 60 or 65 years of age.21 Sixty‐one countries targeted individuals with underlying health conditions such as HIV/AIDS, asthma and chronic heart or lung diseases. Forty‐seven countries (including 27 in Central and South America) recommended seasonal influenza immunization during pregnancy. Fifty‐one countries recommended vaccination of healthcare professionals against influenza. There was no correlation between the development status of the country and free influenza vaccination to healthcare professionals.24, 25 A survey in 2009 showed that 32 of the 35 countries in Central and South America administered influenza vaccines to their healthcare professionals through the public health systems.26 Healthcare professionals were more commonly targeted for vaccination after the 2009 pandemic.25 In Saudi Arabia, the vaccine was mandatory for all health professionals working in the Hajj pilgrimage centres of Mecca and Medina. Saudi Arabia also required all incoming Hajj pilgrims to be vaccinated against influenza.27, 28
Vaccine supply, availability and coverage
Vaccine coverage varied widely across countries (Appendix G). Seasonal influenza vaccine supply increased from 350 million doses in 2006 to around 900 million in 2009.29 It increased by 87% between 2004 and 2011 but by only 3% per annum in the last 3 years.30 Less than 20% of the global seasonal influenza vaccine was produced by regional manufacturers in tropical and subtropical countries.31 In China, despite an 18% annual increase in vaccine supply since 2005, local manufacturers supplied 32·5 million doses of seasonal influenza vaccine in 2008–2009 season against an estimated domestic need of 570 million doses per year.32 Fourteen manufacturers from the tropical and subtropical regions received technology transfer support that was expected to enhance the supply to 795 million doses by 2016.33, 34, 35, 36 Vaccine manufacturers in India, Indonesia and Thailand had since started production of seasonal influenza vaccine. Mexico, South Africa, Egypt, the Islamic Republic of Iran, Thailand and Viet Nam were expected to begin production within 5–10 years.37 There was no production capacity in sub‐Saharan Africa except one facility in South Africa that filled and packaged imported vaccine.38
Influenza vaccine was available through the public sector in 50 countries. In another 28 countries, it was available solely through the private sector. However, usage was low when the vaccine was available only in the private sector.30 Influenza vaccine was available in 14 of the 31 countries surveyed in Africa – six through the private sector (Democratic Republic of the Congo, Senegal, Togo, Uganda, Zambia and Zimbabwe) and the remaining eight through both public and private sectors (Cameroon, Côte d'Ivoire, Egypt, Kenya, Madagascar, Mauritius, Morocco and South Africa).39
Despite a twofold increase in recent years, influenza vaccine coverage was <5 per 1000 general population in tropical and subtropical countries (Figure 4).40 The greatest increase in coverage (2008–2011) was seen in Asia. However, the total number of seasonal influenza vaccine doses distributed was relatively small at 8·2 million in 2011. Coverage, estimated as the proportion of seasonal influenza vaccine doses distributed in the general population, was <1% in 2011 in all of Africa (except Algeria, Mauritius, Morocco, Namibia, Tunisia and South Africa) 39 and Asia (Bangladesh, India, Indonesia, Myanmar, Nepal and Sri Lanka). The coverage was 7–12 per 1000 in Singapore despite influenza vaccines being offered at a cost in public hospitals.41 In contrast, countries in Central and South America (except Guatemala, Guyana, Haiti and Jamaica), Mauritius and China, Hong Kong SAR reported a coverage of more than one dose per 1000 population.30 Seasonal influenza vaccination coverage varied widely across different targeted population groups (Table 1) in the tropics.
Figure 4.

Seasonal influenza vaccine doses distributed in the tropics and subtropics (2011) (Source: adapted from Ref. 32).
Table 1.
Range of estimates for seasonal influenza vaccine coverage in the tropics and subtropics
| Seasonal influenza vaccine coverage | Period | Countries | References |
|---|---|---|---|
| Children | 2014 | Argentina, Belize, Chile, Colombia, Ecuador, El Salvador, Mexico, Nicaragua, Panama: >80%; Bolivia (Plurinational State of), Honduras, Paraguay, Peru, Uruguay: 23–45% | 76 |
| 2006 | Public funded (Chile, China – Province of Taiwan): 23–62%, User paid (Argentina): 8–10% | 77 | |
| 2011–2012 | China – Province of Taiwan: 32–72% | 78 | |
| 2010–2012 | Thailand: <2% | 79 | |
| Elderly |
1993–1997 2004–2008 2014 |
Chile, Costa Rica, Dominican Republic, El Salvador, Honduras, Mexico and Nicaragua: >75%; Argentina, Belize, Bolivia (Plurinational State of), Colombia, Ecuador, Panama, Paraguay, Peru and Uruguay: 20–69% | 26, 76, 80 |
| 2000–2003 | Brazil: 66–90% | 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 | |
| 2006 | Public funded (Argentina, Chile, Republic of Korea): 14–41%; User paid (China, South Africa): <10% | 77 | |
| Pregnant women |
2005–2006 2010–2013 |
India, China, Hong Kong SAR, Thailand: 0–4% | 79, 92, 93, 94 |
| Healthcare professionals |
2001–2005 2012 |
Brazil, China, 20–56% | 95, 96, 97 |
Vaccine efficacy and effectiveness
Vaccine efficacy and effectiveness against different outcomes such as laboratory‐confirmed disease, influenza‐like illness, hospitalization and mortality associated with seasonal influenza varied widely in different high‐risk groups in tropical and subtropical countries (Appendix H–L). The 21 studies in the elderly that were reviewed comprised four randomized and two non‐randomized controlled trials, seven cohort studies, four case–controls and four ecological studies. Seasonal influenza vaccination provided 43–58% protection in the elderly.18, 42, 43 The seven studies in healthy adults comprised one randomized controlled trial, and three cohorts and case–control studies each. Seasonal influenza vaccination provided 50–59% protection in healthy adults.18, 44, 45, 46 The 12 studies in children comprised nine randomized controlled trials, one cohort and two case–control studies. Seasonal influenza vaccination provided 20–77% in children depending on antigenic match, against laboratory‐confirmed influenza (Table 2).18, 47, 48, 49, 50, 51, 52, 53, 54, 55 Vaccinating school children provided 23·3% (66·3–74·9) protection against influenza and indirect protection of 61% (5·8–84·7) for household contacts.56 Vaccinating pregnant women prevented laboratory‐confirmed influenza in both mothers (50%) and their infants up to 6 months of age (49–63%).57, 58 Vaccinating patients with chronic obstructive pulmonary disease provided 70% protection against laboratory‐confirmed influenza in Thailand.59
Table 2.
Range of VE estimates from meta‐analytic reviews and individual studies from the tropics and subtropics
| Outcome | Range of pooled VE estimates from meta‐analytic reviews | References | Range of VE point estimates from individual studies from the tropics and subtropics | References |
|---|---|---|---|---|
| Elderly | ||||
| ILI | 4–59% | 18, 61, 62 | 0–76% | 42, 90, 98, 99, 100, 101 |
| LCI | 43–58% | 18, 61, 62 | 0–42% | 42, 43 |
| Pneumonia | 30–53% | 61, 102, 103 | 43% | 104 |
| Hospitalization | ||||
| Influenza‐related | No effect – 33% | 18, 62, 102, 103, 105, 106, 107 | 31–77%a | 43, 80, 104, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117 |
| All‐cause | 50% | – | ||
| Mortality | ||||
| Influenza‐related | 8–30% | 61, 62, 102, 103, 106, 107 | 20–53%a | 43, 104, 108, 113, 116, 118, 119, 120, 121 |
| All‐cause | 36–68% | No effect – 44% | ||
| Children | ||||
| ILI |
31–45% LAIV: 36% TIV: 27% |
18, 63, 67, 70, 71, 72 | 8–85% | 47, 101 |
| LCI | ||||
| Overall | 67–74% | 18, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 | 20–77% | 47, 48, 49, 50, 51, 52, 53, 54, 55, 114, 122, 123 |
| LAIV | 62–83% | 64–72% | ||
| TIV | 48–72% | 33–62% | ||
| LAIV good match | 61–88% | 70–78% | ||
| TIV good match | 48–81% | |||
| LAIV poor match | 60–87% | |||
| TIV poor match | 49–56% | |||
| One dose | 58% | 58% | ||
| Two doses | 75% | 74%b | ||
| Influenza A | 31–91% | 25–57% | ||
| Influenza B | 45% | 0–50% | ||
| Healthy adults | ||||
| ILI | ||||
| LAIV | 10% | 18, 65, 74, 124 | – | 101, 125, 126, 127 |
| TIV | 20–69% | 39–73% | ||
| LCI | ||||
| TIV good match | 57–80% | 18, 64, 65, 66, 74, 124 | 44, 45, 46 | |
| TIV poor match | 44–52% | |||
| TIV any match | 59–82% | 50–59% | ||
| TIV influenza A | 64% | H1N1pdm – 84%, H3N2 – 33% | ||
| TIV influenza B | 52% | 84% | ||
| Pregnant women | ||||
| ILI in mother | ||||
| Healthy mothers | 0–44% | 128, 129, 130, 131, 132, 133 | 0–36% | 57, 58 |
| HIV‐infected mothers | – | No effect | ||
| LCI in mother | ||||
| Healthy mothers | – | 50% | 58 | |
| HIV‐infected mothers | – | 58% | ||
| ILI in infant | ||||
| Healthy mothers | No effect | 128, 129, 130, 131, 132, 133 | 0–29% | 57, 58 |
| HIV‐infected mothers | – | No effect | ||
| LCI in infant | ||||
| Healthy mothers | – | 49–63% | 57, 58 | |
| HIV‐infected mothers | – | 27% (ns) | ||
| Preterm/IUGR (infant) | 0–72% | 134 | 28–37% (ns) | 57 |
| High‐risk individuals | ||||
| COPD patients | ||||
| ARI | 11% (ns) | 135 | 60–85% | 59, 136, 137 |
| LCI | 81% | 71% | ||
| Hospitalization (ARI) | 67% (ns) | 72% | ||
| Coronary heart disease patients | ||||
| Coronary heart disease mortality | 61% | 138 | 38 (ns) – 66% | 139, 140 |
| HIV‐infected patients | ||||
| ARI | – | 141, 142 | 8 (ns) – 16% (ns) | 143 |
| LCI | 27–78% | 76% | ||
| Healthcare professionals | No effect on LCI, hospitalization or mortality in elderly who received care from healthcare professionals | 144 | 51% against ILI in health workers if good antigenic match; no effect if poor antigenic match | 145 |
| Pilgrims | ||||
| ILI | 72% | 18 | 38–77% | 146, 147 |
ARI, acute respiratory illness; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ILI, influenza‐like illness; LAIV, live attenuated influenza vaccine; LCI, laboratory‐confirmed influenza; TIV, trivalent inactivated influenza vaccine; VE, vaccine efficacy and effectiveness.
No effect in two studies.
One study showed protective effect only after two doses.
Discussion
This paper presents a comprehensive review of influenza policy, availability, coverage, use and effectiveness of seasonal influenza vaccine currently in the tropics and subtropics. Most countries in Europe, United States and the developed world have a national policy on immunization against seasonal influenza.21, 60 In contrast, large parts of sub‐Saharan Africa and the Indian subcontinent are yet to formulate national policies against seasonal influenza; though, the vaccine is available through the private sector in many countries in the region. Historically, the Central and South Americas have often led the introduction of new and underused vaccines including expansion of seasonal influenza vaccine to pregnant women. Even as some countries such as the United States expand their policy to immunize all individuals above 6 months of age against influenza, targeted vaccination of high‐risk population subgroups remains the main strategy to reduce influenza disease burden. Many countries in the tropics recommended the Northern or Southern Hemisphere vaccine formulation based on geographical location rather than on evidence on local seasonality and antigenic evolution patterns.
Overall, seasonal influenza vaccination coverage was <1% in most parts of Africa and Asia. In contrast, reported coverage in the Central and South America was comparable to that seen in high‐income countries.30 Higher vaccination coverage is not correlated with higher level of economic development. Higher coverage, however, is seen when the vaccine is offered at no cost through the public sector.12 Estimating influenza vaccine coverage poses major challenges for countries in the tropics and subtropics – lack of special vaccine coverage studies, suboptimal definition of coverage, absence of reliable denominators for the targeted high‐risk population groups, lack of information on unused or returned or wasted vaccine doses, among others. Vaccine coverage estimated by national immunization programmes is less meaningful as they are based on the number of vaccine doses distributed or administered expressed as a fraction of number of doses procured. Global distribution of vaccines by the major manufacturers has been used as a proxy for vaccine coverage.30 However, these coverage estimates do not account for 21% of the global vaccine supply by developing country manufacturers that do not contribute to this database.
Our review suggests that the 43–58% protection by the vaccine against laboratory‐confirmed influenza in the elderly was slightly lower in the tropics and subtropics compared to 50–77% protection seen in Europe, United States and other developed countries.61, 62 The protection in children (range 20–77%) and in healthy adults (range 50–59%) seen in the tropics and subtropics was comparable with that seen in developed countries.63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 Vaccine protection studies from the tropics and subtropics faced certain limitations. First, the majority of the studies from the tropics and subtropics were observational and prone to selection or ascertainment bias. The outcome for assessing vaccine protection was based on clinical ascertainment or self‐reports without laboratory confirmation of influenza. Three of 16 vaccine efficacy trials were non‐randomized and prone to ascertainment and reporting bias. Second, most studies were from large urban areas with limited national representativeness. Third, few studies matched for antigenic relatedness of the circulating influenza virus with the vaccine virus strain. Vaccine effectiveness is known to be low during seasons of antigenic mismatch as was seen during the 2014–2015 influenza season in the United States when protection was much lower [18% (95% CI: 6–29%)] against A(H3N2) viruses.75
Notwithstanding these challenges, vaccine effectiveness data is critical for countries to introduce and evaluate influenza vaccination programmes. Even as an increasing number of countries from the tropics and subtropics introduce influenza into their immunization programme, there are critical knowledge gaps in these settings. It is not known how the high prevalence of underlying malnutrition and infections such as tuberculosis, malaria and limited access to health care may increase the risk of influenza‐related complications or may influence vaccine effectiveness. Although children and the elderly are at high risk of severe influenza outcomes, few studies from the tropics and subtropics assessed severe outcomes such as influenza‐related hospitalization or mortality. Almost a third of these studies were observational and prone to bias due to self‐assessment of the influenza outcomes. Only a few studies evaluated vaccine effectiveness stratified by antigenic match or reported data on vaccine coverage, influenza incidence and seasonality.64 Finally, waning immunity in the months following vaccination may decrease vaccine benefits in tropical and subtropical regions where influenza activity prevails all year round. Countries need to strengthen their national surveillance for influenza that integrate methodologies to measure vaccine coverage and evaluate vaccine effectiveness using well‐defined denominators.
Our review, though systematic, was subject to several methodological and substantive limitations. First, we excluded articles in languages other than English. However, we took efforts to search regional databases for articles in Spanish language as countries from Central and South America have contributed substantially to influenza research in the recent past. An earlier review of influenza (seasonal and pandemic) vaccine effectiveness in low‐ and middle‐income countries identified 132 articles of a total of 361, in languages other than English.18 More than 75% of these non‐English language articles were from Russia and another 15 articles were from Romania, countries that were outside the scope of our review. Nevertheless, our review may be limited to some extent by the exclusion of potential studies from China.
Second, there was wide disparity in the geographic distribution of studies. For example, of the 26 studies from the tropics and subtropics of vaccine effectiveness among the elderly, 11 (including nine from Brazil,) were from Central and South America, 12 from Asia Pacific (including four each from China and Hong Kong SAR, three from Thailand and two from China – Province of Taiwan), and three from Africa (all from South Africa). Most of sub‐Saharan Africa, the Middle East, Bangladesh, India, Indonesia, Pakistan and Sri Lanka in Asia were underrepresented in the review.
Conclusion
Seventy‐four countries in the tropics and subtropics representing 60% of the world's population did not have a national vaccination policy against seasonal influenza. Guidelines on vaccine composition, priority risk groups and vaccine availability in the public and private sector varied widely. Influenza vaccination coverage was <5 per 1000 general population. The evidence on vaccine effectiveness in the tropics and subtropics was scarce. Countries in the tropics and subtropics need to strengthen and expand their local and regional evidence‐base required for making informed decisions on influenza vaccine introduction and expansion, and how much benefit to expect.
Supporting information
Appendix S1. PRISMA 2009 checklist.
Appendix S2. Strategies and keywords used for literature search.
Appendix S3. List of countries and territories in the tropics and subtropics included in the review.
Appendix S4. Potential risk of bias (shaded in grey) in cohort vaccine effectiveness studies. Risk of bias assessed using Newcastle‐Ottawa Scale.
Appendix S5. Potential risk of bias (shaded in grey) in case control vaccine effectiveness studies – Risk of bias assessed using Newcastle‐Ottawa Scale.
Appendix S6. Potential risk of bias in randomized controlled trials for vaccine efficacy studies – Risk of bias assessed using Cochrance risk of bias assessment tool. High risk of potential bias or lack of information to assess risk of bias is shaded in grey.
Appendix S7. Seasonal influenza vaccine coverage in the tropics and subtropics.
Appendix S8. Seasonal influenza effectiveness in the elderly.
Appendix S9. Seasonal influenza effectiveness in children.
Appendix S10. Seasonal influenza vaccine effectiveness in healthy adults.
Appendix S11. Seasonal influenza vaccine effectiveness in pregnant women.
Appendix S12. Seasonal influenza vaccine effectiveness in high risk individuals.
Acknowledgements
This review was funded through a WHO grant no. OPP1084574 from the Bill & Melinda Gates Foundation, USA. The authors would like to acknowledge the support of the Centers for Disease Control and Prevention, USA, for sharing data from their ongoing work. We thank colleagues in the WHO regional offices, Joachim Hombach and Justin Ortiz in the WHO Initiative for Vaccine Research, for sharing information and for reviewing the manuscript. We value the summary data on seasonal influenza vaccine distribution shared by IFPMA. Lastly, we would like to thank Maja Lievre for creating the maps, Hannah Moak for screening of literature for eligibility and Jack Sternal for independently assessing the studies for bias.
Hirve et al (2016) Seasonal influenza vaccine policy, use and effectiveness in the tropics and subtropics – a systematic literature review. Influenza and Other Respiratory Viruses 10(4), 254–267.
References
- 1. Gerdil C. The annual production cycle for influenza vaccine. Vaccine 2003; 21:1776–1779. [DOI] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K et al Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou H, Thompson WW, Viboud CG et al Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nair H, Brooks WA, Katz M et al Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta‐analysis. Lancet 2011; 378:1917–1930. [DOI] [PubMed] [Google Scholar]
- 5. Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub‐Saharan Africa: a systematic review. Lancet Infect Dis 2011; 11:223–235. [DOI] [PubMed] [Google Scholar]
- 6. WHO Unicef World Bank . State of the World's Vaccines and Immunization, 3rd ed Geneva: World Health Organization, 2009. [Google Scholar]
- 7. World Health Organization . Resolution 56.19. Prevention and Control of Influenza Pandemics and Annual Epidemics. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 8. World Health Organization . Resolution WHA58.5. Strengthening Pandemic‐Influenza Preparedness and Response. Geneva, Switzerland: World Health Organization, 2005. [Google Scholar]
- 9. World Health Organization . Report of the Second WHO Consultation on the Global Action Plan for Influenza Vaccines (GAP), Geneva, Switzerland, 12–14 July 2011. Geneva: World Health Organization, 2012. [Google Scholar]
- 10. Abbott A. Flu jabs urged for developing countries. Nature 2009; 460:156–157. [DOI] [PubMed] [Google Scholar]
- 11. Kieny MP, Tam JS, Hendriks J. Progress update on the global action plan for influenza vaccines (GAP). Options for the control of Influenza VIII; Cape Town, South Africa, 2013; P1–P408.
- 12. Macroepidemiology of Influenza Vaccination Study G . The macro‐epidemiology of influenza vaccination in 56 countries, 1997–2003. Vaccine 2005; 23:5133–5143. [DOI] [PubMed] [Google Scholar]
- 13. van Essen GA, Palache AM, Forleo E, Fedson DS. Influenza vaccination in 2000: recommendations and vaccine use in 50 developed and rapidly developing countries. Vaccine 2003; 21:1780–1785. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization . Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec 2012; 47:461–476. [PubMed] [Google Scholar]
- 15. Saha S, Chadha M, Al Mamun A et al Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and south‐eastern Asia. Bull World Health Organ 2014; 92:318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durand L, Cheng P, Palekar R et al Timing of influenza epidemics and vaccines in the American Tropics, 2002–2008, 2011–2014. Influenza and other respir viruses 2016. doi: 10.1111/irv.12371. [DOI] [PMC free article] [PubMed]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. Open Med 2009; 3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 18. Breteler JK, Tam JS, Jit M, Ket JC, De Boer MR. Efficacy and effectiveness of seasonal and pandemic A (H1N1) 2009 influenza vaccines in low and middle income countries: a systematic review and meta‐analysis. Vaccine 2013; 31:5168–5177. [DOI] [PubMed] [Google Scholar]
- 19. Wells GA, Shea B, O'Connell D et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 28 November 2014).
- 20. Collaboration C . Part 2: General methods for Cochrane reviews. 2011. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Available at http://handbook.cochrane.org/front_page.htm (Accessed 28 November 2014).
- 21. World Health Organization . Global Survey on Seasonal Influenza Vaccine Policy Development and Implementation – 2010: Report of Findings. Geneva: World Health Organization, 2013. [Google Scholar]
- 22. Cortes MdlA, Cardoso D, Fitzgerald J, DiFabio JL. Public vaccine manufacturing capacity in the Latin American and Caribbean region: current status and perspectives. Biologicals 2012; 40:3–14. [DOI] [PubMed] [Google Scholar]
- 23. Mato Chaín G, Mariano Lázaro A, Alcudia Pérez F, Verdejo Bravo C. Vacunación antigripal en personas mayores. Rev Esp Geriatr Gerontol 2011; 46:89–95. [DOI] [PubMed] [Google Scholar]
- 24. Music T. Protecting patients, protecting healthcare workers: a review of the role of influenza vaccination. Int Nurs Rev 2012; 59:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng S, Wu P, Nishiura H, Ip DK, Lee ES, Cowling BJ. An analysis of national target groups for monovalent 2009 pandemic influenza vaccine and trivalent seasonal influenza vaccines in 2009–10 and 2010–11. BMC Infect Dis 2011; 11:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ropero‐Alvarez AM, Kurtis HJ, Danovaro‐Holliday MC, Ruiz‐Matus C, Andrus JK. Expansion of seasonal influenza vaccination in the Americas. BMC Public Health 2009; 9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmed QA, Arabi YM, Memish ZA. Health risks at the Hajj. Lancet 2006; 367:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization . Health conditions for travellers to Saudi Arabia for the pilgrimage to Mecca (Hajj), 2014. Wkly Epidemiol Rec 2014; 89:357–360. [PubMed] [Google Scholar]
- 29. Collin N, de Radigues X, World Health Organization HNVTF . Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine 2009; 27:5184–5186. [DOI] [PubMed] [Google Scholar]
- 30. Palache A, Oriol‐Mathieu V, Abelin A, Music T, Influenza Vaccine Supply task f . Seasonal influenza vaccine dose distribution in 157 countries (2004–2011). Vaccine 2014; 32:6369–6376. [DOI] [PubMed] [Google Scholar]
- 31. Partridge J, Kieny MP, World Health Organization HNivTF . Global production of seasonal and pandemic (H1N1) influenza vaccines in 2009–2010 and comparison with previous estimates and global action plan targets. Vaccine 2010; 28:4709–4712. [DOI] [PubMed] [Google Scholar]
- 32. Feng L, Mounts AW, Feng Y et al Seasonal influenza vaccine supply and target vaccinated population in China, 2004–2009. Vaccine 2010; 28:6778–6782. [DOI] [PubMed] [Google Scholar]
- 33. Hoa LK, Hiep LV, Be LV. Development of pandemic influenza vaccine production capacity in Viet Nam. Vaccine 2011; 29(Suppl 1):A34–A36. [DOI] [PubMed] [Google Scholar]
- 34. Surichan S, Wirachwong P, Supachaturas W et al Development of influenza vaccine production capacity by the Government Pharmaceutical Organization of Thailand: addressing the threat of an influenza pandemic. Vaccine 2011; 29(Suppl 1):A29–A33. [DOI] [PubMed] [Google Scholar]
- 35. Friede M, Palkonyay L, Alfonso C et al WHO initiative to increase global and equitable access to influenza vaccine in the event of a pandemic: supporting developing country production capacity through technology transfer. Vaccine 2011; 29(Suppl 1):A2–A7. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization . Global action plan for influenza vaccines. Global progress report. January 2006–September 2013 March 2014. Available at http://apps.who.int/iris/bitstream/10665/112307/1/9789241507011_eng.pdf?ua=1p (Accessed 28 November 2014).
- 37. Partridge J, Kieny MP. Global production capacity of seasonal influenza vaccine in 2011. Vaccine 2013; 31:728–731. [DOI] [PubMed] [Google Scholar]
- 38. Schoub BD, Gessner BD, Ampofo W, Cohen AL, Steffen CA. Afriflu2–second international workshop on influenza vaccination in the African continent–8 November 2012, Cape Town (South Africa). Vaccine 2013; 31:3461–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duque J, McMorrow ML, Cohen AL. Influenza vaccines and influenza antiviral drugs in Africa: are they available and do guidelines for their use exist? BMC Public Health 2014; 14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis 2006; 194(Suppl 2):S111–S118. [DOI] [PubMed] [Google Scholar]
- 41. Gupta V, Dawood FS, Muangchana C et al Influenza vaccination guidelines and vaccine sales in southeast Asia: 2008–2011. PLoS ONE 2012; 7:e52842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Praditsuwan R, Assantachai P, Wasi C, Puthavatana P, Kositanont U. The efficacy and effectiveness of influenza vaccination among Thai elderly persons living in the community. J Med Assoc Thai 2005; 88:256–264. [PubMed] [Google Scholar]
- 43. De Villiers PJ, Steele AD, Hiemstra LA et al Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine 2009; 28:228–234. [DOI] [PubMed] [Google Scholar]
- 44. Ho HP, Zhao X, Pang J et al Effectiveness of seasonal influenza vaccinations against laboratory‐confirmed influenza‐associated infections among Singapore military personnel in 2010–2013. Influenza Other Respir Viruses 2014; DOI: 10.1111/irv.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ntshoe GM, McAnerney JM, Tempia S et al Influenza epidemiology and vaccine effectiveness among patients with influenza‐like illness, viral watch sentinel sites, South Africa, 2005–2009. PLoS ONE 2014; 9:e94681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McAnerney JM, Walaza S, Buys A et al Effectiveness of seasonal influenza vaccine in preventing laboratory confirmed influenza in a primary health care setting in South Africa, 2010–2012. Options for control of influenza VIII; Cape Town, South Africa, 2013; P1–P409.
- 47. Cowling BJ, Ng S, Ma ES et al Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis 2010; 51:1370–1379. [DOI] [PubMed] [Google Scholar]
- 48. Jain VK, Rivera L, Zaman K et al Vaccine for prevention of mild and moderate‐to‐severe influenza in children. N Engl J Med 2013; 369:2481–2491. [DOI] [PubMed] [Google Scholar]
- 49. Bracco Neto H, Farhat CK, Tregnaghi MW et al Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine‐naive children. Pediatr Infect Dis J 2009; 28:365–371. [DOI] [PubMed] [Google Scholar]
- 50. Tam JS, Capeding MR, Lum LC et al Efficacy and safety of a live attenuated, cold‐adapted influenza vaccine, trivalent against culture‐confirmed influenza in young children in Asia. Pediatr Infect Dis J 2007; 26:619–628. [DOI] [PubMed] [Google Scholar]
- 51. Lum LC, Borja‐Tabora CF, Breiman RF et al Influenza vaccine concurrently administered with a combination measles, mumps, and rubella vaccine to young children. Vaccine 2010; 28:1566–1574. [DOI] [PubMed] [Google Scholar]
- 52. Belshe RB, Edwards KM, Vesikari T et al Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–696. [DOI] [PubMed] [Google Scholar]
- 53. He Q, Xu J, Chen X et al Effectiveness of seasonal influenza vaccine against clinically diagnosed influenza over 2 consecutive seasons in children in Guangzhou, China: a matched case‐control study. Hum Vaccin Immunother 2013; 9:1720–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fu C, He Q, Li Z et al Seasonal influenza vaccine effectiveness among children, 2010–2012. Influenza Other Respir Viruses 2013; 7:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang Z, Dong Z, Fu C. Seasonal influenza vaccine effectiveness among children aged 6 to 59 months in southern China. PLoS ONE 2012; 7:e30424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gattas VL, Cardoso MR, Machado CM, Mondini G, Luna EJ. Indirect effectiveness of vaccinating school children against influenza. Options for the control of influenza VIII; Cape Town, South Africa, 2013; P1–P388.
- 57. Zaman K, Roy E, Arifeen SE et al Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–1564. [DOI] [PubMed] [Google Scholar]
- 58. Madhi SA, Cutland CL, Kuwanda L et al Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371:918–931. [DOI] [PubMed] [Google Scholar]
- 59. Kositanont U, Kanyok R, Wasi C, Wongsurakiat P, Suthamsmai T, Maranetra N. Occurrence and protective level of influenza infections using serology in patients with COPD in vaccination study. J Med Assoc Thai 2004; 87:964–969. [PubMed] [Google Scholar]
- 60. Mereckiene J, Cotter S, D'Ancona F et al Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008–2009. Euro Surveill 2010; pii:19700. [DOI] [PubMed] [Google Scholar]
- 61. Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen‐Van‐Tam JS, Osterhaus AD. Cochrane re‐arranged: support for policies to vaccinate elderly people against influenza. Vaccine 2013; 31:6030–6033. [DOI] [PubMed] [Google Scholar]
- 62. Darvishian M, Gefenaite G, Turner RM et al After adjusting for bias in meta‐analysis seasonal influenza vaccine remains effective in community‐dwelling elderly. J Clin Epidemiol 2014; 67:734–744. [DOI] [PubMed] [Google Scholar]
- 63. Luksic I, Clay S, Falconer R et al Effectiveness of seasonal influenza vaccines in children – a systematic review and meta‐analysis. Croat Med J 2013; 54:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tricco AC, Chit A, Soobiah C et al Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta‐analysis. BMC Med 2013; 11:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta‐analysis. Lancet Infect Dis 2012; 12:36–44. [DOI] [PubMed] [Google Scholar]
- 66. DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non‐elderly adults: a systematic review with meta‐analyses of controlled trials. Vaccine 2012; 31:49–57. [DOI] [PubMed] [Google Scholar]
- 67. Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 2011; 29:9159–9170. [DOI] [PubMed] [Google Scholar]
- 68. Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011; 71:1591–1622. [DOI] [PubMed] [Google Scholar]
- 69. Rhorer J, Ambrose CS, Dickinson S et al Efficacy of live attenuated influenza vaccine in children: a meta‐analysis of nine randomized clinical trials. Vaccine 2009; 27:1101–1110. [DOI] [PubMed] [Google Scholar]
- 70. Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2012, DOI: 10.1002/14651858.CD004879.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: a meta‐analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr Infect Dis J 2007; 26:97–106. [DOI] [PubMed] [Google Scholar]
- 72. Negri E, Colombo C, Giordano L, Groth N, Apolone G, La Vecchia C. Influenza vaccine in healthy children: a meta‐analysis. Vaccine 2005; 23:2851–2861. [DOI] [PubMed] [Google Scholar]
- 73. Ruben FL. Inactivated influenza virus vaccines in children. Clin Infect Dis 2004; 38:678–688. [DOI] [PubMed] [Google Scholar]
- 74. Demicheli V, Di Pietrantonj C, Jefferson T, Rivetti A, Rivetti D. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev, 2007, DOI: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 75. Centers for Disease Control and Prevention . Seasonal influenza vaccine effectiveness, 2005–2015. 2015. Available at http://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm (Accessed 21 December 2015).
- 76. Organización Panamericana de la Salud . Vigilancia regional de la influenza, OPS‐OMS. Influenza y otros virus respiratorios bajo vigilancia, 2010–2014 [Internet]. 2014. Available at http://ais.paho.org/phip/viz/ed_flu.asp (Accessed 28 November 2014).
- 77. de Lataillade C, Auvergne S, Delannoy I. 2005 and 2006 seasonal influenza vaccination coverage rates in 10 countries in Africa, Asia Pacific, Europe, Latin America and the Middle East. J Public Health Policy 2009; 30:83–101. [DOI] [PubMed] [Google Scholar]
- 78. Lee CL, Chen TY, Chih YC, Chou SM, Chen CH, Yang CH. The overview of government‐funded influenza vaccination program during influenza season 2011–2012. Options for control of Influenza VIII; Cape Town, South Africa, 2013; P1–P407.
- 79. Owusu JT, Prapasiri P, Ditsungnoen D et al Seasonal influenza vaccine coverage by risk group in Thailand, 2010–2012. Options for control of influenza VIII; Cape Town, South Africa, 2013; P2–P498.
- 80. Stamboulian D, Bonvehi PE, Nacinovich FM, Ruttimann RW. Immunization against influenza in the elderly: the Argentinian experience, 1993–1997. Vaccine 1999; 17(Suppl 1):S53–S56. [DOI] [PubMed] [Google Scholar]
- 81. Luna EJ, Gattas VL. Effectiveness of the Brazilian influenza vaccination policy, a systematic review. Rev Inst Med Trop Sao Paulo 2010; 52:175–181. [DOI] [PubMed] [Google Scholar]
- 82. Cabral MHdP. A campanha nacional de vacinação de idosos como estartégia de entrada do Programa de Saúde da Família em uma área programática de saúde do município do Rio de Janeiro‐RJ: planejamento, implementação e execução. Cad Saúde Colet 2006; 14:425–434. [Google Scholar]
- 83. Cesar JA, Oliveira‐Filho JA, Bess G et al Perfil dos idosos residentes em dois municípios pobres das regiões Norte e Nordeste do Brasil: resultados de estudo transversal de base populacional. Cad Saude Publica 2008; 24:1835–1845. [DOI] [PubMed] [Google Scholar]
- 84. Donalisio MR, Ruiz T, Cordeiro R. Factors associated with influenza vaccination among elderly persons in Southeastern Brazil. Rev Saude Publica 2006; 40:115–119. [DOI] [PubMed] [Google Scholar]
- 85. Francisco P, Donalisio MR, Barros MBdA, César CLG, Carandina L, Goldbaum M. Fatores associados à vacinação contra a influenza em idosos. Rev Panam Salud Publica 2006; 19:259–264. [DOI] [PubMed] [Google Scholar]
- 86. Francisco P, DonalisioI MR, de Azevedo MB, CésarII CLG, CarandinaIII L, GoldbaumIV M. Fatores associados à doença pulmonar em idosos. Rev Saude Publica 2006; 40:428–435. [DOI] [PubMed] [Google Scholar]
- 87. Francisco PMSB, Donalisio MR, Barros MBdA, Cesar CLG, Carandina L, Goldbaum M. Vacinação contra influenza em idosos por área de residência: prevalência e fatores associados. Rev Bras Epidemiol 2006; 9:162–171. [Google Scholar]
- 88. Lima‐Costa MF. Fatores associados à vacinação contra gripe em idosos na região metropolitana de Belo Horizonte. Rev Saude Publica 2008; 42:100–107. [DOI] [PubMed] [Google Scholar]
- 89. Geronutti DA, Molina AC, Lima SAM. Vacinação de idosos contra a influenza em um centro de saúde escola do interior do estado de São Paulo. Texto Contexto Enferm 2008; 17:336–341. [Google Scholar]
- 90. Gutierrez EB, Li HY, Santos AC, Lopes MH. Effectiveness of influenza vaccination in elderly outpatients in Sao Paulo city, Brazil. Rev Inst Med Trop Sao Paulo 2001; 43:317–320. [DOI] [PubMed] [Google Scholar]
- 91. Santos BRLd, Creutzberg M, Cardoso RF et al Situação vacinal e associação com a qualidade de vida, a funcionalidade ea motivação para o autocuidado em idosos. Rev Bras Epidemiol 2009; 12:533–540. [Google Scholar]
- 92. Koul PA, Bali NK, Ali S et al Poor uptake of influenza vaccination in pregnancy in northern India. Int J Gynaecol Obstet 2014; 127:234–237. [DOI] [PubMed] [Google Scholar]
- 93. Lau JT, Cai Y, Tsui HY, Choi KC. Prevalence of influenza vaccination and associated factors among pregnant women in Hong Kong. Vaccine 2010; 28:5389–5397. [DOI] [PubMed] [Google Scholar]
- 94. Yuet Sheung Yuen C, Yee Tak Fong D, Lai Yin Lee I, Chu S, Sau‐Mei Siu E, Tarrant M. Prevalence and predictors of maternal seasonal influenza vaccination in Hong Kong. Vaccine 2013; 31:5281–5288. [DOI] [PubMed] [Google Scholar]
- 95. Bellei N, Carraro E, Perosa AH, Benfica D, Granato CF. Influenza and rhinovirus infections among health‐care workers. Respirology 2007; 12:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Takayanagi IJ, Cardoso MRA, Costa SF, Araya MES, Machado CM. Attitudes of health care workers to influenza vaccination: why are they not vaccinated? Am J Infect Control 2007; 35:56–61. [DOI] [PubMed] [Google Scholar]
- 97. Chan TC, Hung IF, Luk JK, Woo PC, Chu LW, Chan FH. Prevalence of influenza vaccination and associated factors in Chinese nursing home healthcare workers. J Am Geriatr Soc 2013; 61:1824–1827. [DOI] [PubMed] [Google Scholar]
- 98. Forrest BD, Steele AD, Hiemstra L, Rappaport R, Ambrose CS, Gruber WC. A prospective, randomized, open‐label trial comparing the safety and efficacy of trivalent live attenuated and inactivated influenza vaccines in adults 60 years of age and older. Vaccine 2011; 29:3633–3639. [DOI] [PubMed] [Google Scholar]
- 99. Isahak I, Mahayiddin AA, Ismail R. Effectiveness of influenza vaccination in prevention of influenza‐like illness among inhabitants of old folk homes. Southeast Asian J Trop Med Public Health 2007; 38:841–848. [PubMed] [Google Scholar]
- 100. Plasai V, Lertmaharit S, Viputsiri OA et al Influenza vaccination among the elderly in Bangkok. Southeast Asian J Trop Med Public Health 2006; 37(Suppl 3):140–144. [PubMed] [Google Scholar]
- 101. Jianping H, Xin F, Changshun L et al Assessment of effectiveness of Vaxigrip. Vaccine 1999; 17(Suppl 1):S57–S58. [DOI] [PubMed] [Google Scholar]
- 102. Jefferson T, Di Pietrantonj C, Al‐Ansary L, Ferroni E, Thorning S, Thomas R. Vaccines for preventing influenza in the elderly (Review). Cochrane Database Syst Rev 2010; 2010: Art. No.: CD004876. [DOI] [PubMed] [Google Scholar]
- 103. Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature. Ann Intern Med 1995; 123:518–527. [DOI] [PubMed] [Google Scholar]
- 104. Hung IF, Leung AY, Chu DW et al Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis 2010; 51:1007–1016. [DOI] [PubMed] [Google Scholar]
- 105. Moreno J, De la Hoz F, Rico A, Cotes K, Porras A. Effectiveness of the Influenza vaccine: meta‐analysis of literature. Biomedica 2009; 29:87–97. [PubMed] [Google Scholar]
- 106. Rivetti D, Jefferson T, Thomas R et al Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006; DOI: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 107. Vu T, Farish S, Jenkins M, Kelly H. A meta‐analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine 2002; 20:1831–1836. [DOI] [PubMed] [Google Scholar]
- 108. Façanha MC. Influenza vaccination of individuals over the age of 60: impact on hospital admissions and deaths from respiratory and circulatory diseases in Fortaleza, Brazil. J Bras Pneumol 2005; 31:415–420. [Google Scholar]
- 109. Brondi L, Barbosa J. (eds). Vacina contra influenza: experiencia en Brasil. Resúmenes de lo 12° Congreso Latinoamericano de Pediatria‐taller sobre imunizaciones; 2000.
- 110. Daufenbach LZ, Carmo EH, Duarte EC, Campagna AdS, Teles CAS. Morbidade hospitalar por causas relacionadas à influenza em idosos no Brasil, 1992 a 2006. 2009.
- 111. Ferrer AL, Marcon SS, Santana RG. Hospital morbidity among elderly patients, before and after influenza vaccination in the state of Parana. Rev Lat Am Enfermagem 2008; 16:832–837. [DOI] [PubMed] [Google Scholar]
- 112. Francisco PMSB, Donalisio MR, Latorre MdRDdO. Internações por doenças respiratórias em idosos ea intervenção vacinal contra influenza no Estado de São Paulo. Rev Bras Epidemiol 2004; 7:220–227. [Google Scholar]
- 113. van Vuuren A, Rheeder P, Hak E. Effectiveness of influenza vaccination in the elderly in South Africa. Epidemiol Infect 2009; 137:994–1002. [DOI] [PubMed] [Google Scholar]
- 114. El Omeiri N, Azziz‐Baumgartner E, Clará 'W et al Pilot to evaluate the feasibility of measuring seasonal influenza vaccine effectiveness using surveillance platforms in Central‐America, 2012. BMC Public Health 2015; 15:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dawood FS, Prapasiri P, Areerat P et al Effectiveness of the 2010 and 2011 Southern Hemisphere trivalent inactivated influenza vaccines against hospitalization with influenza‐associated acute respiratory infection among Thai adults aged >/= 50 years. Influenza Other Respir Viruses 2014; 8:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang CS, Wang ST, Lai CT, Lin LJ, Chou P. Impact of influenza vaccination on major cause‐specific mortality. Vaccine 2007; 25:1196–1203. [DOI] [PubMed] [Google Scholar]
- 117. Yung CF, Chan S, Tan A, Leo YS. A novel approach using vaccinated cases only to evaluate influenza vaccine effectiveness against all‐cause hospital admissions in the tropics. Options for control of influenza VIII; Cape Town, South Africa, 2013; P2–P527.
- 118. Yung CF, Win MK, Tan A, Leo YS. Using vaccinated cases only to evaluate influenza vaccine effectiveness against death following admission to the hospital in the tropics. Options for control of influenza VIII; Cape Town, South Africa, 2013; P2–P526.
- 119. Antunes JL, Waldman EA, Borrell C, Paiva TM. Effectiveness of influenza vaccination and its impact on health inequalities. Int J Epidemiol 2007; 36:1319–1326. [DOI] [PubMed] [Google Scholar]
- 120. Francisco PMSB, Donalisio MRdC, Lattorre MdRDd. Impact of influenza vaccination on mortality by respiratory diseases among Brazilian elderly persons. Rev Saude Publica 2005; 39:75–81. [DOI] [PubMed] [Google Scholar]
- 121. Chan TC, Fan‐Ngai Hung I, Ka‐Hay Luk J, Chiu‐Yat Woo P, Chu LW, Hon‐Wai Chan F. Efficacy of trivalent seasonal influenza vaccination in reducing mortality and hospitalization in Chinese nursing home older adults. J Am Med Dir Assoc 2013; 14:889–894. [DOI] [PubMed] [Google Scholar]
- 122. Victor JC, Diallo A, Niang MN et al Effectiveness of seasonal influenza vaccination of children in tropical developing Africa: a cluster‐randomized trial. Options for control of influenza VIII; Cape Town, South Africa, 2013; O–860.
- 123. Kittikraisak W, Suntarattiwong P, Levy J et al Influenza vaccination coverage and effectiveness in young children in Thailand, 2011–2013. Influenza Other Respir Viruses 2015; 9:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al‐Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010; 7: DOI: 10.1002/14651858.CD001269.pub4. [DOI] [PubMed] [Google Scholar]
- 125. Hui LS, Rashwan H, bin Jaafar MH, Hussaini HM, Isahak DI. Effectiveness of influenza vaccine in preventing influenza‐like illness among Faculty of Dentistry staff and students in Universiti Kebangsaan Malaysia. Healthc Infect 2008; 13:4–9. [Google Scholar]
- 126. Samad AH, Usul MH, Zakaria D et al Workplace vaccination against influenza in Malaysia: does the employer benefit? J Occup Health 2006; 48:1–10. [DOI] [PubMed] [Google Scholar]
- 127. Morales A, Martinez MM, Tasset‐Tisseau A, Rey E, Baron‐Papillon F, Follet A. Costs and benefits of influenza vaccination and work productivity in a Colombian company from the employer's perspective. Value Health 2004; 7:433–441. [DOI] [PubMed] [Google Scholar]
- 128. Galvao TF, Silva MT, Zimmermann IR, Lopes LA, Bernardo EF, Pereira MG. Influenza vaccination in pregnant women: a systematic review. ISRN Prev Med 2013; 2013:879493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Omer SB, Bednarczyk R, Madhi SA, Klugman KP. Benefits to mother and child of influenza vaccination during pregnancy. Hum Vaccin Immunother 2012; 8:130–137. [DOI] [PubMed] [Google Scholar]
- 130. Blanchard‐Rohner G, Siegrist CA. Vaccination during pregnancy to protect infants against influenza: why and why not? Vaccine 2011; 29:7542–7550. [DOI] [PubMed] [Google Scholar]
- 131. Skowronski DM, De Serres G. Is routine influenza immunization warranted in early pregnancy? Vaccine 2009; 27:4754–4770. [DOI] [PubMed] [Google Scholar]
- 132. Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev 2006; 28:47–53. [DOI] [PubMed] [Google Scholar]
- 133. Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44–52. [DOI] [PubMed] [Google Scholar]
- 134. Steinhoff MC, MacDonald N, Pfeifer D, Muglia LJ. Influenza vaccine in pregnancy: policy and research strategies. Lancet 2014; 383:1611–1613. [DOI] [PubMed] [Google Scholar]
- 135. Poole PJ, Chacko E, Wood‐Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; DOI: 10.1002/14651858.CD002733.pub2. [DOI] [PubMed] [Google Scholar]
- 136. Menon B, Gurnani M, Aggarwal B. Comparison of outpatient visits and hospitalisations, in patients with chronic obstructive pulmonary disease, before and after influenza vaccination. Int J Clin Pract 2008; 62:593–598. [DOI] [PubMed] [Google Scholar]
- 137. Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004; 125:2011–2020. [DOI] [PubMed] [Google Scholar]
- 138. Keller T, Weeda VB, van Dongen CJ, Levi M. Influenza vaccines for preventing coronary heart disease. Cochrane Database Syst Rev 2008; DOI: 10.1002/14651858.CD005050.pub2. [DOI] [PubMed] [Google Scholar]
- 139. Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J 2011; 32:1730–1735. [DOI] [PubMed] [Google Scholar]
- 140. Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J 2004; 25:25–31. [DOI] [PubMed] [Google Scholar]
- 141. Anema A, Mills E, Montaner J, Brownstein JS, Cooper C. Efficacy of influenza vaccination in HIV‐positive patients: a systematic review and meta‐analysis. HIV Med 2008; 9:57–61. [DOI] [PubMed] [Google Scholar]
- 142. Atashili J, Kalilani L, Adimora AA. Efficacy and clinical effectiveness of influenza vaccines in HIV‐infected individuals: a meta‐analysis. BMC Infect Dis 2006; 6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Madhi SA, Maskew M, Koen A et al Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis 2011; 52:128–137. [DOI] [PubMed] [Google Scholar]
- 144. Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long‐term care institutions. Cochrane Database Syst Rev 2013; 7:CD005187. [DOI] [PubMed] [Google Scholar]
- 145. Kheok SW, Chong CY, McCarthy G et al The efficacy of influenza vaccination in healthcare workers in a tropical setting: a prospective investigator blinded observational study. Ann Acad Med Singapore 2008; 37:465–469. [PubMed] [Google Scholar]
- 146. Qureshi H, Gessner BD, Leboulleux D, Hasan H, Alam SE, Moulton LH. The incidence of vaccine preventable influenza‐like illness and medication use among Pakistani pilgrims to the Haj in Saudi Arabia. Vaccine 2000; 18:2956–2962. [DOI] [PubMed] [Google Scholar]
- 147. Mustafa AN, Gessner BD, Ismail R et al A case‐control study of influenza vaccine effectiveness among Malaysian pilgrims attending the Haj in Saudi Arabia. Int J Infect Dis 2003; 7:210–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. PRISMA 2009 checklist.
Appendix S2. Strategies and keywords used for literature search.
Appendix S3. List of countries and territories in the tropics and subtropics included in the review.
Appendix S4. Potential risk of bias (shaded in grey) in cohort vaccine effectiveness studies. Risk of bias assessed using Newcastle‐Ottawa Scale.
Appendix S5. Potential risk of bias (shaded in grey) in case control vaccine effectiveness studies – Risk of bias assessed using Newcastle‐Ottawa Scale.
Appendix S6. Potential risk of bias in randomized controlled trials for vaccine efficacy studies – Risk of bias assessed using Cochrance risk of bias assessment tool. High risk of potential bias or lack of information to assess risk of bias is shaded in grey.
Appendix S7. Seasonal influenza vaccine coverage in the tropics and subtropics.
Appendix S8. Seasonal influenza effectiveness in the elderly.
Appendix S9. Seasonal influenza effectiveness in children.
Appendix S10. Seasonal influenza vaccine effectiveness in healthy adults.
Appendix S11. Seasonal influenza vaccine effectiveness in pregnant women.
Appendix S12. Seasonal influenza vaccine effectiveness in high risk individuals.


