Abstract
Malassezia pachydermatis is a relatively rare agent of bloodstream infections. We describe an unusual case of Malassezia fungemia in an adult patient hospitalized for Staphylococcus aureus bacteremia who was also found to have multibacillary leprosy. Treatment of the patient required extensive medical management but resulted in a good outcome.
Keywords: Malassezia pachydermatis, Fungemia, Hansen's disease
1. Introduction
Lipophilic yeasts of the genus Malassezia have been recognized as members of the normal skin flora as well as agents of numerous dermatologic and systemic infectious conditions [1]. Malassezia pachydermatis has mostly been associated with infections in neonates. Intravascular catheters, administration of lipid supplemented parenteral nutrition, prematurity, and use of broad-spectrum antibiotics have been implicated as risk factors for infection [2]. M. pachydermatis is a relatively rare agent of bloodstream infections in adults. To our knowledge, only two cases of fungemia due to this organism have been described to date in adults. In both prior cases, the patients were being treated for acute myeloid leukemia [3], [4]. We describe a case of M. pachydermatis fungemia in a man who was also diagnosed with lepromatous leprosy during his hospitalization.
2. Case
A 53 year old Caucasian male from rural east Texas presented to the Emergency Department of our tertiary care University hospital (day 0) with pleuritic chest pain, dyspnea, and fever that started 1 d prior to admission. He had a past medical history of endocarditis with mitral valve annular repair and bioprosthetic aortic valve replacement, hepatitis C, epilepsy, congestive heart failure, and bipolar disorder. He was hospitalized a week earlier at a different medical center where he was treated for possible Staphylococcus aureus pneumonia and discharged on clindamycin and metronidazole. His home medications included aspirin, carvedilol, lisinopril, spironolactone, levetiracetam and trazodone. His social history was notable for intravenous use of various drugs including methamphetamine and cocaine.
At presentation his temperature was 37.6 °C, blood pressure 119/66 mm Hg, pulse 90 beats/min, and respirations 16 breaths/min with an oxygen saturation of 99% on 2 L nasal cannula. He appeared alert and was in no acute distress. Cardiovascular exam revealed a 3/6 systolic murmur in the right upper sternal border and axilla. Lungs were clear to auscultation and his abdominal exam was normal. He had mild peripheral edema bilaterally without jugular venous distension. His skin was notable for a rash with multiple erythematous patches and plaques on his forearms, chest, abdomen, and thighs. Laboratory tests revealed normocytic anemia (hemoglobin 10.3 g/dL); leukocytosis (13,100/mm3); thrombocytosis (357,000/mm3); elevated NT-proBNP (1090 pg/mL); and hypoalbuminemia (3.1 g/dL). He had preserved renal function, normal liver enzymes, and HIV antibody testing was negative. A chest radiograph was normal and electrocardiogram showed normal sinus rhythm with a heart rate of 95 and a prolonged QTc interval of 515 ms. He was admitted to the internal medicine service for further evaluation.
On day 2 the patient became febrile, hemodynamically unstable, and multiple sets of blood cultures flagged positive on the automated blood culture system in use at our institution (BACTEC FX, BD). The organism was identified as methicillin susceptible Staphylococcus aureus (MSSA). The patient was started on intravenous (IV) nafcillin for MSSA sepsis. Transthoracic and transesophageal echocardiograms did not reveal findings suggestive of recurrence of endocarditis.
On day 3 the patient improved. Repeat blood cultures obtained on day 7 were sterile and a midline catheter was placed in his right arm for ongoing antibiotic therapy. His rash was evaluated further by punch biopsy and showed panniculitis with marked inflammation and abundant Fite positive bacilli consistent with mycobacteria (Fite's method of staining acid fast bacilli takes longer than the traditional Ziehl-Neelsen method, but has higher sensitivity for detecting organisms). Multibacillary leprosy was suspected, so tissue samples were sent to the National Hansen's Disease Program (NHDP) reference lab (Baton Rouge, LA) for further evaluation. On further questioning, the patient admitted to handling armadillos (a known reservoir for Mycobacterium leprae) as a young man.
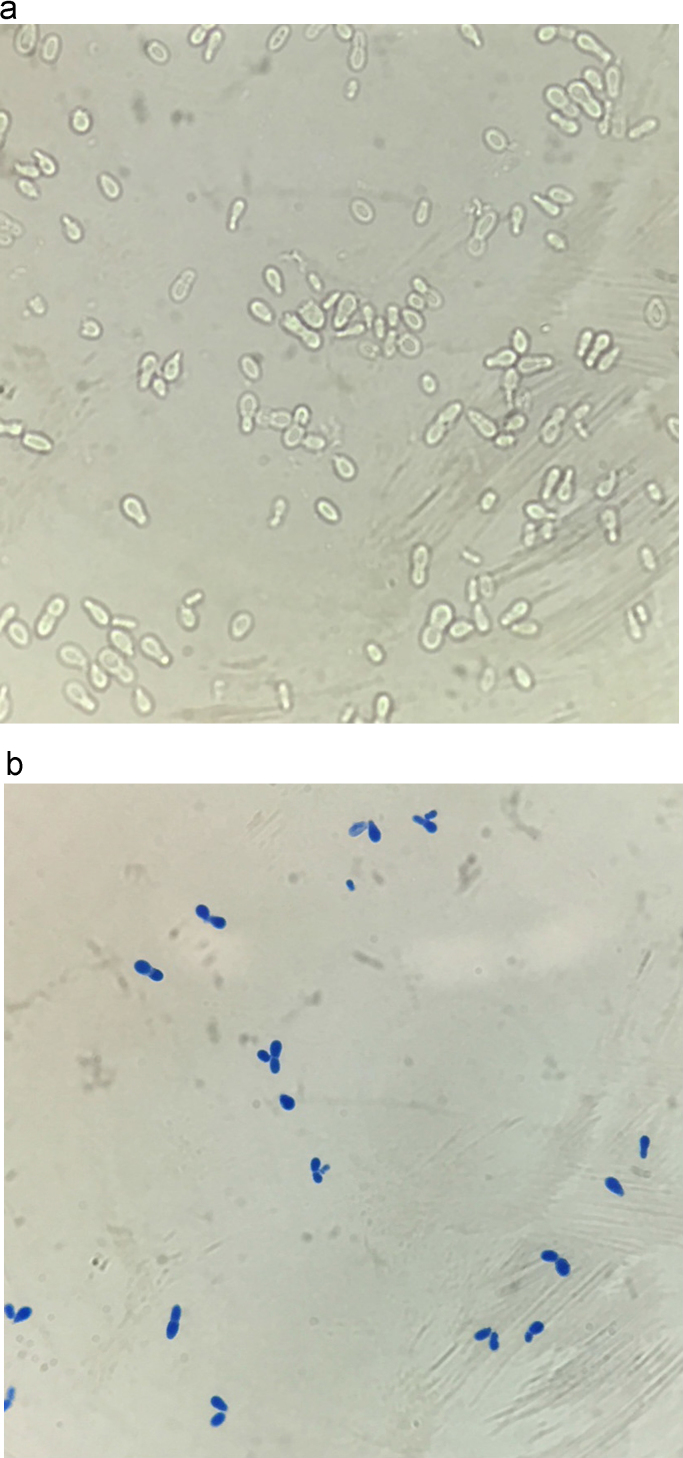
While on IV nafcillin the patient once again developed fever on day 17. Repeat blood cultures were obtained and his midline catheter was removed. On day 22 an aerobic blood culture grew unipolar budding yeast cells (Fig. 1). The yeast was initially suspected to be Candida species and the patient was started on micafungin 100 mg IV daily. On day 24 the clinical microbiology laboratory identified the fungus as Malassezia pachydermatis by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (BioMérieux). MALDI-TOF yielded results with 99.9% certainty. Antifungal therapy was changed to liposomal amphotericin B 400 mg IV daily (approximately 5 mg/kg). Multiple repeat blood cultures were sterile and the patient improved clinically. He completed therapy with a 4 week course of nafcillin for the MSSA bacteremia and 7 days of liposomal amphotericin B.
Fig. 1.
Unipolar budding yeast (1000× magnification) seen on (a) wet mount and (b) lactophenol cotton blue staining. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
After discharge, the Fite stains at the NHDP showed large numbers of acid-fast organisms within histiocytes at various levels of the dermis as well as within cutaneous nerves. The latter findings are pathognomonic for Hansen's disease and the patient was referred to a nearby satellite NHDP clinic for therapy.
3. Discussion
The pathogenic role of Malassezia yeasts is subject to controversy with both immunocompetent and immunosuppressed patients being susceptible to infection. The spectrum of disease ranges from asymptomatic skin colonization to life-threatening sepsis. In immunocompromised patients, such as those with acquired immune-deficiency syndrome, recipients of solid organ and bone marrow transplants, and those with hematologic ma lignancies, Malassezia has been linked to sepsis, catheter related fungemia, and invasive infections [5]. The actual number of human infections is uncertain. As Malassezia represents an uncommon cause of fungemia, a high index of suspicion is needed to make the diagnosis. Malassezia species can be detected in blood and other specimens by direct microscopic examination, culture, and molecular methods. The diagnosis of M. pachydermatis infection is complicated by the fact the organism can be misidentified or not identified at all by some commercial identification systems [6]. The MALDI-TOF system in use at our institution identified Malassezia to species level with 99.9% certainty. Subsequently, the diagnosis was confirmed by sequencing the internal transcribed spacer region performed in-house based on a validated protocol. The sequence was submitted to the National Center for Biotechnology Information GenBank (accession number KX246671).
In vitro studies of Malassezia isolates have shown consistent susceptibility to both triazole antifungal agents and amphotericin B, with echinocandins and flucytosine being less active against the genus [7], [8], [9]. In light of experimental and comparative clinical data, Tragiannidis and colleagues have recommended the use of intravenous triazoles (fluconazole or voriconazole) as first-line options for treatment of invasive Malassezia infections with amphotericin B as back-up for refractory or life-threatening infections [5].
To our knowledge, only 2 cases of fungemia due to M. pachydermatis have been described to date in adults. The first reported case occurred in a 21 year old male with acute myeloid leukemia (AML) who underwent allogeneic bone marrow transplantation [3]. The patient had two central catheters in place for approximately 40 days and had not received any parenteral nutrition. Cultures from blood and one of the catheter tips grew M. pachydermatis. Removal of both catheters and treatment with amphotericin B was started. Subsequent blood cultures were negative and the patient died of unrelated causes. The second reported case occurred in a 69 year old male undergoing induction chemotherapy for AML while on posaconazole prophylaxis [4]. M. pachydermatis was cultured from a peripherally inserted central catheter that had been in place for 2 weeks. Removal of the catheter and treatment with amphotericin B resulted in clearance of the infection.
Dog owners have been implicated as carriers for M. pachydermatis [10]. In one study, the organism was shown to be introduced into an intensive care nursery via the hands of healthcare workers after being colonized from pet dogs at home [11]. In the absence of other risk factors in our case, such as contact with dogs or use of total parenteral nutrition, the protracted use of a catheter was implicated as the main risk factor for infection. While the catheter is the most probable culprit for infection, we can’t help but hypothesize that infection with Mycobacterium leprae was also a contributory factor. We hypothesize that the change in the skin microbiota due to leprosy placed the patient at an increased risk for M. pachydermatis fungemia. As a member of the normal mycobiota of the skin, Malassezia usually exists as a commensal yeast. However, it has been suggested that Malassezia can become invasive whenever there is an alteration in host defense or in the skin surface microclimate [12]. The human microbiota plays an essential role in immune responses [13] and leprosy has been shown to shift the microbiota towards colonization with potentially pathogenic microorganisms [14]. It is possible that leprous lesions impair the skin barrier protection and facilitate the access of microorganisms normally absent in healthy skin. The alteration in the skin microclimate induced by M. leprae in conjunction with the protracted use of a vascular catheter could escalate the risk of a systemic fungal infection. This report represents a unique case in which infection with M. leprae could have contributed to an invasive infection with M. pachydermatis.
Conflict of interest
None.
Acknowledgements
None.
References
- 1.A. Prohic, T.J. Sadikovic, M. Krupalija-Fazlic, S. Kuskunovic-Vlahovljak, Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol., 2015. December 29. (Epub ahead of print). [DOI] [PubMed]
- 2.Guillot J., Bond R. Malassezia pachydermatis: a review. Med. Mycol. 1999;37:295–306. doi: 10.1046/j.1365-280x.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 3.Lautenbach E., Nachamkin I., Schuster M. Malassezia pachydermatis Infections. N. Engl. J. Med. 1998;339:270–271. doi: 10.1056/NEJM199807233390414. [DOI] [PubMed] [Google Scholar]
- 4.Choudhury S., Marte R.L. Malassezia pachydermatis fungaemia in an adult on posaconazole prophylaxis for acute myeloid leukaemia. Pathology. 2014;46:466–467. doi: 10.1097/PAT.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 5.Tragiannidis A., Bisping G., Koehler G., Groll A.H. Minireview: Malassezia infections in immunocompromised patients. Mycoses. 2010;53:187–195. doi: 10.1111/j.1439-0507.2009.01814.x. [DOI] [PubMed] [Google Scholar]
- 6.Meletiadis J., Arabatzis M., Bompola M., Tsiveriotis K., Hini S., Petinaki E. Comparative evaluation of three commercial identification systems using common and rare bloodstream yeast isolates. J. Clin. Microbiol. 2011;49:2722–2727. doi: 10.1128/JCM.01253-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garau M., Pereiro M., Palacio A.D. In vitro susceptibilities of Malassezia species to a new triazole, albaconazole (UR-9825), and other antifungal compounds. Antimicrob. Agents Chemother. 2003;47:2342–2344. doi: 10.1128/AAC.47.7.2342-2344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda K.C., de Araujo C.R., Costa C.R., Passos X.S., Fernandes O.D.F.L, Silva M.D.R.R. Antifungal activities of azole agents against the Malassezia species. Int. J. Antimicrob. Agents. 2007;29:281–284. doi: 10.1016/j.ijantimicag.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A.K., Kohli Y., Li A., Faergemann J., Summerbell R.C. In vitro susceptibility of the seven Malassezia species to ketoconazole, voriconazole, itraconazole and terbinafine. Br. J. Dermatol. 2000;142:758–765. doi: 10.1046/j.1365-2133.2000.03294.x. [DOI] [PubMed] [Google Scholar]
- 10.Morris D.O., O’Shea K., Shofer F.S., Rankin S. Malassezia pachydermatis carriage in dog owners. Emerg. Infect. Dis. 2005;11:83–88. doi: 10.3201/eid1101.040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang H.J., Miller H.L., Watkins N., Arduino M.J., Ashford D.A., Midgley G. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N. Engl. J. Med. 1998;338:706–711. doi: 10.1056/NEJM199803123381102. [DOI] [PubMed] [Google Scholar]
- 12.Cabañes F.J. Malassezia yeasts: How many species infect humans and animals? PLoS Pathog. 2014;10:e1003892. doi: 10.1371/journal.ppat.1003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva P.E.S., Costa P.S., Ávila M.P., Suhadolnik M.L.S., Reis M.P., Salgado A.P.C. Leprous lesion presents enrichment of opportunistic pathogenic bacteria. SpringerPlus. 2015;4:187. doi: 10.1186/s40064-015-0955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]