Abstract
There is a broad range of possible diagnoses for an elevated beta human chorionic gonadotropin (β-hCG) in the absence of intrauterine or ectopic pregnancy. When women of child bearing potential undergo evaluation for clinical trial, it is often unclear what course of evaluation to take when a pregnancy test is positive. We describe the clinical course of a patient with widely metastatic mucinous ovarian carcinoma with metastasis to the peritoneum, lymph nodes and liver. The patient was found to have a mildly elevated β-hCG during initial evaluation for clinical trial. Extensive work up for ectopic pregnancy, trophoblastic disease, and phantom β-hCG were negative. The patient's β-hCG levels continued to rise until initiation of therapy. She was treated on a phase I protocol with restaging scans revealing a partial response. The β-hCG was retested and declined in conjunction with her response, consistent with paraneoplastic β-hCG. Here, we propose a decision making algorithm to evaluate a patient with an elevated β-hCG undergoing assessment for clinical trial.
Keywords: Paraneoplastic syndrome, Ovarian cancer, Phantom hCG, Clinical trial, Tumor heterogeneity, β-hCG heterophilic antibody
Highlights
-
•
Evaluation of positive pregnancy test during clinical trial accrual is difficult.
-
•
We describe a case of a woman with metastatic ovarian cancer.
-
•
The patient was found to have an elevated β-hCG during clinical trial evaluation.
-
•
We propose a decision making algorithm to evaluate these patients.
1. Introduction
When evaluating patients for clinical trial, intrauterine pregnancy is commonly considered an exclusion criteria, however guidelines do not exist to determine what to do should a pregnancy test result as positive.
We describe a clinical case of a patient with metastatic mucinous ovarian carcinoma. The patient underwent standard-of-care therapy upfront with progression of disease and was referred to investigational therapeutics for phase I treatment. She was found to have an elevated β-hCG consistent with pregnancy. Pelvic ultrasound and β-hCG heterophilic antibody testing were negative ruling out intrauterine and ectopic pregnancy or phantom hCG. Her elevated β-hCG appeared consistent with paraneoplastic syndrome reflected in a decline in β-hCG that correlated with a response to treatment.
2. Case report
A 34-year-old African American female with metastatic mucinous ovarian carcinoma presented with a pelvic mass discovered during her annual gynecological examination. An ultrasound showed a 7.7 × 8.7 cm left ovarian mass with complex appearance. The right ovary appeared normal. Laparoscopic left salpingo-oophorectomy showed a moderately differentiated mucinous ovarian carcinoma, stage IC1, due to intraoperative rupture of the ovarian mass. Tumor cells were diffusely positive for keratin 7 and very focally positive for keratin 20. There was patchy expression of PAX-8 and DPC4 expression was preserved. The patient was taken for diagnostic laparoscopy with appendectomy, omentectomy, and multiple peritoneal biopsies by a gynecologic oncologist; all of which were negative for malignancy.
Eight weeks later the patient started adjuvant chemotherapy with one cycle of capecitabine and oxaliplatin (XELOX), transitioned to four months of fluorouracil and oxaliplatin (FOLFOX) due to insurance issues. The CT abdomen and pelvis post FOLFOX chemotherapy showed an enhancing 2.6 cm nodule in rectus abdominis muscle. The patient underwent an exploratory laparotomy with excision of a 5 × 6 cm abdominal mass involving fascia and muscle, consistent with metastatic adenocarcinoma. The surgical resection margins were negative for malignancy. After recovery, the patient received chemotherapy with carboplatin and paclitaxel for 6 months. The CT abdomen and pelvis performed following 6 months of carboplatin and paclitaxel showed progression of disease with new nodal and perihepatic implants.
The patient subsequently consulted with the MD Anderson Cancer Center Department of Investigational Cancer Therapeutics for phase I clinical trial options. The patient was treated with a phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitor plus a Mitogen/Extracellular signal-regulated Kinase (MEK) inhibitor with progression of disease after 5 months. She then started on a second phase I trial with a polymeric micellar nanoparticle of the substance DACH-Pt (1,2-diaminocyclohexane platinum), an in vivo metabolite of oxaliplatin, with progression following 1 cycle of therapy. The patient was then enrolled in a trial with paclitaxel, bevacizumab and temsirolimus (PAT). On the day of clearance to start treatment, she was found to have a positive urine and serum pregnancy test. Her β-hCG was 103. The patient was having regular menstrual cycles and using two forms of birth control. Her husband previously had a vasectomy.
The initial pelvic ultrasound showed no sonographic evidence of intrauterine or tubal gestation. The left ovary was surgically absent however the right ovary was enlarged with complex cystic mass with solid components measuring 9.5 × 6.6 cm. The work-up for false positive elevation of β-hCG including serial dilutions, incorporation of animal serum and outside institutional validation testing for β-hCG heterophile antibody, was negative. Therefore, the β-hCG elevation was not thought to be related to a phantom hCG.
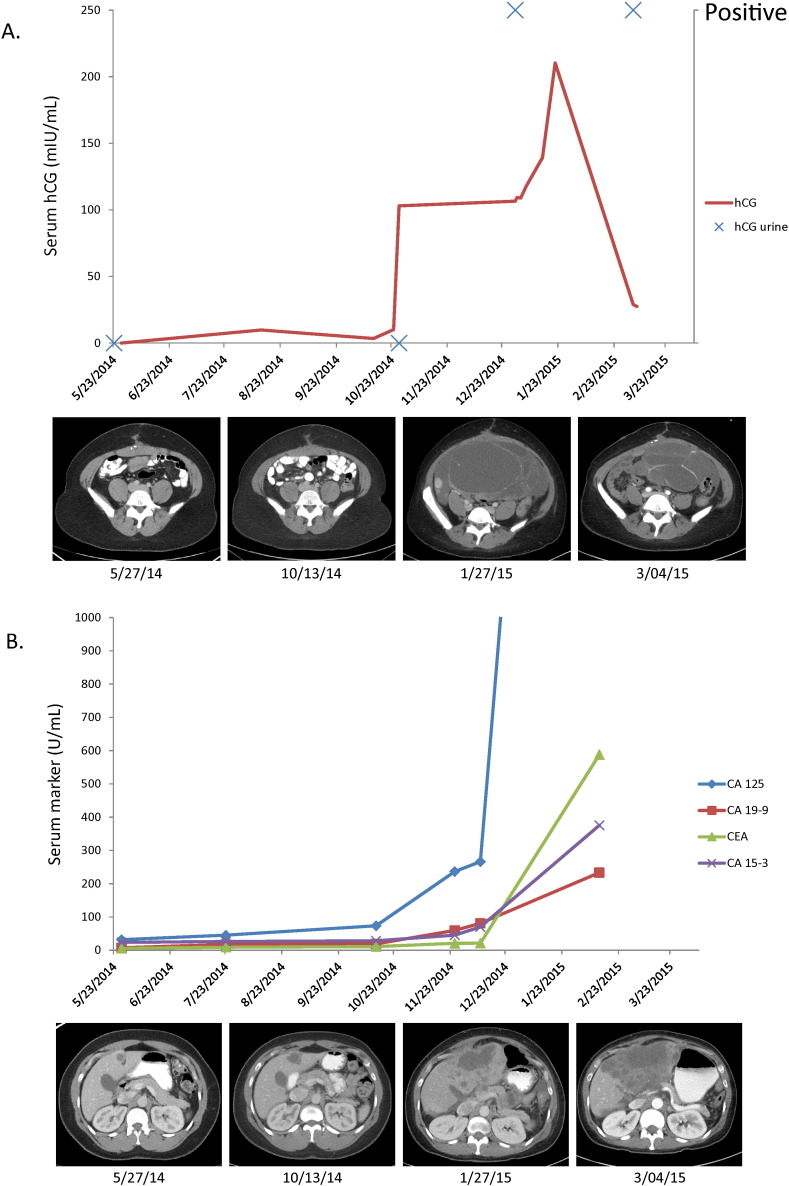
Dilation and curettage to rule out trophoblastic disease was planned, but not completed due to discovery of a deep vein thrombosis and initiation of therapeutic anticoagulation. As no intrauterine or extrauterine pregnancy could be identified, after several weeks, the patient returned to clinic and was cleared to start treatment. At that time, her β-hCG was 210.3. Three weeks post treatment with PAT, the β-hCG decreased to 27.5 and CT scans showed partial response (Fig. 1A,B). Shortly thereafter, the patient was admitted to the intensive care unit for weakness due to Guillain Barre syndrome. She eventually transitioned to hospice care.
Fig. 1.
A. Corresponding serum (IU/l) and urine (positive or negative) hCG assays with treatment response over time. B. Corresponding serum tumor markers with treatment response over time. Tumor markers correspond with disease progression in some tissues while hCG corresponds with disease response in other tissues.
3. Discussion
Nearly all female patients undergoing enrollment on clinical trial are evaluated for pregnancy as part of standard exclusion criteria. An elevated β-hCG in the absence of viable pregnancy can occur for multiple reasons and has a broad differential diagnosis including miscarriage, ectopic pregnancy, pituitary hCG production, trophoblastic disease and phantom hCG. Ectopic pregnancy is frequently suspected when β-hCG levels plateau or fail to double within 48 h without evidence of intrauterine pregnancy with ultrasound. When intrauterine and extrauterine pregnancy are ruled out, other causes should be investigated.
Pituitary hCG may be produced in perimenopausal or postmenopausal women. As estrogen and progesterone production decreases, releasing gonadotropin releasing hormone (GnRH) from negative feedback, luteinizing hormone (LH) and follicular stimulating hormone (FSH) rise. The β subunit gene of LH is found in a sequence of 7 hCG β subunit genes and therefore uncontrolled GnRH stimulation may lead to hCG production by pituitary gonadotrope cells (Cole, 2005). Using an FSH level of 45.0 mIU/ml identified hCG of perimenopausal women, ages 41–55 years, with mildly increased serum hCG concentrations (5.0–14.0 mIU/ml), an FSH cutoff of 45.0 mIU/ml identified hCG of placental origin with 100% sensitivity and 75% specificity. FSH levels greater than 45 mIU/ml were never observed when hCG was of placental origin. When hCG is less than 5.0 mIU/ml, FSH testing is not performed as pregnancy is unlikely. When hCG is greater than 14.0 mIU/ml, FSH testing is not performed as this is consistent with pregnancy (Gronowski et al., 2008). In one study, two weeks of estrogen-progesterone therapy suppressed pituitary hCG and may help confirm diagnosis (Cole et al., 2007).
Gestational trophoblastic disease is a group of conditions that may be classified as either premalignant, in the case of a partial or complete hydatidiform mole, or malignant, including invasive mole, choriocarcinoma, placental-site trophoblastic tumor and epithelioid trophoblastic tumor. Although not always the case, β-hCG is commonly found to be elevated in these conditions and these diseases are considered high on the differential diagnosis list when found.
Phantom hCG is a phenomenon first described in 1998 by Laurence Cole, Ph.D., where patients have persistent mild elevations of β-hCG, occasionally following miscarriage. The elevated β-hCG often falsely interpreted and misleads physicians to evaluate or incorrectly treat patients with cytotoxic agents for trophoblast disease (Cole, 1998). Heterophilic antibodies against mouse, goat, rabbit, cow, horse, or sheep antigens can interfere with two-site immunoassays by creating complexes between the two anti-β-hCG antibodies without β-hCG being present. This leads to a false-positive result (Vladutiu et al., 1982). People can develop heterophilic antibodies by exposure to the serum, tissue, or other antigens of nonhuman species. The incidence of these antibodies in the general population is uncertain. Heterophilic antibodies do not appear to be excreted in the urine and therefore urine assays can be used to identify false-positive serum results. Serial dilutions may also be performed to assess for interference. To avoid false positives, manufacturers have incorporated animal serum and nonspecific animal antibodies into all their test ingredients. The excess of nonspecific antibodies saturates heterophilic antibodies in human serum and usually eliminates their interference with the assay (Johnson et al., 2000). Additionally, serum samples can be sent to outside laboratories with different assay systems to validate results. In our patient's case, the patient continued to have normal menstrual cycles and was using two forms of contraception with her husband who had history of vasectomy. The work up, including pelvic ultrasound and CT scans, was negative for pregnancy and trophoblastic disease. The testing for heterophilic hCG antibodies was also negative. The patient additionally had a positive urine pregnancy test inconsistent with phantom hCG. It was therefore believed that the patient's β-hCG production was due to the patient's tumor, consistent with a paraneoplastic syndrome.
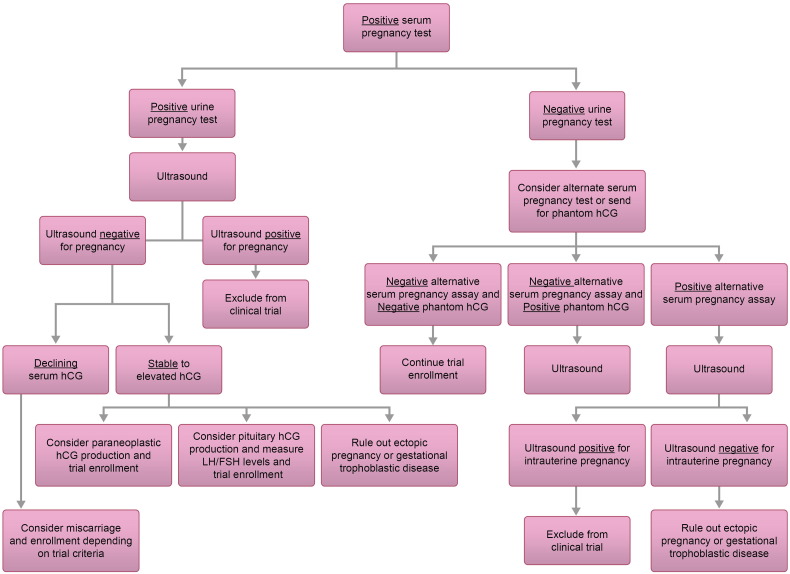
Paraneoplastic syndromes occur in about 8% of cancer patients and frequently develop with advanced disease. They may appear earlier than symptoms of the primary tumor itself, leading to new diagnosis of cancer (Pelosof and Gerber, 2010). β-hCG is primarily produced by placental syncytiotrophoblast cells during pregnancy. It can also be found in the testis, liver, lung, colon, and stomach in small amounts. Malignancies in these organs may lead to an increased serum levels (Demirtas et al., 2007). A gastric origin is the most frequent, ranging from 11% to 17% of this rare subset (Germann et al., 2002). In our case, the patient had a primary ovarian neoplasm with metastases within the abdomen. β-hCG levels rose while the patient was on a treatment break, nearly impeding her from further treatment. With re-initiation of therapy, the β-hCG declined, corresponding to a partial response per Response Evaluation Criteria in Solid Tumors (RECIST) on radiographic imaging. Urine hCG remained positive despite decline in serum hCG as the level was still over the detection threshold of 25 IU/l. Interesting enough, other tumor markers (CA 125, CA 15-3, CA 19.9 and CEA) all increased. The decreased size of tumor within the pelvis corresponded with decreasing β-hCG levels while increased hepatic metastasis corresponded to increases in other markers. This lends evidence to the idea that the patient had tumoral heterogeneity with different markers produced by tumors in different areas of the body. This is the first case of paraneoplastic β-hCG production from an ovarian adenocarcinoma reported in the literature. We provide a treatment algorithm to evaluate for paraneoplastic hCG production when assessing for clinical trial eligibility (Fig. 2). Paraneoplastic syndrome should remain in the differential diagnosis in patients found to have elevated levels of β-hCG without evidence of intra- or extra-uterine pregnancy.
Fig. 2.
Decision making algorithm for elevated hCG found during evaluation for clinical trial.
Consent
Informed consent to publish the information was granted from the patient.
None of the authors have conflict of interest or competing interest.
Authors' contributions
JG participated in the design of the manuscript, analyzed the biomarker data and drafted the manuscript. PP JG participated in the design of the manuscript, analyzed the biomarker data and drafted the manuscript. NF participated in the design of the manuscript and helped to draft the manuscript. SW participated in the design of the manuscript and helped to draft the manuscript. SP conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Jennifer Goldstein, Email: jbgoldstein@mdanderson.org.
Prasamsa Pandey, Email: ppandey@mdanderson.org.
Nicole Fleming, Email: nfleming@mdanderson.org.
Shannon Westin, Email: swestin@mdanderson.org.
Sarina Piha-Paul, Email: spihapau@mdanderson.org.
References
- Cole L.A. Phantom hCG and phantom choriocarcinoma. Gynecol. Oncol. 1998;71:325–329. doi: 10.1006/gyno.1998.5181. [DOI] [PubMed] [Google Scholar]
- Cole L.A. “Background” human chorionic gonadotropin in healthy, nonpregnant women. Clin. Chem. 2005;51:1765–1766. doi: 10.1373/clinchem.2005.056507. [DOI] [PubMed] [Google Scholar]
- Cole L.A., Sasaki Y., Muller C.Y. Normal production of human chorionic gonadotropin in menopause. N. Engl. J. Med. 2007;356:1184–1186. doi: 10.1056/NEJMc066500. [DOI] [PubMed] [Google Scholar]
- Demirtas E., Krishnamurthy S., Tulandi T. Elevated serum beta-human chorionic gonadotropin in nonpregnant conditions. Obstet. Gynecol. Surv. 2007;62:675–679. doi: 10.1097/01.ogx.0000281557.04956.61. (quiz 91) [DOI] [PubMed] [Google Scholar]
- Germann N., Gross-Goupil M., Wasserman E. The chemotherapy of metastatic gastric adenocarcinomas with hypersecretion of alpha-fetoprotein or beta-human chorionic gonadotrophin: report of two cases. Ann. Oncol.: Official Journal of the European Society for Medical Oncology/ESMO. 2002;13:632–636. doi: 10.1093/annonc/mdf026. [DOI] [PubMed] [Google Scholar]
- Gronowski A.M., Fantz C.R., Parvin C.A. Use of serum FSH to identify perimenopausal women with pituitary hCG. Clin. Chem. 2008;54:652–656. doi: 10.1373/clinchem.2007.100305. [DOI] [PubMed] [Google Scholar]
- Johnson G.F.F.R., Beranek J.M., Roberts P.L. Two cases of immunoassay interference with clinical consequences. Clin. Chem. 2000;46:A37. [Google Scholar]
- Pelosof L.C., Gerber D.E. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin. Proc. 2010;85:838–854. doi: 10.4065/mcp.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladutiu A.O., Sulewski J.M., Pudlak K.A., Stull C.G. Heterophilic antibodies interfering with radioimmunoassay. A false-positive pregnancy test. J. Am. Med. Assoc. 1982;248:2489–2490. [PubMed] [Google Scholar]