Abstract
Background
Bambusa vulgaris (Family: Poaceae) used in Ayurveda for paralytic complaints, inflammatory disorders and externally to skin disorders. It has various medicinal uses with good nutritional composition and a rich source of vitamins, proteins, amino acid, beta-carotene and phenolic compounds.
Objective
The present study was aimed to evaluate wound healing and anti-inflammatory potential of ethanol extract of B. vulgaris leaves in rats.
Materials and methods
The B. vulgaris leaves were evaluated for wound healing on incision and excision wound methods. Anti-inflammatory effect was evaluated by measurement of paw edema in carrageenan-induced inflammation in rats. Ethyl acetate (BVL-A) and aqueous (BVL-B) fractions from the ethanol extract of leaves were screened for wound healing effects by measuring tensile strength and biochemical parameters in incision wound method. The wound contraction area, antioxidant status and histopathological studies were done in excision wound method.
Results
Tensile strength and hydroxyproline level of 5% w/w ointment of BVL-A and BVL-B treated groups were found significantly (P < 0.01) higher and comparable to the reference group. The histopathological study showed the proliferation of collagen, fibrous tissue, and capillaries with epidermal covering at the margin of the wound. The percent inhibition of paw edema was significantly decrease by increasing concentration of BVL-A and BVL-B fractions. In addition, it was found that B. vulgaris possesses antioxidant properties, by its ability to increase antioxidants level.
Conclusions
The results obtained in the present study were indicated that ethyl acetate fraction of B. vulgaris leaves inhibits paw edema and accelerates cutaneous wound healing.
Keywords: Anti-inflammatory, Bambusa vulgaris, Wound healing
1. Introduction
Wound healing is a normal biological process in the human body that includes three overlapping phases: inflammation, tissue formation, and remodeling. It involves soluble mediators, blood cells, parenchymal cells, and extracellular matrix [1]. As the blood components fall into the injury site, the platelets move toward into contact with exposed collagen. This result, the platelets releases clotting factors and essential growth factors. In hemostasis, the neutrophils go in to the wound site and begin phagocytosis to remove bacteria, foreign materials, and damaged tissue. The macrophages appear in the inflammatory phase and continue the phagocytosis process. Once the wound site is cleaned out, fibroblasts migrate to start the tissue formation and deposit new extracellular matrix. The new collagen matrix organized by cross-linking during the final remodeling phase [2].
Herbal medicines are being used by about 80% of the world population for primary health care due to their efficacy, safety, cultural acceptability, and less side effects. The plant constituents are a part of the physiological function of living flora and hence they have better compatibility with the human body [3]. Scientists who are trying to develop newer drugs from natural resources are looking toward the Indian traditional system of medicine, Ayurveda. Numerous drugs from plant, animal and mineral origin are described in the Ayurveda for their wound healing properties under the term Vranaropaka. According to the Ayurveda, Vrana (wounds) is the discontinuation of the lining membrane that leaves a scar for life after healing, closely similar to the modern definition. Similarly, inflammation is an early phase in the pathogenesis of wounds termed Vranashotha. Due to a defect in human functional units, different types of wounds are mentioned in Ayurveda, such as Vata, Pitta, and Kapha types or exogenous due to trauma, such as Chinna (cut wound), Bhinna (perforated wound), Kshata (lacerated wound), Viddha (punctured wound), Picchita (contusion), and Ghrista (abrasion wound). These steps have striking similarities with wounds described in modern medicine [4].
The current treatment for the wounds are the application of silver products, steroids, advanced dressings and skin substitutes, negative pressure wound devices, growth factor, and hyperbaric oxygen [5]. All these therapies are including issues of resistance and are costly. The plant constituents have a dual mechanism of action, not only they enhance wound quality and closure rates, but they can also act as an antimicrobial agent which is an important clinical property in wound healing.
Bambusa vulgaris, commonly known as bamboo is taxonomically a grass, but its habit is tree-like. The leaves of B. vulgaris revealed that it contains crude protein of 10.1%, phosphorus 86.0 mg/100 g, iron 13.4 mg/100 g, vitamin B1 0.1 mg/100 g, vitamin B2 2.54 mg/100 g, and carotene 12.32 mg/100 g [6]. Bamboo leaves have been claimed to be used as an astringent, ophthalmic solution, emmenagogue, vulnerary, and febrifuge to heal the wounds and to control diarrhea in cattle. In ayurvedic medicine, leaves are traditionally used in paralytic complaints and to treat various inflammatory conditions [7]. Evaluation of various plant products according to their traditional uses and medicinal value based on their therapeutic efficacy leads to the discovery of newer and cost-effective drugs for treating various ailments. B. vulgaris has an impressive range of medicinal uses with high nutritional value and serves as a good source of vitamins, proteins, amino acid, beta-carotene, and various phenolics [6], [8]. It was reported to possess antimicrobial action [9], [10], antidiabetic [11], antioxidant [12], and abortifacient properties [13]. It has also been reported to possess antidiabetic activity through antioxidant nature. Stem decoction of B. vulgaris is used to control menstrual pain [14]. Leaves are used in against fever and diabetes [15]. B. vulgaris is used for stomach problems, pain, and internal parasites. It is used for skin problems in Trinidad and Tobago [16]. In Nigerian folklore medicine, bamboo is used as an emmenagogue, abortifacient, appetizer, and for managing respiratory diseases as well as gonorrhea [17]. Leaves and stems are used by the tribes of Raisen, Madhya Pradesh, to treat healing of skin injuries topically. Though the plant and its extracts have been used in the folklore medicine extensively, there is no scientific evidence for wound healing activity of the plant. The present work, therefore, was aimed to evaluate wound healing activity of different fractions of B. vulgaris leaves in rats.
2. Materials and methods
2.1. Animals
Wistar albino rats (150–200 g) of either sex were selected for the experiment. Animals were procured from Defense Research and Development Establishment, Gwalior, MP, India. They were housed individually in well-ventilated, temperature controlled (26 ± 2 °C) animal room for 7 days of the period prior experiment. The animals were given the standard commercial pellet rodent diet (Hindustan Lever Pvt., Ltd., Bengaluru, India) and water ad libitum. The procedures were reviewed and approved by the Institutional Animal Ethics Committee (Reg. No. 1471/P0/a/11/CPCSEA).
2.2. Chemicals and reagents
Petroleum ether (60–80 °C), ethyl acetate, glacial acetic acid, sodium tartrate, copper sulfate, sodium carbonate, hydrochloric acid, chloroform, and perchloric acid were purchased from Ranbaxy Fine Chemicals Ltd., Thane, India. All these chemicals were of analytical grade. Folin-Ciocalteu reagent, Ehlrich reagent, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH) were purchased from Sigma Chemical Co., Ltd., USA. Hydroxyproline, carrageenan, chloramine-T, and trichloracetic acid (TCA) were purchased from Hi Media Laboratories Pvt., Ltd., Mumbai, India.
2.3. Plant material
The leaves of B. vulgaris were collected from village Kukri kheda near to the college campus, during the month of January. Plant material was identified by Dr. M. K. Shrivastava, Department of Botany, Jawaharlal Nehru Krishi Vishwavidyalaya, Jabalpur, M.P., India, and a voucher Specimen (No. BV/472/13) was submitted in the department. The plant materials were dried in the shade, powdered moderately, and pass through sieve No. 10.
2.4. Extraction and fractionation
The powdered leaves (2 kg) were defatted with petroleum ether (60–80 °C) and extracted with ethyl alcohol (95% w/w) for 24 h in soxhlet apparatus. The dried ethyl alcohol extract was suspended in distilled water and extracted with ethyl acetate in a separating funnel repeatedly. The removal of solvent from ethyl acetate fraction yielded a brown powdered product which gave positive Shinoda tests (magnesium hydrochloride reduction test) for the presence of flavonoids. The aqueous layer of the fraction was dried and stored for study. The dried ethyl acetate extract of leaves was referred as BVL-A, and aqueous fraction of leaves was referred as BVL-B, which were subjected to preliminary screening for wound healing and anti-inflammatory activity.
2.5. Phytochemical studies
The phytochemical screenings of ethanol extract were performed to detect the presence of different phytoconstituents by qualitative chemical tests. High-performance thin layer chromatography (HPTLC) fingerprinting of both fractions of B. vulgaris was carried out to study the presence of different phytoconstituents and detect the presence of ascorbic acid in the fractions.
2.6. High-performance thin layer chromatography fingerprinting and quantification of ascorbic acid
The HPTLC fingerprinting of ethyl acetate and aqueous fractions was carried out on a pre-coated silica gel plate (0.2 mm, Merck 60 F-254, Germany) as the stationary phase and chloroform: methanol:glacial acetic acid:water (8:6:1:2) as a mobile phase. The dried fractions were dissolved in methanol (10 mg/ml) and filtered the solutions. The samples (10 μL) of fractions and standard ascorbic acid (Sigma–Aldrich, USA) were spotted in the form of bands of width 6 mm with a 100 μL Hamilton syringe on pre-coated silica gel aluminum plate (10 cm × 10 cm) with the help of Linomat 5 applicator. The applicator was attached to HPTLC system CAMAG which was operated through winCATS software (CAMAG Scientific Inc., USA).
The linear ascending development was carried out in a 20 cm × 10 cm twin through glass chamber saturated with the mobile phase. The developed plate was dried by hot air to evaporate solvents from the plate. The plate was placed in the ultraviolet (UV) chamber and observed at 254 nm. The plate was kept in densitometer (CAMAG Scanner 3) under UV light at 254 nm. The Rf values and finger print data were recorded by WIN CATS software. The operating conditions were as follows: Syringe delivery speed, 10 s/μL; injection volume, 10 μL; band width, 6 mm; space between two bands, 15 mm; start position, 15 mm; lamp, D2; and distance from bottom of plate, 15 mm.
2.7. Experimental protocol and preparation of test sample
The fractions were formulated in 5% w/w ointment by fusion method [18] using simple ointment base BP [19]. Each fraction ointment and povidone iodine ointment USP (Zenith Drugs Pvt., Ltd., India) was applied twice daily to treat different groups of animals. Six animals were taken in each group for study. The Group I was referred as a control group, received only simple ointment base while Group II and III received BVL-A faction ointment with 2.5% and 5% w/w, respectively. Group IV and V were given BVL-B fraction ointment with 2.5% and 5% w/w, respectively. Group VI denoted as reference group that received 5% w/w povidone iodine ointment. The healing property was assessed in terms of physical, biochemical parameters and histopathological study.
2.8. Wound healing activity
2.8.1. Incision wound creation
All animals were anesthetized before wound creation and a 1.5 cm long incision was made through the skin at dorsal portion of rat skin. No local or systemic antimicrobials were used throughout the experiment. The both edges of wound kept together and stitched with black silk surgical thread (No. 000) and a curved needle (No. 22) was used for stitching. Both wound edges were tightened for the good closure of the wound and after stitching, the wound was left undressed. All fractions and reference drug ointment were applied daily up to 9 days; when wounds were healed thoroughly the sutures were removed on the 9th day, and tensile strength of cured wound skin was measured using tensiometer [20].
2.8.2. Excision wound creation
An excision wound was inflicted by cutting away a 500 mm2 full thickness of skin from a traced area and left undressed to the open environment [21]. Wound contraction was measured as percent contraction in each 2 days after wound formation. The wounds were left undressed to the open environment and observed daily. The treatments were applied topically twice a day, starting from the wound induction until complete healing to cover all wounds. Small skin samples were collected on the 18th day, and biochemical estimation (hydroxyproline and protein estimation), and the histopathological study was performed.
2.8.3. Wound breaking strength measurement
Breaking strength is the resistance to breaking under tension that indicates how much the repaired tissue resists to break under tension. It indicated the repaired tissue condition. Before testing, the animals were anesthetized with an open mask. The sutures were removed from the stitched wounds of rats after recovery, and tensile strength was measured. The newly repaired tissue including scar was used to measure the tensile strength by using tensiometer [22]. The tensile strength increment indicates better wound healing stimulation by the applied medicines.
2.8.4. Percent wound contraction and epithelialization time
After wound creation the wound margin was traced at 2 days intervals on transparent graph paper having a millimeter scale that was measured by a caliper with a accuracy of 1/20 mm measurements were continued up to complete healing. Each 2 days interval, healed area was calculated by subtracting initial wound area to the unhealed area [23]. The contraction was represented as percent wound contraction and epithelialization time was observed after complete healing. The rate of healing expressed as percentage contraction:
2.8.5. Protein estimation
On the post-wounding days 18th, the protein content of skin tissues was determined by the method of Lowry et al. [24] The tissue lysate was treated with a mixture of sodium tartrate, copper sulfate and sodium carbonate. The mixture was left to stand for 10 min and then treated with Folin-Ciocalteu reagent that resulted in a bluish color in 20–30 min. The absorbance was taken at 650 nm using Spectrophotometer.
2.8.6. Collagen content measurement
Wound tissues were analyzed on 18th day for hydroxyproline content that is, a basic constituent of collagen. Tissues were dried at 60–70 °C to constant weight in a hot air oven and hydrolyzed in 6 N HCl at 130 °C for 4 h in sealed tubes. The hydrolyzate was neutralized (pH 7) then subjected to Chloramine-T oxidation for 20 min [25]. The reaction was terminated by addition of 0.4 M perchloric acid and a color developed with Ehlrich reagent at 60 °C was read at 557 nm in UV Spectrophotometer (Agilent Technologies, USA).
2.8.7. Enzymatic and non-enzymatic antioxidant assay
The skin tissues were collected from all treated animals and analyzed for antioxidants assay. The tissues were homogenized in phosphate buffer (pH, 7.0) and centrifuged under the cold situation. The clear supernatant was taken to assay of antioxidants level. SOD was assayed [26] based on the inhibition of epinephrine autoxidation by the enzyme. CAT was estimated following the breakdown of hydrogen peroxide [27]. Reduced GSH level was determined by the method of Moron et al. [28] Homogenates mixture was instantly precipitate out with 25% TCA (0.1 ml) and after centrifugation, the precipitate was removed. Free-SH groups were assayed by the addition of 0.6 mM DTNB (2 ml) and 0.9 ml of 0.2 mM sodium phosphate buffer (pH 8.0) to the supernatant (0.1 ml) and the absorbance was read at 412 nm at UV spectrophotometer.
2.8.8. Histopathological study
Animals were anesthetized before taking skin sample using diethyl ether. On the 18th day wound tissue specimen from control, treatment, and reference group were collected, and store in 10% formalin after that usual processing 6 μm thick sections were cut and stained with hematoxylin and eosin [29]. Sections were qualitatively assessed under light microscope and were observed collagen maturation, fibroblast proliferation, epithelialization and angiogenesis.
2.9. Anti-inflammatory activity
Carrageenan-induce rat paw edema model was used for anti-inflammatory study [30] on albino rats. The prepared fractions were applied to the plantar surface of the left hind paw by gently rubbing. After 1 h, inflammation was induced by subplantar injection of 0.1 ml of 1% carrageenan solution in normal saline into the treated paw of all rats. The paw volume of each rat was measured in milliliter using a plethysmometer up to 24 h post-carrageenan injections. The percentage anti-inflammatory activity was calculated using the following equation:
Where, Vt is the paw volume of the test group, and Vc is the paw volume of the control group.
2.10. Statistical analysis
Pharmacological data were represented as the mean ± standard error of the mean for six rats and data were evaluated using the Tukey test. Values of P < 0.01 were considered to be statistically significant.
3. Results
3.1. Phytochemical studies and quantification of ascorbic acid by high-performance thin layer chromatography
The phytochemical analyses of ethanol extract of leaves were gives a positive test for terpenoids, flavonoids, carbohydrates, proteins, tannins, and glycosides. The yield of BVL-A and BVL-B fractions were found 1.13 and 1.82% w/w, respectively.
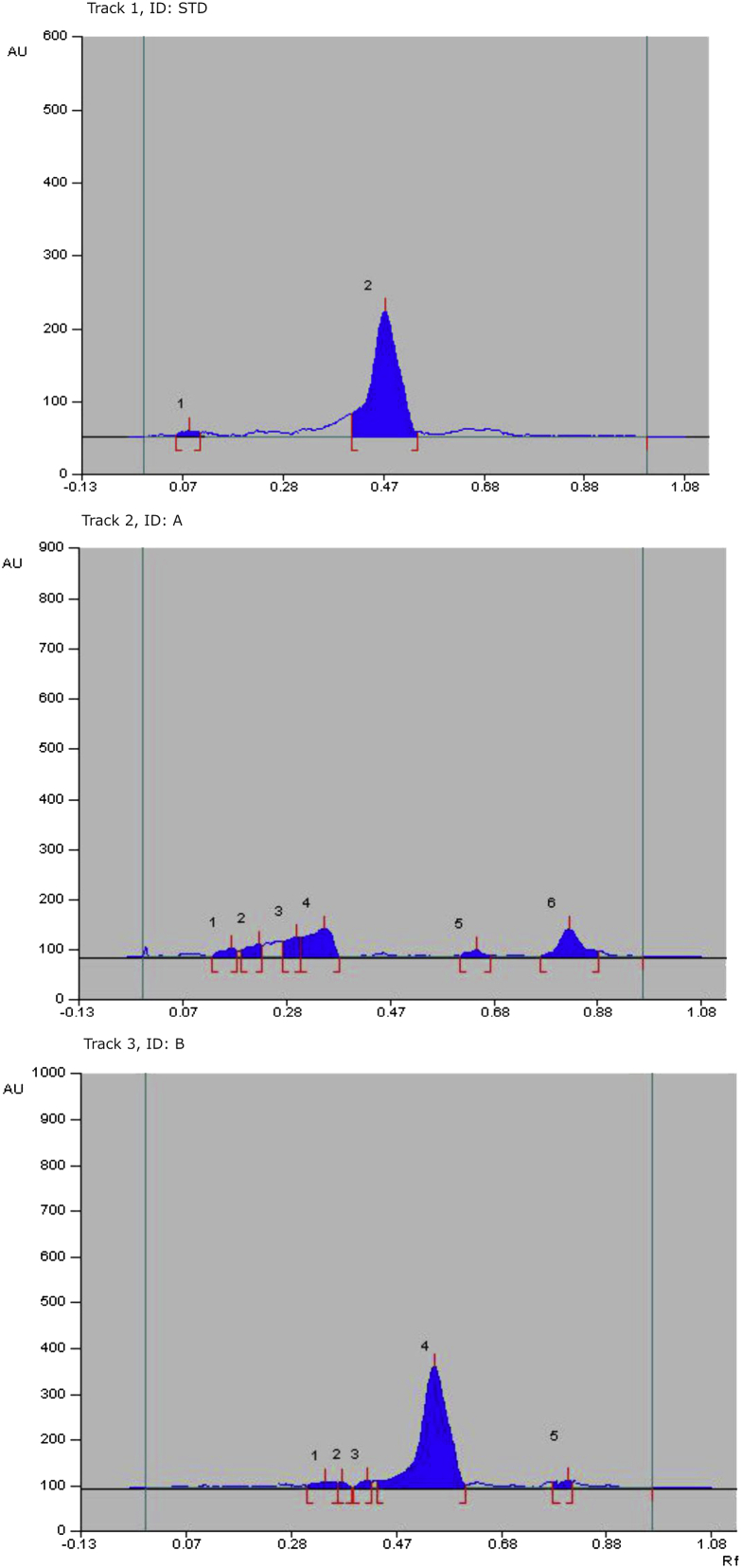
The HPTLC fingerprinting of both fractions of B. vulgaris leaves were revealed several peaks under UV 254 nm and recorded in Table 1 [Fig. 1]. The standard compound ascorbic acid gave single major spot at Rf value 0.48. The BVL-B revealed seven peaks with Rf values in the range of 0.05–0.75 and BVL-A gives nine peaks with Rf range from 0.03 to 0.93. The compound with Rf value 0.48 is identify as ascorbic acid. The densitometry scan was performed for all tracks at 265 nm and identity the presence of ascorbic acid in an aqueous fraction of leaves (BVL-B) of B. vulgaris. The amount of ascorbic acid per gram of leaves extract was found 54.7 mg.
Table 1.
HPTLC fingerprint of different fractions of Bambusa vulgaris leaves.
| Sample | Peak | Maximum Rf | Area |
|---|---|---|---|
| Track 1 – Standard ascorbic acid | 1 | 0.09 | 254.5 |
| 2 | 0.48 | 17503.2 | |
| Track 2 – A (BVL-A) | 1 | 0.1 | 487.4 |
| 2 | 0.17 | 1007.6 | |
| 3 | 0.31 | 1384.1 | |
| 4 | 0.38 | 2404.5 | |
| 5 | 0.62 | 625.3 | |
| 6 | 0.82 | 1935.2 | |
| Track 3 – B (BVL-B) | 1 | 0.25 | 302.0 |
| 2 | 0.31 | 576.4 | |
| 3 | 0.38 | 452.6 | |
| 4 | 0.48 | 19181.4 | |
| 5 | 0.80 | 328.2 |
Fig. 1.
High-performance thin layer chromatography fingerprint of different fractions with standard ascorbic acid. Track 1: Standard ascorbic acid; Track 2: A (BVL-A); Track 3: B (BVL-B).
3.2. Wound healing activity
3.2.1. Wound breaking strength measurement
The tensile strength indicates how much the repaired tissue resists to breaking under tension and may indicate in part the quality of repaired tissue. The results of the measurement of tensile strength on day 9th were shown in Table 2. The tensile strength of the animals treated with 5% w/w of BVL-A was found significantly greater than the control group and comparable to the reference group.
Table 2.
Effect of different fractions of Bambusa vulgaris on tensile strength of incision wound in rats.
| Groups | Breaking strength (gm/cm2) |
|---|---|
| Control (Base) | 437.3 ± 8.16 |
| BVL-A (2.5%w/w) | 735.7 ± 13.52* |
| BVL-A (5%w/w) | 939.4 ± 22.74** |
| BVL-B (2.5%w/w) | 596.3 ± 11.28 |
| BVL-B (5%w/w) | 685.4 ± 12.38* |
| Povidone-iodine ointment | 942.7 ± 23.52** |
n = 6 albino rats per group, the value represents mean ± SEM. *P < 0.01, **P < 0.001, when compared each treated group with the control group. SEM: Standard error of mean.
3.2.2. Determination of percent wound contraction
The measurement takes in each 2 days interval after extracts application continued until the wound was completely healed. On day 6–12 wound treated with 5% w/w of BVL-A exhibited significant (P < 0.01) increase in the percent wound contraction as compared to control group [Table 3]. Wound treated with BVL-B from 2 to 8 days, was not increased contraction significantly in comparison to control. From 14 to 18th day, the group treatment with BVL-A was observed significant (P < 0.01) difference in the percentage of wound contraction when compared to control group and comparable to the reference group of animals. On day 18th no scars were observed in animal treated with 5% w/w of BVL-A and reference group, which was an indication for complete healing.
Table 3.
Effect of different fractions of Bambusa vulgaris on percentage wound contraction and epithelialization period of excision wound in rats.
| Animal groups | Post wounding days |
Epithelialization period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | ||
| Control | 12.13 ± 1.82 | 19.11 ± 1.34 | 28.19 ± 1.54 | 38.68 ± 2.51 | 44.92 ± 2.81 | 49.76 ± 2.83 | 53.47 ± 2.76 | 58.65 ± 2.48 | 67.67 ± 2.86 | 24 |
| BVL-A (2.5% w/w) | 13.47 ± 1.76 | 21.53 ± 1.72 | 30.28 ± 2.46 | 42.31 ± 2.84 | 50.15 ± 2.67 | 57.28 ± 2.32 | 68.76 ± 3.20 | 77.64 ± 3.26 | 85.23 ± 3.62 | 20 |
| BVL-A (5% w/w) | 15.12 ± 1.29 | 35.28 ± 1.85 | 41.29 ± 2.61 | 55.82 ± 2.94 | 68.37 ± 2.85 | 79.24 ± 2.68 | 86.34 ± 3.72 | 94.28 ± 4.46* | 100.27 ± 4.27* | 18 |
| BVL-B (2.5% w/w) | 14.51 ± 1.25 | 23.20 ± 1.84 | 30.76 ± 1.87 | 39.65 ± 2.16 | 54.84 ± 2.76 | 61.35 ± 2.34 | 65.42 ± 3.18 | 71.27 ± 3.43 | 80.53 ± 3.52* | 22 |
| BVL-B (5% w/w) | 14.47 ± 1.27 | 25.57 ± 1.94 | 31.24 ± 1.92 | 41.02 ± 2.61 | 52.17 ± 2.94 | 59.54 ± 2.46 | 65.64 ± 3.21 | 72.28 ± 3.21 | 78.37 ± 3.18 | 22 |
| Povidone iodine ointment | 15.49 ± 1.35 | 32.57 ± 1.83 | 44.15 ± 2.15 | 53.84 ± 2.57 | 65.92 ± 2.98 | 77.19 ± 3.05 | 88.38 ± 3.52 | 96.28 ± 4.38* | 100.35 ± 3.94* | 18 |
n = 6 albino rats per group, tabular value represents mean ± SEM. *P < 0.01 as compared to group control and reference at respective days. SEM: Standard error of mean.
3.2.3. Protein estimation
The protein content of granulation tissues indicates the level of protein synthesis and cellular proliferation. The group 5% w/w of BVL-A (92.38 ± 4.82) and reference ointment (89.97 ± 4.10) treated group of animals were found significantly higher protein content when compared to control group (45.49 ± 1.57) of animals. The higher protein content of treated animals suggest that BVL-A through an unknown mechanism, stimulate cellular proliferation [Table 4].
Table 4.
Effect of Bambusa vulgaris fractions on hydroxyproline and protein content of tissues from incision wound in rats.
| Animal groups | Hydroxyproline content (mg/g tissues) | Protein content (mg/g tissues) |
|---|---|---|
| Control (Base) | 31.37 ± 0.82 | 45.49 ± 1.57 |
| BVL-A (2.5% w/w) | 51.42 ± 1.97 | 73.54 ± 2.90* |
| BVL-A (5% w/w) | 78.63 ± 3.28* | 92.38 ± 4.82** |
| BVL-B (2.5% w/w) | 55.26 ± 2.24 | 59.37 ± 2.51 |
| BVL-B (5% w/w) | 61.54 ± 2.17 | 63.57 ± 2.48 |
| Povidone iodine ointment | 75.14 ± 2.89* | 89.97 ± 4.10** |
n = 6 albino rats per group, value represents mean ± SEM. *P < 0.01, **P < 0.001, when compared each treated group with control group in respective parameters. SEM: Standard error mean.
3.2.4. Collagen content measurement
The breakdown of collagen liberates free hydroxyproline and its peptide. Measurement of hydroxylproline, therefore, has been used as an index of collagen turnover. The hydroxyproline content was determined in small tissue specimen collected from each group of animals. The hydroxyproline level was found significantly increase in the group treated with 5% w/w of BVL-A and BVL-B (5% w/w), when compared to control group [Table 4].
3.2.5. Enzymatic and non-enzymatic antioxidant assay
The level of antioxidant substances in skin tissues are given in Table 5. A significant decrease in antioxidants level was observed in the control group as well as in 2.5% w/w of BVL-A, and 2.5% w/w of BVL-B ointment treated animals when compared with the reference group. The slightly increased in the level of SOD, CAT, and GSH were found in the treatment group of 5% w/w BVL-B ointment. The antioxidants (SOD and GSH) level of BVL-A (5% w/w) and reference ointment treated animals were improved significantly up on complete healing. BVL-B (5% w/w) treatment group also showed increased level of SOD and CAT.
Table 5.
Effect of Bambusa vulgaris fractions on antioxidants level of tissues from excision wound.
| Animal groups | Enzymatic and nonenzymatic antioxidant levels |
||
|---|---|---|---|
| SOD (μg/50 mg tissue) | CAT (μmol/50 mg tissue) | GSH (μmol/50 mg tissue) | |
| Control (Base) | 14.27 ± 0.21 | 12.72 ± 0.09 | 16.43 ± 0.82 |
| BVL-A (2.5% w/w) | 25.31 ± 0.76 | 19.13 ± 0.42 | 20.28 ± 0.94 |
| BVL-A (5% w/w) | 30.85 ± 1.24** | 24.08 ± 1.62** | 35.52 ± 1.83** |
| BVL-B (2.5% w/w) | 18.62 ± 0.82 | 14.64 ± 0.73 | 21.76 ± 1.27 |
| BVL-B (5% w/w) | 22.94 ± 0.96* | 17.43 ± 0.54* | 24.34 ± 1.64* |
| Povidone iodine ointment | 32.54 ± 1.35** | 27.07 ± 1.69** | 39.53 ± 2.40** |
n = 6 albino rats per group, the value represents mean ± SEM. *P < 0.01, **P < 0.001, when compared each treated group with the control group in respective parameters. SEM: Standard error of mean, SOD: Superoxide dismutase, CAT: Catalase, GSH: Glutathione.
3.2.6. Histopathological study
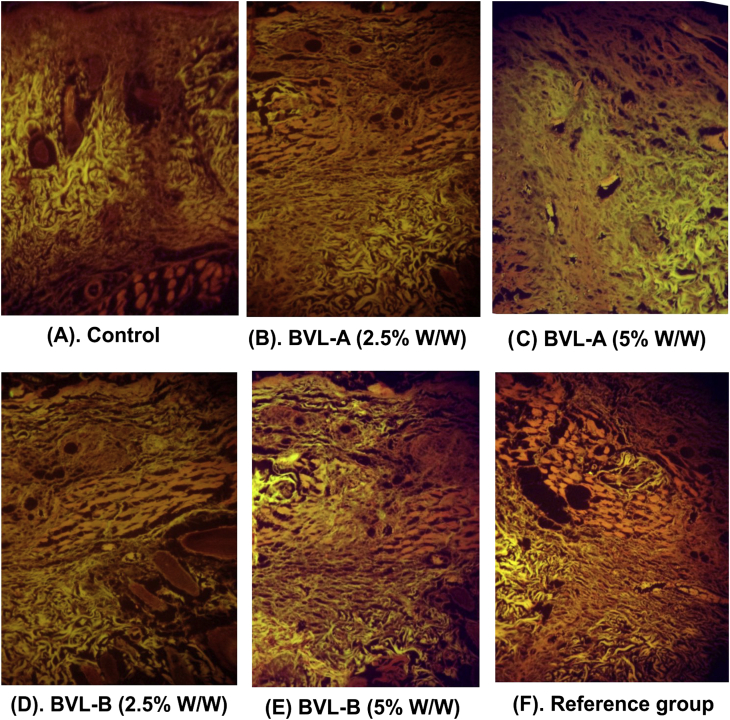
In the histopathological study, on day 18th the skin tissues treated with 5% w/w BVL-A as well as reference ointment were found to cover by new epidermal layers. The proliferation of fibrous tissue, collagen and capillaries with epidermal covering at the margin of the wound was observed. A large number of fibroblast was clearly observed in the dermis, and the process of epidermal regeneration was complete. The control group presented monocyte cells, edema, and cellular necrosis [Fig. 2].
Fig. 2.
Photomicrograph of skin tissues after 18th post-wounding days with different fraction treatment in rats.
3.3. Anti-inflammatory activity
The anti-inflammatory effects were observed in carrageenan-induced paw edema in rats. All fractions and reference ointment were applied topically to rat paw and percent inhibition was observed significantly increase in 5% w/w BVL-A ointment group and comparable to reference group of animals on 3 to 5th h after induction. Indomethacin showed a 61.9% of inhibitory effect at 3 h and inhibition of edema of 73.9% at time 4 h after induction of inflammation [Table 6].
Table 6.
Effect of Bambusa vulgaris fractions on carrageenan-induced rat paw edema in rats.
| Animal groups | Carrageenan-induced rat paw edema mean ± SEM (percentage inhibition of paw volume) |
|||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 24 h | |
| Control | 0.71 ± 0.05 | 0.84 ± 0.06 | 1.13 ± 0.09 | 1.15 ± 0.10 | 0.89 ± 0.07 | 0.67 ± 0.04 |
| BVL-A (2.5% w/w) | 0.49 ± 0.02 (30.9) | 0.52 ± 0.04 (38.0) | 0.60 ± 0.01 (46.9) | 0.48 ± 0.06 (58.2)* | 0.44 ± 0.01 (50.5) | 0.41 ± 0.03 (38.8) |
| BVL-A (5% w/w) | 0.29 ± 0.01 (59.1) | 0.32 ± 0.02 (61.9) | 0.41 ± 0.03 (63.7) | 0.28 ± 0.01 (75.6)* | 0.23 ± 0.01 (74.1) | 0.35 ± 0.02 (47.7) |
| BVL-B (2.5% w/w) | 0.54 ± 0.05 (23.9) | 0.67 ± 0.06 (20.2) | 0.74 ± 0.04 (34.5) | 0.62 ± 0.05 (46.0) | 0.49 ± 0.02 (44.9) | 0.45 ± 0.03 (32.8) |
| BVL-B (5% w/w) | 0.52 ± 0.03 (26.7) | 0.65 ± 0.04 (22.6) | 0.72 ± 0.05 (36.2) | 0.58 ± 0.03 (49.5)* | 0.47 ± 0.03 (47.2) | 0.51 ± 0.03 (23.8) |
| Povidone iodine ointment | 0.33 ± 0.02 (53.5) | 0.37 ± 0.02 (55.9) | 0.43 ± 0.03 (61.9) | 0.3 ± 0.02 (73.9)* | 0.33 ± 0.02 (62.9) | 0.28 ± 0.02 (58.2) |
n = 6 albino rats per group, the value represents mean ± SEM. *P < 0.01, when compared each treated group with the control group in respective parameters. SEM: Standard error mean.
4. Discussion
The objective of present study was to evaluate the healing property of ethanol extract of B. vulgaris leaves in rats. The phytochemical work concluded that ethanol extract of B. vulgaris leaves contains flavonoids, which have been documented to have antibacterial activity and free radical scavenging effect [31].
In the present study, the effect of B. vulgaris extract was observed on tissue regeneration and strength using experimental animal models. No clinical data have been reported with this plant product for wound healing property. The main objective of wound management is to heal the injury in the shortest possible time with minimal pain and discomfort to the patient. At the site of the wound, a flexible fine scar with maximal tensile strength is desired. The wound was treated topically with prepared ointments and observed that after 4th day, dead tissue was removed. After 8 days, the swelling and redness was minimized which indicates that the prepared ointment has tissue debride effect at the wound site. According to Acharya Sushruta, “Kalka” (application of paste) is a major dosage form for wound healing located in muscle and included slough. The paste makes both functions of “Shodhana” (cleaning) and “Ropana” (healing) in Dushta Vrana [32]. Kashaya rasa has Grahi and Stambhana properties which helped to stop discharge from the wound. Madhura Rasa (sweet taste) gives nutrition to the wound tissue, which support in granulation tissue development [33]. B. vulgaris has Kashaya rasa (astringent) which provides Lekhana (scraping) that helps to slough out necrosed tissue and preparing the wound for healing. B. vulgaris has slight sweet-bitter taste due to presence of carbohydrates, vitamins, and proteins [6]. Initially at the time of injury, the patient had pain, discharge, redness, and swelling at the surrounding area with predominating tridosha (Vata, Kapha and Pitta) [34]. Madhura Rasa of B. vulgaris reduced the vitiated Vata Dosha, which leads to reduced pain and enhanced wound healing. Thus, B. vulgaris has healing properties to the skin wounds by virtue of its Sodhana (purification), Ropana (healing), and Sandhana (union) actions.
Moisture provides a transparent medium for essential growth factors during epithelialization. Wound healing in ayurveda is based on moisture preservation. Rasa is the key nutritive element for the regeneration of all tissues. Maintaining Rasa integrity entails adequate preservation of moisture at the wound site [35]. Use of simple ointment base could be beneficial due to the properties such as Picchaila (lubricous), Madhura (balances the skin pH), Balya (improves tensile strength of the skin), Grahi (moisture holding capacity of skin), and useful in Vata disorders including dryness [36]. Collagen imparts tensile strength and elasticity to healed skin. An increase in collagen content in the treated wounds corresponds with the significantly increased tensile strength in both treated groups of incision wounds over that in the untreated wounds [37]. Therefore, the tensile strength of a wound can be related to its collagen formation and maturation. The protein content of granulation tissue is said to indicate the levels of protein synthesis and cell proliferation. An increase in protein content is due to an increase in collagen synthesis [38], [39]. In the present study, the total protein content of treatment groups was increased; it implies that the treatment stimulates cell proliferation. In the histopathological study, the injury treated with BVL-A as well as reference ointment was found to be covered by the new epidermis. The proliferation of collagen and capillaries with epidermal covering at the margin of the wound was observed. Formation of dense fibrous tissue and arrangement of blood capillaries was perpendicular to the fibrous tissue; mature connective tissue was observed in BVL-A and reference group of animals.
The microenvironment of the wound is quite hypoxic due to vascular disruption and high oxygen consumption by metabolically active cells. Several systemic conditions, including old age and diabetes, can cause impaired vascular flow that produced poor tissue oxygenation. This overlay of poor perfusion creates a hypoxic wound [40]. The proper oxygen level is crucial for optimum wound healing. Hypoxia is required to stimulate wound healing at initial stages, such as angiogenesis and release of growth factors while oxygen is needed to continue the healing process [41]. In summary, the impaired healing that occurs in diabetic condition involves hypoxia, dysfunction in fibroblasts, neovascularization, impaired angiogenesis and high levels of metalloproteases. The antioxidants are known to quench the superoxide radical and thus prevent the damage of cells caused by free radicals. There is plenty of evidence to suggest that increased production of reactive oxygen, lipid peroxidation and ineffective scavenging play a crucial role in various skin lesions and in the modulation of fibroblast proliferation [42]. Cutaneous wounding causes a depression in the overall antioxidant status making it more vulnerable to oxygen radical attack [43], [44]. All these finding indicate that antioxidants may play an important role in wound healing.
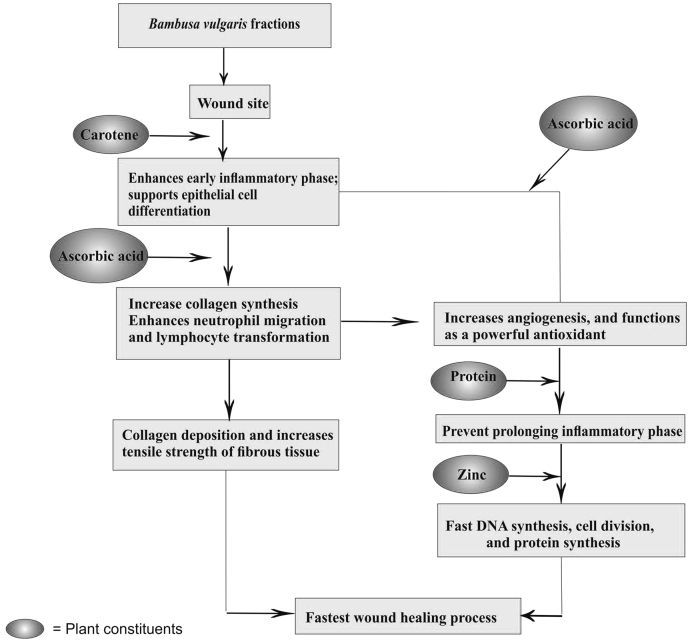
A significant increase in hydroxyproline content, which was a reflection of increased collagen levels, that was further supported by histopathological evidence. This indicates improved collagen maturation by increased cross-linking. Flavonoids have been shown to increase collagen synthesis, support the cross-linking of collagen, and decrease the degradation of soluble collagen. This accelerates the conversion of soluble collagen to insoluble collagen, and inhibit the catabolism of soluble collagen [45], [46]. From a clinical aspects, collagen deposition in the wound is the most essential phase of healing. Facilitating oxygen diffusion, diminishing oxygen free radical overproduction, and increases collagen synthesis were together found for improve healing [47]. Collagen is a major protein of the extracellular matrix and is the component that ultimately contributes to wound strength. Breakdown of collagen produces free hydroxyproline. Measurement of this hydroxylproline, therefore, has been used as an index of collagen turnover [48]. The increased hydroxyproline content of the excision wound has indicated faster collagen turnover leading to rapid wound healing. The differences in severity of vascular and cellular changes between the wounds treated with various medicaments might be due to different chemical constituents in medicaments. Ascorbic acid is an essential cofactor for the collagen synthesis, proteoglycans, and other organic components of the intracellular matrix of tissues such as skin, capillary walls, bones and other connective tissues. It promotes adhesion of endothelium cells and increases the tensile strength of the fibrous tissue. Ascorbic acid is necessary for the hydroxylation of proline and lysine residues in procollagen, which is required for its release and conversion to collagen [Fig. 3]. In addition to collagen production, ascorbic acid increases angiogenesis, enhances neutrophil function and functions as a powerful antioxidant. Zn required for DNA synthesis, cell division, and protein synthesis [49]. Increases in the level of SOD, CAT, and GSH were observed in tissues collected from excision wounds. These enzymes are also known to quench the superoxide radical and thus prevent the damage of cells caused by free radicals. The antioxidant and anti-inflammatory properties of B. vulgaris, makes it an exclusive plant for wound healing [12], [50]. B. vulgaris is a rich source of vitamin C (ascorbic acid), which is a potent antioxidant [8]. It is potent anti-aging drug (vayasthaprana); it has properties like Rasayana (adaptogenic), ayushprada (prolongs cell life), ajara (usefulness in pre-mature aging) and sandhana karaka (improves cell migration and cell binding) [51]. In the present study, topical application of leaves extract caused acceleration in wound healing with marked collagen synthesis and fully regenerated epithelial layer in comparison to the control group. It may be due to the decreased expression of inflammatory cytokines and increased levels of anti-inflammatory cytokines at the wound site. The anti-inflammatory activity of B. vulgaris fractions was further confirmed by in vivo testing using carrageenan-induced edema. Based on these findings, we propose that the anti-inflammatory and antioxidant potential of B. vulgaris accelerated wound healing in rats.
Fig. 3.
Schematic diagram of possible mechanism for wound healing by topical application of Bambusa vulgaris leaves fractions.
5. Conclusions
The observations and results obtained in the present study indicated that flavonoid fraction of B. vulgaris leaves possesses a definite healing action. The aqueous fraction of leaves was containing amino acids, vitamins and ascorbic acid have also reported healing promoter by the researchers. This is demonstrated by a significant increase in the rate of wound contraction and by enhanced epithelization. Thus, the present study reveals that one of the mechanisms of the wound healing by this plant may be its antioxidant property. These findings could justify, the inclusion of this plant in the management of wound healing in folk medicine.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge to Madhya Pradesh Council of Science and Technology (File No. 3587/CST/R&D/Bio. Proj./2012), Bhopal (India), for financial support for this research work.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Falabella A.F., Kirsner R.S. Taylor & Francis Group; Boca Raton: 2005. Wound healing: historical aspects of wound healing; pp. 1–2. [Google Scholar]
- 2.Diegelmann R.F., Evans M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 3.Kamboj V.P. Herbal medicine. Curr Sci. 2000;78:35–51. [Google Scholar]
- 4.Biswas T.K., Mukherjee B. Plant medicines of Indian origin for wound healing activity: a review. Int J Low Extrem Wounds. 2003;2:25–39. doi: 10.1177/1534734603002001006. [DOI] [PubMed] [Google Scholar]
- 5.Murphy P.S., Evans G.R. Advances in wound healing: a review of current wound healing products. Plast Surg Int. 2012;2012:190436. doi: 10.1155/2012/190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamolang F.N., Lopez F.R., Semana J.A., Casin R.F., Espiloy Z.B. Properties and utilization of Philippine bamboos. In: Lessard G., Chouinard A., editors. Bamboo research in Asia. IDRC; Ottawa: 1980. pp. 189–200. [Google Scholar]
- 7.Kirtikar K.R., Basu B.D. International Book Distributors; New Delhi: 1990. Indian medicinal plants; pp. 2724–2727. [Google Scholar]
- 8.Abidemi O.O. Proximate composition and vitamin levels of seven medicinal plants. Int J Eng Sci Invent. 2013;2:47–50. [Google Scholar]
- 9.Owokotomo I.A., Owoeye G. Proximate analysis and antimicrobial activities of Bambusa vulgaris L. leaves' beverage. Afr J Agric Res. 2011;6:5030–5032. [Google Scholar]
- 10.Kennard W.C., Freyre R.H. The edibility of shoots of some bamboos growing in Puerto Rico. Econ Bot. 1957;11:235–243. [Google Scholar]
- 11.Senthilkumar M.K., Sivakumar P., Faisal C., Rajesh V., Perumal P. Evaluation of anti-diabetic activity of Bambusa vulgaris leaves in streptozotocin induced diabetic rats. Int J Pharm Sci Drug Res. 2011;3:208–210. [Google Scholar]
- 12.Goyal A.K., Middha S.K., Sen A. Bambusa vulgaris Schrad. ex J. C. Wendl. var. vittata Riviere & C. Riviere leaves attenuate oxidative stress – an in vitro biochemical assay. Indian J Nat Prod Resour. 2013;4:436–440. [Google Scholar]
- 13.Yakubu M.T., Bukoye B.B. Abortifacient potentials of the aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Contraception. 2009;80:308–313. doi: 10.1016/j.contraception.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Rai R., Nath V. World forestry congress XII, 21 to 28 September 2003 in Québec, Canada. 2003. Use of medicinal plants by traditional herbal healers in Central India. [Google Scholar]
- 15.Pullaiah T., Naid C.K. Daya Books; New Delhi: 2003. Antidiabetic plants in India and herbal based antidiabetic research; p. 98. [Google Scholar]
- 16.Lans C. Comparison of plants used for skin and stomach problems in Trinidad And Tobago with Asian ethnomedicine. J Ethnobiol Ethnomed. 2007;3:3. doi: 10.1186/1746-4269-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill L.S. Uniben Press; Benin, Nigeria: 1992. Ethnomedical uses of plants in Nigeria; pp. 35–36. [Google Scholar]
- 18.Jain N.K., Sharma S.N. 4th ed. Vallabh Prakashan; New Delhi: 1998. A textbook of professional pharmacy; pp. 261–270. [Google Scholar]
- 19.Anonymous . The Pharmaceutical Press; 1953. British pharmacopoeia, General Medical Council. 17 Bloomsbury Square London. WCI 396. [Google Scholar]
- 20.Hemalata S., Subramanian N., Ravichandran V., Chinnaswamy K. Wound healing activity of Indigofera ennaphylla Linn. Indian J Pharm Sci. 2001;63:331–333. [Google Scholar]
- 21.Taranalli A.D., Tipare S.V., Kumar S., Torgal S.S. Wound healing activity of Oxalis corniculata whole plant extract in rats. Indian J Pharm Sci. 2004;66:444–446. [Google Scholar]
- 22.Rashed A.N., Afifi F.U., Disi A.M. Simple evaluation of wound healing activity of a crude extract of Portuloca oleracea L. (growing in Jorden) in Mus musculus JVI-1. J Ethnopharmacol. 2003;88:131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 23.Kuwano H., Yano K., Ohano S., Ikebe M., Kitampura K., Toh Y. Dipyridamole inhibits early wound healing in rat skin incisions. J Surg Res. 1994;56:267–270. doi: 10.1006/jsre.1994.1042. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Woessner J.F. The determination of hydroxyproline in tissue and protein samples containing small portion of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 26.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 27.Beers R.F., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 28.Moron M.A., Depierre J.W., Mannervick B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 29.Bancroft J.D., Gamble M. 6th ed. Churchill Livingstone, Elsevier; Philadelphia, PA: 2008. Theory and practice of histological techniques; pp. 93–133. [Google Scholar]
- 30.Maswadeh H.M., Semreen M.H., Naddaf A.R. Anti-inflammatory activity of Achillea and Ruscus topical gel on carrageenan-induced paw edema in rats. Acta Pol Pharm Drug Res. 2006;63:277–280. [PubMed] [Google Scholar]
- 31.Devi P.S., Shyamala D.C.S. Protective effect of quercetin in cisplatin induced cell injury in the kidney. Indian J Pharmacol. 1999;31:422. [Google Scholar]
- 32.Ajmeer A.S., Dudhamal T.S., Gupta S.K., Mahanta V. Katupila (Securinega leucopyrus) as a potential option for diabetic wound management. J Ayurveda Integr Med. 2014;5:60–63. doi: 10.4103/0975-9476.128872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudhamal T.S., Gupta S.K., Bhuyan C. Role of honey (Madhu) in the management of wounds (Dushta Vrana) Int J Ayurveda Res. 2010;1:271–273. doi: 10.4103/0974-7788.76793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dash B., Kashyap L. Concept Publishing Company; New Delhi: 1980. Basic principles of Ayurveda; pp. 212–213. [Google Scholar]
- 35.Vinaya P.N., Prasad J.S. Time framework and insights from Ayurveda on wound management. Int J Res Ayurveda Pharm. 2014;5:561–565. [Google Scholar]
- 36.Datta H.S., Mitra S.K., Patwardhan B. Wound healing activity of topical application forms based on Ayurveda. Evid Based Complement Altern Med. 2011;2011:134378. doi: 10.1093/ecam/nep015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer A.J., Clark R.A. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 38.Bourguignon G.J., Bourguignon L.Y. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J. 1987;1:398–402. doi: 10.1096/fasebj.1.5.3678699. [DOI] [PubMed] [Google Scholar]
- 39.Rasik A.M., Raghubir R., Gupta A., Shukla A., Dubey M.P., Srivastava S. Healing potential of Calotropis procera on dermal wounds in Guinea pigs. J Ethnopharmacol. 1999;68:261–266. doi: 10.1016/s0378-8741(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 40.Tandara A.A., Mustoe T.A. Oxygen in wound healing – more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 41.Bishop A. Role of oxygen in wound healing. J Wound Care. 2008;17:399–402. doi: 10.12968/jowc.2008.17.9.30937. [DOI] [PubMed] [Google Scholar]
- 42.Murrell G.A., Francis M.J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukla A., Rasik A.M., Patnaik G.K. Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defence enzymes in a healing cutaneous wound. Free Radic Res. 1997;26:93–101. doi: 10.3109/10715769709097788. [DOI] [PubMed] [Google Scholar]
- 44.Lodhi S., Pawar R.S., Jain A.P., Jain A., Singhai A.K. Effect of Tephrosia purpurea (L) Pers. on partial thickness and full thickness burn wounds in rats. J Complement Integr Med. 2010;7:13. [Google Scholar]
- 45.Stipcevic T., Piljac J., Vanden Berghe D. Effect of different flavonoids on collagen synthesis in human fibroblasts. Plant Foods Hum Nutr. 2006;61:29–34. doi: 10.1007/s11130-006-0006-8. [DOI] [PubMed] [Google Scholar]
- 46.Galicka A., Nazaruk J. Stimulation of collagen biosynthesis by flavonoid glycosides in skin fibroblasts of osteogenesis imperfecta type I and the potential mechanism of their action. Int J Mol Med. 2007;20:889–895. [PubMed] [Google Scholar]
- 47.Inan A., Sen M., Koca C., Akpinar A., Dener C. The Effect of purified micronized flavonoid fraction on the healing of anastomoses in the colon in rats. Surg Today. 2006;36:818–822. doi: 10.1007/s00595-006-3251-4. [DOI] [PubMed] [Google Scholar]
- 48.Lodhi S., Jain A.P., Sharma V.K., Singhai A.K. Wound-healing effect of flavonoid-rich fraction from Tephrosia purpurea Linn. on streptozotocin-induced diabetic rats. J Herbs Spices Med Plants. 2013;19:191–205. [Google Scholar]
- 49.MacKay D., Miller A.L. Nutritional support for wound healing. Altern Med Rev. 2003;8:359–377. [PubMed] [Google Scholar]
- 50.Carey W.M., Dasi J.M., Rao N.V., Gottumukkala K.M. Anti-inflammatory activity of methanolic extract of Bambusa vulgaris leaves. Int J Green Pharm. 2009;3:234–238. [Google Scholar]
- 51.Sharma L., Agarwal G., Kumar A. Medicinal plants for skin and hair care. Indian J Tradit Knowl. 2003;2:62–68. [Google Scholar]