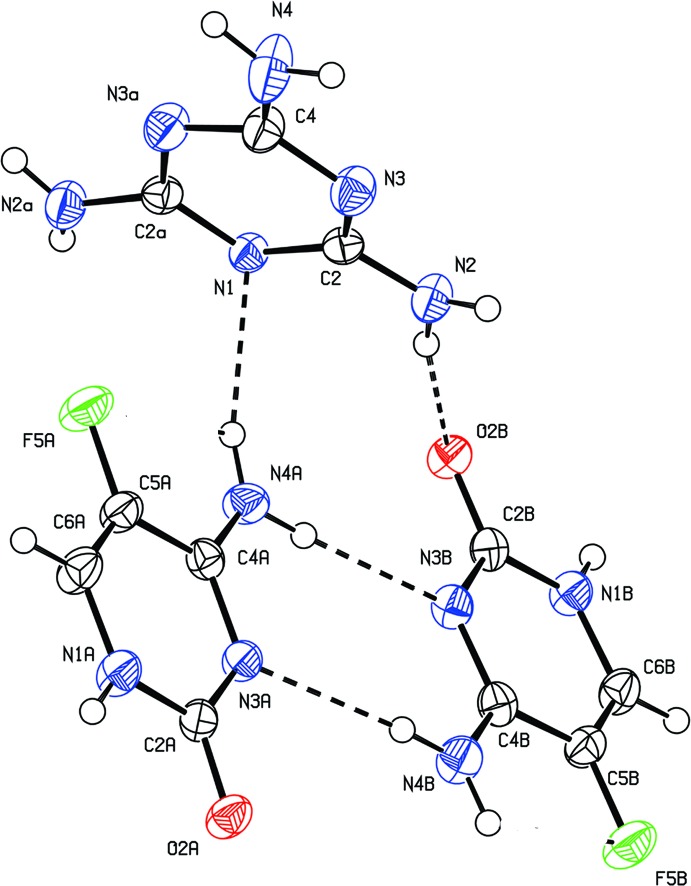
The asymmetric unit of the title compound comprises two independent 5-fluorocytosine molecules and one half-molecule of melamine. The 5-fluorocytosine molecules are linked through two different homosynthons; one is formed via a pair of N—H⋯O hydrogen bonds and the second via a pair of N—H⋯N hydrogen bonds. The 5-fluorocytosine and melamine molecules interact via N—H⋯O, N—H⋯N and N—H⋯O, N—H⋯N, C—H⋯F hydrogen bonds.
Keywords: crystal structure, 5-fluorocytosine, melamine, homosynthons, hydrogen bonding
Abstract
The asymmetric unit of the title compound, 4C4H4FN3O·C3H6N6, comprises of two independent 5-fluorocytosine (5FC) molecules (A and B) and one half-molecule of melamine (M). The other half of the melamine molecule is generated by a twofold axis. 5FC molecules A and B are linked through two different homosynthons [R 2 2(8) ring motif]; one is formed via a pair of N—H⋯O hydrogen bonds and the second via a pair of N—H⋯N hydrogen bonds. In addition to this pairing, the O atoms of 5FC molecules A and B interact with the N2 amino group on both sides of the melamine molecule, forming a DDAA array of quadruple hydrogen bonds and generating a supramolecular pattern. The 5FC (molecules A and B) and two melamine molecules interact via N—H⋯O, N—H⋯N and N—H⋯O, N—H⋯N, C—H⋯F hydrogen bonds forming R 6 6(24) and R 4 4(15) ring motifs. The crystal structure is further strengthened by C—H⋯F, C—F⋯π and π–π stacking interactions.
Chemical context
Pyrimidine derivatives are used in the treatment of antiviral, antifungal, antitumor and cardiovascular diseases. 5-Fluorocytosine (5FC), a synthetic antimycotic compound, first synthesized in 1957 and widely used as an antitumor agent as a cytosine derivative (Tassel & Madoff, 1968 ▸; Benson & Nahata, 1988 ▸; Bennet, 1977 ▸; Polak & Scholer, 1980 ▸). It is active against fungal infection and was released in the year 1968 (Vermes et al., 2000 ▸). It becomes active by deamination of 5FC into 5-fluorouracil by the enzyme cytosine deaminase (CD) and inhibits RNA and DNA synthesis (Larsen et al., 2003 ▸; Mullen et al., 1994 ▸; Morschhäuser, 2003 ▸). Melamine is a triazine derivative. It shows antitumor activity as well as biological activities such as antiangiogenesis and antimicrobial effects. Triazine derivatives are useful synthons in supramolecular chemistry. In particular, aminotriazines have been used for the formation of supramolecular architectures using hydrogen bonds (Russell et al., 1998 ▸; MacDonald & Whitesides, 1994 ▸; Whitesides et al., 1991 ▸). The organic and inorganic salts develop well-defined non-covalent molecular recognition via multiple hydrogen bonds by self assembly of components which contain a complementary array of hydrogen-bonding sites (Desiraju, 1989 ▸). The present work is focused on the supramolecular hydrogen-bonding patterns exhibited by the co-crystal of 5-fluorocytosine with melamine.
Structural commentary
The asymmetric unit comprises two independent 5-fluorocytosine (5FC) molecules (A and B) and half a molecule of melamine (M). The twofold axis of melamine coincides with the crystallographic twofold axis. An ORTEP view of the crystal structure is shown in Fig. 1 ▸. The values for the C—F bond distance in the two molecules [1.3491 (18) in 5FC A and 1.3492 (18) Å in 5FC B and the corresponding internal angles at the carbon-carrying fluorine atom [C2A—N3A—C4A = 119.96 (13) in 5FC A and C2B—N3B—C4B = 119.92 (13)° in 5FC B] agree with those reported in the literature (Louis et al., 1982 ▸).
Figure 1.
The asymmetric unit of the title compound, showing 30% probability displacement ellipsoids. Dashed lines indicate hydrogen bonds. Atoms with the suffix a are generated by the symmetry operation 1 − x, y, {\script{1\over 2}} − z.
Supramolecular features
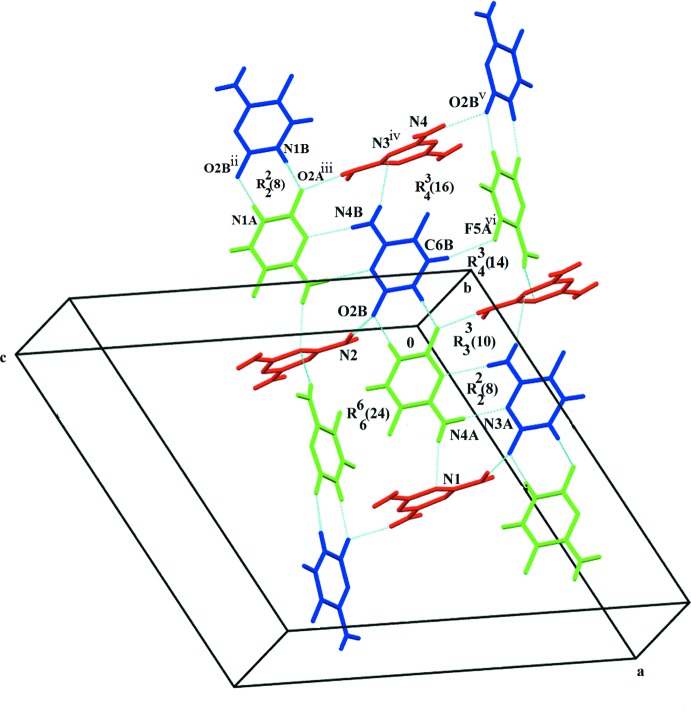
Two different homosynthons are assembled via a pair of N—H⋯O and N—H⋯N hydrogen bonds (Table 1 ▸) to render a robust  (8) ring motif. The first type of homosynthon is formed by the interaction of the protonated N1 and O atoms of 5FC molecules A and B through N—H⋯O hydrogen bonds. Another type of homosynthon is formed via the N4-amino and N3-pyrimidine ring nitrogen atoms of the 5FC A and B molecules through a pair of N—H⋯N hydrogen bonds (da Silva et al., 2013 ▸; Tutughamiarso et al., 2012 ▸). The melamine molecule and 5FC (molecules A and B) are involved in the generation of a quadruple hydrogen-bonded DDAA array having a fused-ring sequence of
(8) ring motif. The first type of homosynthon is formed by the interaction of the protonated N1 and O atoms of 5FC molecules A and B through N—H⋯O hydrogen bonds. Another type of homosynthon is formed via the N4-amino and N3-pyrimidine ring nitrogen atoms of the 5FC A and B molecules through a pair of N—H⋯N hydrogen bonds (da Silva et al., 2013 ▸; Tutughamiarso et al., 2012 ▸). The melamine molecule and 5FC (molecules A and B) are involved in the generation of a quadruple hydrogen-bonded DDAA array having a fused-ring sequence of  (10),
(10),  (8) and
(8) and  (10). The
(10). The  (10) motif is formed on both sides via N—H⋯O and N—H⋯N hydrogen bonds. These quadruple arrays are further linked by three large ring motifs:
(10) motif is formed on both sides via N—H⋯O and N—H⋯N hydrogen bonds. These quadruple arrays are further linked by three large ring motifs:  (24),
(24),  (16) and
(16) and  (14). The
(14). The  (24) ring motifs are formed by the interaction of two 5FC A molecules, two 5FC B molecules and two melamine molecules through several N—H⋯O and N—H⋯N hydrogen bonds, generating a hexameric supermolecule. The
(24) ring motifs are formed by the interaction of two 5FC A molecules, two 5FC B molecules and two melamine molecules through several N—H⋯O and N—H⋯N hydrogen bonds, generating a hexameric supermolecule. The  (16) ring motif links the one 5FC A molecule, two 5FC B molecules and one melamine molecule through N—H⋯O, N—H⋯N and C—H⋯F hydrogen bonds, generating a tetrameric supermolecule. Similarly, the
(16) ring motif links the one 5FC A molecule, two 5FC B molecules and one melamine molecule through N—H⋯O, N—H⋯N and C—H⋯F hydrogen bonds, generating a tetrameric supermolecule. Similarly, the  (14) ring motifs are formed by the interaction of two 5FC A molecules, one 5FC B molecule and one melamine molecule through N—H⋯O, N—H⋯N and C—H⋯F hydrogen bonds, generating another tetrameric supermolecule. The association of these
(14) ring motifs are formed by the interaction of two 5FC A molecules, one 5FC B molecule and one melamine molecule through N—H⋯O, N—H⋯N and C—H⋯F hydrogen bonds, generating another tetrameric supermolecule. The association of these  (8), DDAA array and
(8), DDAA array and  (24),
(24),  (16) and
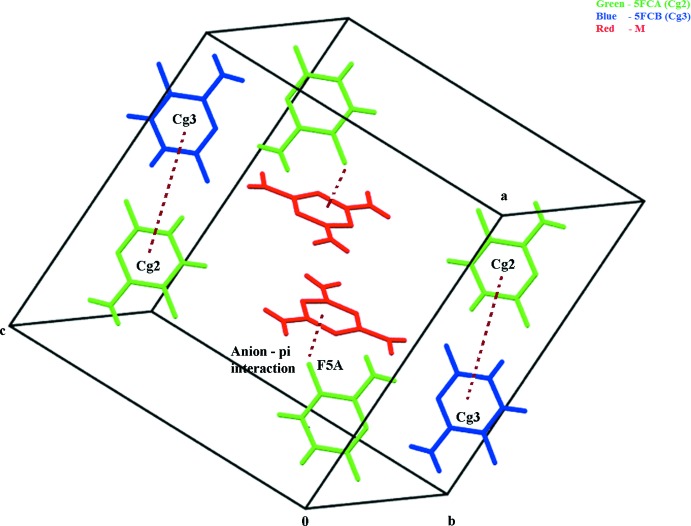
(16) and  (14) motifs leads to the formation of supramolecular patterns (Fig. 2 ▸). The crystal structure is also stabilized by weak C—H⋯F hydrogen bonds and π–π stacking interactions between 5FC A and B molecules with an interplanar distance of 3.475 (6) Å, centroid-to-centroid distance of 3.6875 (11) Å, and slip angle of 19.52°. The crystal structure is further strengthened by a C—F⋯π interaction [3.4541 (14) Å] between 5-fluorocytosinium molecule A and the melamine molecule (Fig. 3 ▸).
(14) motifs leads to the formation of supramolecular patterns (Fig. 2 ▸). The crystal structure is also stabilized by weak C—H⋯F hydrogen bonds and π–π stacking interactions between 5FC A and B molecules with an interplanar distance of 3.475 (6) Å, centroid-to-centroid distance of 3.6875 (11) Å, and slip angle of 19.52°. The crystal structure is further strengthened by a C—F⋯π interaction [3.4541 (14) Å] between 5-fluorocytosinium molecule A and the melamine molecule (Fig. 3 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4A—H4A1⋯F5A | 0.86 (2) | 2.47 (2) | 2.7560 (18) | 100.0 (18) |
| N4A—H4A1⋯N1 | 0.86 (2) | 2.23 (2) | 3.0664 (18) | 164 (2) |
| N1A—H1A⋯O2B ii | 0.88 | 1.90 | 2.773 (2) | 173 |
| N1B—H1B⋯O2A iii | 0.88 | 1.88 | 2.7545 (19) | 175 |
| N4A—H4A2⋯N3B | 0.91 (2) | 2.10 (2) | 2.992 (2) | 169 (2) |
| N2—H2A⋯O2B | 0.89 (2) | 2.10 (2) | 2.9689 (19) | 167.6 (18) |
| N2—H2B⋯O2A iv | 0.84 (2) | 2.15 (2) | 2.8949 (19) | 149 (2) |
| N4B—H4B1⋯N3A | 0.88 (2) | 2.20 (2) | 3.060 (2) | 169 (2) |
| N4B—H4B2⋯F5B | 0.86 (2) | 2.42 (2) | 2.7459 (19) | 103 (2) |
| N4B—H4B2⋯N3iv | 0.86 (2) | 2.53 (2) | 3.360 (2) | 162 (2) |
| N4—H4A⋯O2B v | 0.89 (2) | 2.09 (2) | 2.9600 (15) | 165 (2) |
| C6B—H6B⋯F5A vi | 0.95 | 2.43 | 3.2444 (19) | 143 |
Symmetry codes: (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  .
.
Figure 2.
A view of the supramolecular pattern involving two synthons formed by N—H⋯O hydrogen bonds. 5FC A molecules are shown in green, 5FC B molecules in blue and melamine in red. Blue dashed lines indicate hydrogen bonds. Symmetry codes are given in Table 1 ▸.
Figure 3.
A view of C—F⋯π and aromatic π–π stacking interactions (dashed lines) between 5FC molecules A and B and melamine.
In this co-crystal, 5FC molecules A and B form two types of homosynthons (two types of base pairing) while the melamine molecule interacts with them via N—H⋯O and N—H⋯N hydrogen bonds, generating the supramolecular architecture.
Database survey
The crystal structure of 5-fluorocytosine monohydrate (Louis et al., 1982 ▸; Portalone & Colapietro, 2006 ▸; Portalone, 2011 ▸), polymorphs (Hulme & Tocher, 2006 ▸; Tutughamiarso et al., 2009 ▸), salts (Perumalla et al., 2013a ▸,b ▸) and co-crystals (Tutughamiarso et al., 2012 ▸; Da Silva et al., 2013 ▸) have been reported in the literature. From our laboratory, 5-fluorocytosinium salicylate (Prabakaran et al., 2001 ▸) and 5-fluorocytosinium 3-hydroxypicolinate (Karthikeyan et al., 2014 ▸) have been reported. Various salts, co-crystals and metal complexes of melamine have also been reported (Janczak & Perpétuo, 2001a ▸,b ▸, 2002 ▸, 2004 ▸; Perpétuo et al., 2005 ▸; Zerkowski & Whitesides, 1994 ▸; Wang et al., 2007 ▸).
Synthesis and crystallization
Hot aqueous solutions of 5-fluorocytosine (32 mg) and melamine (31 mg) were mixed in a 1:1 molar ratio. The resulting solution was warmed to 353 K using a water bath for half an hour and kept at room temperature for crystallization. After one week, colourless crystals were obtained.
Refinement details
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The hydrogen atoms of amino (N2, N4, N4A, N4B) groups were located in a difference Fourier map and refined freely. The other hydrogen atoms were positioned geometrically (C—H = 0.95, N—H = 0.88 Å) and were refined using a riding model with U iso(H) = 1.2U eq(parent atom).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | 4C4H4FN3O·C3H6N6 |
| M r | 642.55 |
| Crystal system, space group | Monoclinic, C2/c |
| Temperature (K) | 200 |
| a, b, c (Å) | 18.343 (4), 7.9591 (16), 19.680 (4) |
| β (°) | 114.65 (3) |
| V (Å3) | 2611.3 (11) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.14 |
| Crystal size (mm) | 0.20 × 0.20 × 0.20 |
| Data collection | |
| Diffractometer | Rigaku AFC–8S |
| Absorption correction | Multi-scan (CrystalClear; Rigaku/MSC, 2008 ▸) |
| T min, T max | 0.972, 0.972 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 10071, 2564, 2362 |
| R int | 0.019 |
| (sin θ/λ)max (Å−1) | 0.617 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.045, 0.123, 1.07 |
| No. of reflections | 2564 |
| No. of parameters | 233 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.44, −0.34 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S205698901600476X/hg5470sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901600476X/hg5470Isup2.hkl
Supporting information file. DOI: 10.1107/S205698901600476X/hg5470Isup3.cml
CCDC reference: 1469709
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
MM thanks the UGC–BSR, India, for the award of an RFSMS. PTM thanks the UGC–BSR faculty fellowship for a one-time grant.
supplementary crystallographic information
Crystal data
| 4(C4H4FN3O)·C3H6N6 | F(000) = 1320 |
| Mr = 642.55 | Dx = 1.634 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 2564 reflections |
| a = 18.343 (4) Å | θ = 2.4–26.0° |
| b = 7.9591 (16) Å | µ = 0.14 mm−1 |
| c = 19.680 (4) Å | T = 200 K |
| β = 114.65 (3)° | Prism, colorless |
| V = 2611.3 (11) Å3 | 0.20 × 0.20 × 0.20 mm |
| Z = 4 |
Data collection
| Rigaku AFC–8S diffractometer | 2564 independent reflections |
| Radiation source: fine focus sealed tube | 2362 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.019 |
| Detector resolution: 14.6199 pixels mm-1 | θmax = 26.0°, θmin = 2.4° |
| ω scans | h = −22→18 |
| Absorption correction: multi-scan (CrystalClear; Rigaku/MSC, 2008) | k = −9→9 |
| Tmin = 0.972, Tmax = 0.972 | l = −24→24 |
| 10071 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.123 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0741P)2 + 1.8751P] where P = (Fo2 + 2Fc2)/3 |
| 2564 reflections | (Δ/σ)max < 0.001 |
| 233 parameters | Δρmax = 0.44 e Å−3 |
| 0 restraints | Δρmin = −0.34 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.50000 | 0.1866 (2) | 0.25000 | 0.0244 (5) | |
| N2 | 0.48606 (8) | 0.18336 (19) | 0.12847 (8) | 0.0326 (4) | |
| N3 | 0.48592 (8) | −0.07579 (17) | 0.18444 (7) | 0.0342 (4) | |
| N4 | 0.50000 | −0.3280 (3) | 0.25000 | 0.0501 (8) | |
| C2 | 0.49100 (8) | 0.09524 (18) | 0.18883 (7) | 0.0248 (4) | |
| C4 | 0.50000 | −0.1589 (3) | 0.25000 | 0.0300 (6) | |
| F5A | 0.32327 (5) | 0.12206 (14) | 0.26116 (5) | 0.0420 (3) | |
| O2A | 0.06416 (6) | 0.34719 (15) | 0.03132 (6) | 0.0326 (3) | |
| N1A | 0.11896 (8) | 0.17904 (17) | 0.13300 (7) | 0.0315 (4) | |
| N3A | 0.19980 (7) | 0.34775 (15) | 0.09362 (7) | 0.0252 (3) | |
| N4A | 0.33640 (8) | 0.35220 (17) | 0.16222 (8) | 0.0299 (4) | |
| C2A | 0.12590 (8) | 0.29396 (19) | 0.08380 (8) | 0.0253 (4) | |
| C4A | 0.26467 (8) | 0.29295 (18) | 0.15183 (8) | 0.0243 (4) | |
| C5A | 0.25656 (9) | 0.17587 (19) | 0.20310 (8) | 0.0286 (4) | |
| C6A | 0.18349 (9) | 0.1215 (2) | 0.19258 (9) | 0.0332 (5) | |
| F5B | 0.21480 (6) | 0.72438 (14) | −0.15032 (5) | 0.0419 (3) | |
| O2B | 0.46711 (6) | 0.54980 (13) | 0.09792 (6) | 0.0280 (3) | |
| N1B | 0.41539 (7) | 0.70320 (16) | −0.00875 (7) | 0.0278 (3) | |
| N3B | 0.33298 (7) | 0.53026 (15) | 0.02705 (7) | 0.0261 (3) | |
| N4B | 0.19773 (8) | 0.51382 (18) | −0.04738 (8) | 0.0328 (4) | |
| C2B | 0.40666 (8) | 0.59203 (17) | 0.04058 (8) | 0.0240 (4) | |
| C4B | 0.26965 (9) | 0.57389 (18) | −0.03540 (8) | 0.0259 (4) | |
| C5B | 0.27992 (9) | 0.68402 (19) | −0.08772 (8) | 0.0285 (4) | |
| C6B | 0.35239 (9) | 0.74902 (19) | −0.07308 (8) | 0.0298 (4) | |
| H2A | 0.4807 (12) | 0.294 (3) | 0.1263 (11) | 0.037 (5)* | |
| H2B | 0.4701 (12) | 0.135 (3) | 0.0868 (12) | 0.042 (5)* | |
| H4A | 0.5134 (13) | −0.381 (3) | 0.2935 (11) | 0.048 (6)* | |
| H4A1 | 0.3799 (13) | 0.308 (3) | 0.1947 (11) | 0.040 (5)* | |
| H1A | 0.07100 | 0.14180 | 0.12550 | 0.0380* | |
| H4A2 | 0.3380 (13) | 0.418 (3) | 0.1253 (13) | 0.049 (6)* | |
| H6A | 0.17710 | 0.04390 | 0.22640 | 0.0400* | |
| H1B | 0.46300 | 0.74610 | 0.00150 | 0.0330* | |
| H4B1 | 0.1965 (13) | 0.453 (3) | −0.0107 (12) | 0.047 (6)* | |
| H4B2 | 0.1569 (13) | 0.543 (3) | −0.0874 (13) | 0.045 (6)* | |
| H6B | 0.35960 | 0.82540 | −0.10690 | 0.0360* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0248 (8) | 0.0253 (8) | 0.0219 (8) | 0.0000 | 0.0085 (7) | 0.0000 |
| N2 | 0.0389 (8) | 0.0345 (8) | 0.0244 (7) | −0.0006 (6) | 0.0133 (6) | 0.0007 (5) |
| N3 | 0.0418 (8) | 0.0307 (7) | 0.0290 (7) | −0.0010 (5) | 0.0138 (6) | −0.0013 (5) |
| N4 | 0.098 (2) | 0.0262 (10) | 0.0346 (11) | 0.0000 | 0.0362 (13) | 0.0000 |
| C2 | 0.0196 (6) | 0.0293 (7) | 0.0231 (7) | 0.0011 (5) | 0.0064 (5) | −0.0001 (5) |
| C4 | 0.0387 (11) | 0.0260 (10) | 0.0245 (10) | 0.0000 | 0.0124 (9) | 0.0000 |
| F5A | 0.0276 (5) | 0.0583 (7) | 0.0320 (5) | 0.0010 (4) | 0.0043 (4) | 0.0153 (4) |
| O2A | 0.0229 (5) | 0.0448 (7) | 0.0268 (5) | −0.0027 (4) | 0.0070 (4) | 0.0085 (5) |
| N1A | 0.0245 (6) | 0.0405 (7) | 0.0289 (7) | −0.0059 (5) | 0.0106 (5) | 0.0071 (5) |
| N3A | 0.0222 (6) | 0.0289 (6) | 0.0244 (6) | −0.0020 (5) | 0.0096 (5) | 0.0018 (5) |
| N4A | 0.0216 (6) | 0.0349 (7) | 0.0318 (7) | −0.0005 (5) | 0.0099 (6) | 0.0054 (5) |
| C2A | 0.0245 (7) | 0.0299 (7) | 0.0219 (7) | −0.0018 (5) | 0.0101 (6) | −0.0004 (5) |
| C4A | 0.0250 (7) | 0.0249 (7) | 0.0239 (7) | −0.0008 (5) | 0.0112 (6) | −0.0031 (5) |
| C5A | 0.0261 (7) | 0.0334 (8) | 0.0226 (7) | 0.0005 (6) | 0.0065 (6) | 0.0033 (6) |
| C6A | 0.0312 (8) | 0.0387 (8) | 0.0283 (8) | −0.0037 (6) | 0.0111 (6) | 0.0090 (6) |
| F5B | 0.0300 (5) | 0.0569 (6) | 0.0302 (5) | −0.0019 (4) | 0.0041 (4) | 0.0115 (4) |
| O2B | 0.0250 (5) | 0.0298 (5) | 0.0269 (5) | −0.0040 (4) | 0.0085 (4) | 0.0023 (4) |
| N1B | 0.0246 (6) | 0.0310 (6) | 0.0282 (6) | −0.0051 (5) | 0.0114 (5) | 0.0032 (5) |
| N3B | 0.0245 (6) | 0.0270 (6) | 0.0279 (6) | −0.0055 (5) | 0.0119 (5) | −0.0003 (5) |
| N4B | 0.0258 (7) | 0.0408 (8) | 0.0294 (7) | −0.0066 (5) | 0.0092 (6) | 0.0010 (6) |
| C2B | 0.0267 (7) | 0.0226 (6) | 0.0241 (7) | −0.0030 (5) | 0.0119 (6) | −0.0030 (5) |
| C4B | 0.0274 (7) | 0.0254 (7) | 0.0259 (7) | −0.0032 (5) | 0.0122 (6) | −0.0045 (5) |
| C5B | 0.0267 (7) | 0.0341 (8) | 0.0217 (7) | 0.0007 (6) | 0.0072 (6) | 0.0014 (6) |
| C6B | 0.0320 (8) | 0.0328 (8) | 0.0257 (7) | −0.0021 (6) | 0.0130 (6) | 0.0040 (6) |
Geometric parameters (Å, º)
| F5A—C5A | 1.3491 (18) | N4A—C4A | 1.331 (2) |
| F5B—C5B | 1.3492 (18) | N1A—H1A | 0.8800 |
| O2A—C2A | 1.2462 (19) | N4A—H4A2 | 0.91 (2) |
| O2B—C2B | 1.2539 (19) | N4A—H4A1 | 0.86 (2) |
| N1—C2 | 1.3563 (16) | N1B—C2B | 1.3714 (19) |
| N1—C2i | 1.3563 (16) | N1B—C6B | 1.361 (2) |
| N2—C2 | 1.350 (2) | N3B—C4B | 1.338 (2) |
| N3—C2 | 1.365 (2) | N3B—C2B | 1.356 (2) |
| N3—C4 | 1.3751 (18) | N4B—C4B | 1.329 (2) |
| N4—C4 | 1.346 (3) | N1B—H1B | 0.8800 |
| N2—H2A | 0.89 (2) | N4B—H4B1 | 0.88 (2) |
| N2—H2B | 0.84 (2) | N4B—H4B2 | 0.86 (2) |
| N4—H4A | 0.89 (2) | C4A—C5A | 1.426 (2) |
| N4—H4Ai | 0.89 (2) | C5A—C6A | 1.340 (3) |
| N1A—C2A | 1.376 (2) | C6A—H6A | 0.9500 |
| N1A—C6A | 1.352 (2) | C4B—C5B | 1.423 (2) |
| N3A—C4A | 1.335 (2) | C5B—C6B | 1.341 (2) |
| N3A—C2A | 1.356 (2) | C6B—H6B | 0.9500 |
| F5A···C2i | 3.134 (2) | C4B···C4Bix | 3.340 (2) |
| F5A···C6Bii | 3.2444 (19) | C5A···C5Bv | 3.541 (2) |
| F5A···N4A | 2.7560 (18) | C5B···C6Av | 3.432 (2) |
| F5B···N4B | 2.7459 (19) | C5B···C5Av | 3.541 (2) |
| F5B···C6Aiii | 3.148 (2) | C5B···C4Bix | 3.500 (2) |
| F5A···H4A1 | 2.47 (2) | C6A···F5Bii | 3.148 (2) |
| F5A···H6Bii | 2.4300 | C6A···C5Bv | 3.432 (2) |
| F5B···H4B2 | 2.42 (2) | C6B···N3Aix | 3.325 (2) |
| O2A···N1Biv | 2.7545 (19) | C6B···F5Aiii | 3.2444 (19) |
| O2A···N2v | 2.8949 (19) | C6B···N4Bix | 3.442 (2) |
| O2B···N4vi | 2.9600 (15) | C2···H4A1 | 2.69 (2) |
| O2B···N2 | 2.9689 (19) | C2···H4A1i | 3.03 (2) |
| O2B···N4vii | 2.9600 (15) | C2···H4B2v | 2.84 (2) |
| O2B···N1Aviii | 2.773 (2) | C2A···H4B1 | 2.96 (2) |
| O2A···H1Biv | 1.8800 | C2A···H6Bix | 3.0600 |
| O2A···H2Bv | 2.15 (2) | C2A···H1Biv | 2.7700 |
| O2B···H4Avii | 2.09 (2) | C2B···H1Aviii | 2.8000 |
| O2B···H4A2 | 2.84 (3) | C2B···H4Avii | 2.98 (2) |
| O2B···H1Aviii | 1.9000 | C2B···H4A2 | 2.84 (2) |
| O2B···H2A | 2.10 (2) | C2B···H2A | 2.90 (2) |
| N1···N4A | 3.0664 (18) | C4A···H6Axii | 2.9600 |
| N1···N4Ai | 3.0664 (18) | H4A1···C2 | 2.69 (2) |
| N1A···O2Biv | 2.773 (2) | H4A1···N1 | 2.23 (2) |
| N1B···O2Aviii | 2.7545 (19) | H4A1···N2 | 2.93 (2) |
| N2···O2B | 2.9689 (19) | H4A1···N1 | 2.23 (2) |
| N2···O2Av | 2.8949 (19) | H4A1···F5A | 2.47 (2) |
| N3A···N4B | 3.060 (2) | H4A1···C2i | 3.03 (2) |
| N3A···C6Bix | 3.325 (2) | H1A···C2Biv | 2.8000 |
| N3B···N4A | 2.992 (2) | H1A···H1Biv | 2.5600 |
| N4···O2Bx | 2.9600 (15) | H1A···O2Biv | 1.9000 |
| N4···O2Bxi | 2.9600 (15) | H1B···C2Aviii | 2.7700 |
| N4A···N1 | 3.0664 (18) | H1B···O2Aviii | 1.8800 |
| N4A···N1 | 3.0664 (18) | H1B···H1Aviii | 2.5600 |
| N4A···C2 | 3.356 (2) | H4A2···O2B | 2.84 (3) |
| N4A···N3B | 2.992 (2) | H4A2···N3B | 2.10 (2) |
| N4A···F5A | 2.7560 (18) | H4A2···C2B | 2.84 (2) |
| N4B···C4Av | 3.442 (2) | H2A···C2B | 2.90 (2) |
| N4B···N3A | 3.060 (2) | H2A···O2B | 2.10 (2) |
| N4B···F5B | 2.7459 (19) | H2B···O2Av | 2.15 (2) |
| N4B···C6Bix | 3.442 (2) | H4B1···N3A | 2.20 (2) |
| N1···H4A1 | 2.23 (2) | H4B1···C2A | 2.96 (2) |
| N1···H4A1i | 2.23 (2) | H4B2···F5B | 2.42 (2) |
| N2···H4A1 | 2.93 (2) | H4B2···N3v | 2.53 (2) |
| N3···H4B2v | 2.53 (2) | H4B2···C2v | 2.84 (2) |
| N3A···H6Bix | 2.8700 | H4A···O2Bx | 2.09 (2) |
| N3A···H4B1 | 2.20 (2) | H4A···C2Bx | 2.98 (2) |
| N3B···H4A2 | 2.10 (2) | H6A···N4Axiii | 2.7700 |
| N4A···H6Axii | 2.7700 | H6A···C4Axiii | 2.9600 |
| C2···N4A | 3.356 (2) | H6B···F5Aiii | 2.4300 |
| C2···F5Ai | 3.134 (2) | H6B···N3Aix | 2.8700 |
| C4A···N4Bv | 3.442 (2) | H6B···C2Aix | 3.0600 |
| C4B···C5Bix | 3.500 (2) | ||
| C2—N1—C2i | 115.16 (14) | N3—C4—N3i | 122.49 (19) |
| C2—N3—C4 | 116.06 (14) | N3—C4—N4 | 118.75 (11) |
| C2—N2—H2B | 119.3 (16) | O2A—C2A—N1A | 119.37 (15) |
| H2A—N2—H2B | 115 (2) | N1A—C2A—N3A | 119.33 (14) |
| C2—N2—H2A | 121.7 (13) | O2A—C2A—N3A | 121.30 (14) |
| C4—N4—H4A | 118.2 (15) | N3A—C4A—N4A | 119.11 (14) |
| H4A—N4—H4Ai | 124 (2) | N3A—C4A—C5A | 120.14 (15) |
| C4—N4—H4Ai | 118.2 (15) | N4A—C4A—C5A | 120.73 (14) |
| C2A—N1A—C6A | 122.07 (15) | F5A—C5A—C6A | 121.59 (14) |
| C2A—N3A—C4A | 119.96 (13) | F5A—C5A—C4A | 118.77 (15) |
| C6A—N1A—H1A | 119.00 | C4A—C5A—C6A | 119.64 (15) |
| C2A—N1A—H1A | 119.00 | N1A—C6A—C5A | 118.83 (15) |
| C4A—N4A—H4A1 | 121.3 (16) | N1A—C6A—H6A | 121.00 |
| C4A—N4A—H4A2 | 116.3 (16) | C5A—C6A—H6A | 121.00 |
| H4A1—N4A—H4A2 | 120 (2) | O2B—C2B—N3B | 120.96 (14) |
| C2B—N1B—C6B | 121.81 (14) | N1B—C2B—N3B | 119.73 (14) |
| C2B—N3B—C4B | 119.92 (13) | O2B—C2B—N1B | 119.31 (14) |
| C2B—N1B—H1B | 119.00 | N3B—C4B—C5B | 119.89 (16) |
| C6B—N1B—H1B | 119.00 | N4B—C4B—C5B | 120.96 (15) |
| C4B—N4B—H4B2 | 119.0 (17) | N3B—C4B—N4B | 119.15 (14) |
| H4B1—N4B—H4B2 | 126 (2) | C4B—C5B—C6B | 120.04 (14) |
| C4B—N4B—H4B1 | 114.6 (16) | F5B—C5B—C4B | 118.25 (15) |
| N2—C2—N3 | 119.01 (13) | F5B—C5B—C6B | 121.68 (14) |
| N1—C2—N2 | 116.19 (14) | N1B—C6B—C5B | 118.54 (14) |
| N1—C2—N3 | 124.81 (13) | N1B—C6B—H6B | 121.00 |
| N3i—C4—N4 | 118.75 (11) | C5B—C6B—H6B | 121.00 |
| C2i—N1—C2—N2 | −176.73 (13) | C4B—N3B—C2B—O2B | −178.38 (14) |
| C2i—N1—C2—N3 | 3.98 (19) | C2B—N3B—C4B—N4B | −179.21 (14) |
| C4—N3—C2—N1 | −7.5 (2) | C2B—N3B—C4B—C5B | 0.5 (2) |
| C4—N3—C2—N2 | 173.24 (13) | C4B—N3B—C2B—N1B | 2.1 (2) |
| C2—N3—C4—N4 | −176.55 (11) | N3A—C4A—C5A—F5A | 179.63 (13) |
| C2—N3—C4—N3i | 3.45 (18) | N3A—C4A—C5A—C6A | −0.1 (2) |
| C6A—N1A—C2A—O2A | −177.48 (15) | N4A—C4A—C5A—F5A | −2.1 (2) |
| C6A—N1A—C2A—N3A | 2.2 (2) | N4A—C4A—C5A—C6A | 178.19 (15) |
| C2A—N1A—C6A—C5A | −1.3 (2) | F5A—C5A—C6A—N1A | −179.48 (14) |
| C4A—N3A—C2A—O2A | 177.67 (14) | C4A—C5A—C6A—N1A | 0.3 (2) |
| C4A—N3A—C2A—N1A | −2.0 (2) | N3B—C4B—C5B—F5B | 179.56 (14) |
| C2A—N3A—C4A—N4A | −177.35 (14) | N3B—C4B—C5B—C6B | −2.6 (2) |
| C2A—N3A—C4A—C5A | 1.0 (2) | N4B—C4B—C5B—F5B | −0.8 (2) |
| C6B—N1B—C2B—N3B | −2.7 (2) | N4B—C4B—C5B—C6B | 177.13 (15) |
| C2B—N1B—C6B—C5B | 0.6 (2) | F5B—C5B—C6B—N1B | 179.78 (14) |
| C6B—N1B—C2B—O2B | 177.75 (14) | C4B—C5B—C6B—N1B | 2.0 (2) |
Symmetry codes: (i) −x+1, y, −z+1/2; (ii) x, −y+1, z+1/2; (iii) x, −y+1, z−1/2; (iv) x−1/2, y−1/2, z; (v) −x+1/2, −y+1/2, −z; (vi) x, y+1, z; (vii) −x+1, y+1, −z+1/2; (viii) x+1/2, y+1/2, z; (ix) −x+1/2, −y+3/2, −z; (x) −x+1, y−1, −z+1/2; (xi) x, y−1, z; (xii) −x+1/2, y+1/2, −z+1/2; (xiii) −x+1/2, y−1/2, −z+1/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4A—H4A1···F5A | 0.86 (2) | 2.47 (2) | 2.7560 (18) | 100.0 (18) |
| N4A—H4A1···N1 | 0.86 (2) | 2.23 (2) | 3.0664 (18) | 164 (2) |
| N1A—H1A···O2Biv | 0.88 | 1.90 | 2.773 (2) | 173 |
| N1B—H1B···O2Aviii | 0.88 | 1.88 | 2.7545 (19) | 175 |
| N4A—H4A2···N3B | 0.91 (2) | 2.10 (2) | 2.992 (2) | 169 (2) |
| N2—H2A···O2B | 0.89 (2) | 2.10 (2) | 2.9689 (19) | 167.6 (18) |
| N2—H2B···O2Av | 0.84 (2) | 2.15 (2) | 2.8949 (19) | 149 (2) |
| N4B—H4B1···N3A | 0.88 (2) | 2.20 (2) | 3.060 (2) | 169 (2) |
| N4B—H4B2···F5B | 0.86 (2) | 2.42 (2) | 2.7459 (19) | 103 (2) |
| N4B—H4B2···N3v | 0.86 (2) | 2.53 (2) | 3.360 (2) | 162 (2) |
| N4—H4A···O2Bx | 0.89 (2) | 2.09 (2) | 2.9600 (15) | 165 (2) |
| C6B—H6B···F5Aiii | 0.95 | 2.43 | 3.2444 (19) | 143 |
Symmetry codes: (iii) x, −y+1, z−1/2; (iv) x−1/2, y−1/2, z; (v) −x+1/2, −y+1/2, −z; (viii) x+1/2, y+1/2, z; (x) −x+1, y−1, −z+1/2.
References
- Bennet, J. E. (1977). Ann. Intern. Med. 86, 319–21. [DOI] [PubMed]
- Benson, J. M. & Nahata, M. C. (1988). Clin. Pharm. 7, 424–438. [PubMed]
- Cason, C. J. (2004). POV-RAY for Windows. Persistence of Vision, Raytracer Pvt Ltd, Victoria, Australia. URL: http://www.povray.org.
- Desiraju, G. R. (1989). Crystal Engineering. The Design of Organic Solids. Amsterdam: Elsevier.
- Hulme, A. T. & Tocher, D. A. (2006). Cryst. Growth Des. 6, 481–487.
- Janczak, J. & Perpétuo, G. J. (2001a). Acta Cryst. C57, 1431–1433. [DOI] [PubMed]
- Janczak, J. & Perpétuo, G. J. (2001b). Acta Cryst. C57, 1120–1122. [DOI] [PubMed]
- Janczak, J. & Perpétuo, G. J. (2002). Acta Cryst. C58, o339–o341. [DOI] [PubMed]
- Janczak, J. & Perpétuo, G. J. (2004). Acta Cryst. C60, o211–o214. [DOI] [PubMed]
- Karthikeyan, A., Thomas Muthiah, P. & Perdih, F. (2014). Acta Cryst. E70, 328–330. [DOI] [PMC free article] [PubMed]
- Larsen, R. A., Kauffman, C. A., Pappas, P. G., Sobel, J. D. & Dismukes, W. E. (2003). In Essentials of Clinical Mycology, 2nd ed., pp. 57–60. Oxford University Press. UK.
- Louis, T., Low, J. N. & Tollin, P. (1982). Cryst. Struct. Commun. 11, 1059–1064.
- MacDonald, J. C. & Whitesides, G. M. (1994). Chem. Rev. 94, 2383–2420.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Morschhäuser, J. (2003). Pharm. Unserer Zeit, 32, 124–128. [DOI] [PubMed]
- Mullen, C. A., Coale, M. M., Lowe, R. & Blaese, R. M. (1994). Cancer Res. 54, 1503–1506. [PubMed]
- Perpétuo, G. J., Ribeiro, M. A. & Janczak, J. (2005). Acta Cryst. E61, o1818–o1820.
- Perumalla, S. R., Pedireddi, V. R. & Sun, C. C. (2013a). Cryst. Growth Des. 13, 429–432.
- Perumalla, S. R., Pedireddi, V. R. & Sun, C. C. (2013b). Mol. Pharm. 10, 2462–2466. [DOI] [PubMed]
- Polak, A. & Scholer, H. J. (1980). Rev. Inst. Pasteur Lyon. 13, 233–244.
- Portalone, G. (2011). Chem. Cent. J. 5, 51. [DOI] [PMC free article] [PubMed]
- Portalone, G. & Colapietro, M. (2006). Acta Cryst. E62, o1049–o1051.
- Prabakaran, P., Murugesan, S., Muthiah, P. T., Bocelli, G. & Righi, L. (2001). Acta Cryst. E57, o933–o936. [DOI] [PubMed]
- Rigaku/MSC (2008). CrystalClear. Rigaku Americas Corporation, The Woodlands, Texas, USA.
- Russell, K. C., Lehn, J. M., Kyritsakas, N., DeCian, A. & Fischer, J. (1998). New J. Chem. 22, 123–128.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Silva, C. C. P. da, de Oliveira, R., Tenorio, J. C., Honorato, S., Ayala, A. P. & Ellena, J. (2013). Cryst. Growth Des. 13, 4315–4322.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tassel, D. & Madoff, A. (1968). J. Am. Med. Assoc. 206, 830–832.
- Tutughamiarso, M., Bolte, M. & Egert, E. (2009). Acta Cryst. C65, o574–o578. [DOI] [PubMed]
- Tutughamiarso, M., Wagner, G. & Egert, E. (2012). Acta Cryst. B68, 431–443. [DOI] [PubMed]
- Vermes, A., Guchelaar, H. J. & Dankert, J. (2000). J. Antimicrob. Chemother. 46, 171–179. [DOI] [PubMed]
- Wang, G., Wu, W. & Zhuang, L. (2007). Acta Cryst. E63, m2552–m2553.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Whitesides, G. M., Mathias, J. P. & Seto, C. T. (1991). Science, 254, 1312–1319. [DOI] [PubMed]
- Zerkowski, J. A. & Whitesides, G. M. (1994). J. Am. Chem. Soc. 116, 4298–4304.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S205698901600476X/hg5470sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901600476X/hg5470Isup2.hkl
Supporting information file. DOI: 10.1107/S205698901600476X/hg5470Isup3.cml
CCDC reference: 1469709
Additional supporting information: crystallographic information; 3D view; checkCIF report