Abstract
Background:
Brain acid soluble protein 1 (BASP1) is identified as a novel potential tumor suppressor in several cancers. However, its role in thyroid cancer has not been investigated yet. In the present study, the antitumor activities of BASP1 against the growth and migration of thyroid cancer cells were evaluated.
Methods:
BASP1 expression in thyroid cancer tissues and normal tissues were examined by immunohistochemical staining and the association between its expression and prognosis was analyzed. pcDNA-BASP1 carrying full length of BASP1 cDNA was constructed to restore the expression of BASP1 in thyroid cancer cell lines (BHT-101 and KMH-2). The cell proliferation in vitro and in vivo was evaluated by WST-1 assay and xenograft tumor models, respectively. Cell cycle distribution after transfection was analyzed using flow cytometry. Cell apoptosis after transfection was examined by annexin V/propidium iodide assay. The migration was examined using transwell assay.
Results:
BASP1 expression was abundant in normal tissues while it is significantly decreased in cancer tissues (P = 0.000). pcDNA-BASP1 restored the expression of BASP1 and significantly inhibited the growth of BHT-101 and KMH-2 cells as well as xenograft tumors in nude mice (P = 0.000). pcDNA-BASP1 induced G1 arrest and apoptosis in BHT-101 and KMH-2 cells. In addition, pcDNA-BASP1 significantly inhibited the cell migration.
Conclusions:
Downregulation of BASP1 expression may play a role in the tumorigenesis of thyroid cancer. Restoration of BASP1 expression exerted extensive antitumor activities against growth and migration of thyroid cancer cells, which suggested that BASP1 gene might act as a potential therapeutic agent for the treatment of thyroid cancer.
Keywords: Brain Acid Soluble Protein 1, Gene Transfection, Proliferation, Thyroid Cancer
INTRODUCTION
Thyroid cancer is the most common malignancy of the neck and head. Increasing epidemiology studies showed an increased incidence of thyroid cancer, which is responsible for more deaths than all other endocrine cancers.[1,2] Malignant tumors of the thyroid are subdivided into thyroid-specific tumors which are exclusively origined from the thyroid including papillary, follicular, and medullary carcinomas and those which are also found in other organs including lymphomas and sarcomas. Majority of differentiated thyroid cancer (including papillary and follicular) are indolent neoplasms with better prognosis after treatment with surgery and adjuvant radioactive iodine, while poorly differentiated and undifferentiated carcinomas (including medullary and anaplastic) which display aggressive biological and clinical features have a poor prognosis with survival <1 year in most cases. Moreover, due to high propensity to recur and metastasize, there are few therapeutic options for poorly differentiated and undifferentiated cases.[3,4] Over the past few decades, many studies mainly focused on the treatment for thyroid cancer; few studies investigated the tumorigenesis and pathogenesis of thyroid cancer which should be paid more attention. Activation of oncogenes and inactivation of tumor suppressor genes play an important role in tumorigenesis of thyroid cancer. As the earliest discovery of epigenetic modification, DNA methylation is a main cause for inactivation of tumor suppressor gene. In normal cells, the transcription level of tumor suppressor gene is stable, which is able to perform antitumor effect and maintain the normal state and functions of cells. However, in tumor cells, the CpG islands in the promoter of tumor suppressor gene are always hypermethylated which block the transcription, inactivate tumor suppressor gene, and lead to tumorigenesis.[5,6]
Brain acid soluble protein 1 (BASP1) is a 23,000 protein originally isolated from brain extracts. BASP1 contains an effector domain that dynamically binds to the plasma membrane and involves in neuronal sprouting process.[7] Later studies have identified BASP1 as a component of the WT1 co-suppressor.[8] WT1 gene is abnormally high expressed in a variety of tumors including nonsmall cell lung cancer, thyroid cancer, breast cancer, and head and neck squamous cell carcinoma, which is considered to have the characteristics of oncogene.[9,10,11,12] WT1 can promote migration, invasion and angiogenesis of tumors through promoting tumor cell proliferation, inhibiting apoptosis, and interacting with cytoskeletal proteins.[13] However, the relationship between BASP1 gene and biology of thyroid cancer has not been reported yet. In the present study, we examined the BASP1 expression level in thyroid cancer and analyzed its association with clinical features. We also investigated the role of BASP1 in thyroid cancer proliferation, apoptosis and migration through over-expression of BASP1 by cDNA transfection.
METHODS
Patient samples
Forty-four patients (16 males and 28 females) with thyroid cancer were enrolled from Department of General Surgery, Changzheng Hospital, Second Military Medical University between 2011 and 2012, the average age of these patients was 61.6 ± 8.9 years. Fresh samples of tumor tissue and normal tissue adjacent to carcinoma were obtained from these patients who underwent radical thyroid resection plus neck lymph nodes clean-up surgery. The samples were stored at −80°C immediately after resection. Among these patients, 24 patients had local infiltration and cervical lymph node metastases. No patients were treated with chemotherapy prior to surgery. Normal tissues were used as negative controls. All patients were followed up 3 years after the operation. This study was approved by the Ethical Committee of Second Military Medical University, and all patients provided informed written consent before participating in this study.
Immunohistochemistry
Frozen sections were cut at 5 μm thickness and fixed in cold acetone for 15 min at 4°C and then rinsed with phosphate buffered saline (PBS) for 5 min. Then, the slides were treated with 3% hydrogen peroxide for 20 min for blocking peroxidase in the tissue and subsequently rinsed well with PBS. The slides then were incubated with 10 mmol/L sodium citrate solution for antigen retrieval. The sections were preincubated with goat serum, then incubated with monoclonal antibody to BSAP1 overnight at 4°C. After washing in PBS for 5 min, the slides were incubated for 30 min with the secondary antibody. Then, after three washing in PBS for 5 min, a brown staining was produced by treating the slides with 3,3-diaminobenzidine.
Cell culture and treatment
Human anaplastic thyroid carcinoma cell lines BHT-101 and KMH-2 were purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China).[14,15] Normal human thyroid follicular cell line Nthy-ori 3-1 was purchased from the Institute of European Collection of Cell Cultures (ECACC, UK). Nthy-ori 3-1 was grown at 37°C in complete RPMI 1640 (GIBCO Inc., MD, USA). BHT-101 and KMH-2 cells were maintained at 37°C in Dulbecco's modified Eagle's medium (GIBCO Inc.). All media were supplemented with 10% fetal bovine serum (Hyclone, UT, USA), 1 mmol/L nonessential amino acid and 1% penicillin/streptomycin (Sigma-Aldrich Co., MO, USA) in 5% CO2 incubator. Subcultures were maintained at 80% confluence and passaged by 0.25% Trypsin (GIBCO Inc.).
Plasmids vectors and transfection
Full-length human BASP1 were excised and cloned into the pcDNA3.1 vectors (Invitrogen, CA, USA). Plasmids were isolated from appropriate colonies, and the correct plasmid constructs were confirmed by DNA sequencing. In brief, to produce BASP1 overexpressed cells, BHT-101 and KMH-2 cells were cultured in 12-well plates for 24 h, then 5 μg/ml pcDNA-BASP1 or pcDNA3.1 was added. After 24 h transfection, cells were cultured in complete media overnight. To screen out stable transfected cells lines, cells were subcultured in 25 cm2 flasks with 5 μg/ml puromycin (Sigma-Aldrich Co.) for 2 weeks. Stable cell lines expressing the empty pcDNA3.1 plasmid were used as controls.
Reverse transcription-polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen, CA, USA), and cDNA was synthesized using the SuperScript First-Strand Synthesis System (Invitrogen) for reverse transcription-polymerase chain reaction (RT-PCR) according to the manufacturer's instructions. The resulting cDNA was amplified by PCR using gene specific primers with PrimeScriptTM RT Master Mix (Takara Bio Inc., Japan). Reactions were run on an ABI PRISM 5700 Sequence Detector (PE Applied Biosystems, Foster City, CA, USA). The cycling conditions comprised 10 min polymerase activation at 95°C and 40 cycles at 95°C for 10 s and 60°C for 20 s. BASP1 primer sequences were as fellow: forward, 5’-CTT CAG ACT CAA AAC CCG GC-3’; reverse, 5’-ACG GTT TGG TCG GAA TTA GC-3’(684 bp).
WST-1 assay
The viability of cells was analyzed using a colorimetric assay for quantification of the cleavage of the tetrazolium salt WST-1 (Beyotime Institute of Biotechnology, Shanghai, China) by mitochondrial dehydrogenases. The resulting dye can be quantified by a spectrophotometer and directly correlated with the number of metabolically active cells in the culture. For the test, 1 × 104 BHT-101 and KMH-2 cells were seeded in each well of 96-well microplates for 12 h. Cells were divided into three groups: cells treated with pcDNA-BASP1, cells treated with pcDNA3.1, and blank control. After incubation for 24 h, 48 h, and 72 h at 37°C, the culture medium was removed and the cells were rinsed twice with PBS. Then, 100 μl of culture medium containing 10 vol. % WST-1 reagent was added to each well. The absorbance of WST-1-derived formazan was measured using a Model 550 Microplate Reader (Bio-Rad Laboratories, Hercules, CA, USA) at 450 nm. Cell survival rate = (OD experiment group − OD background)/(OD control group − OD background) ×100%.
Xenografts in nude mice
To evaluate the effects of BASP1 on proliferation of BHT-101 and KMH-2 cells in vivo, mouse models with BHT-101 and KMH-2 xenografts were used. Animal care and experimental procedures at the animal experiment center of Second Military Medical University were performed according with Council Directive 86/609/EEC of 24 November 1986 and were approved by the Ethical Commission of the Second Military Medical University. A total of 36 BALB/c nude male mice (4-week-old) were used in the experiments. They were maintained in a 12 h light/dark cycle and were given free access to a standard food and water at all times in specific-pathogen-free (SPF) environment. Animals were housed for at least 7 days before being used for experiments under isoflurane anesthesia. Mice were divided into three groups: group treated with stable pcDNA-BASP1 transfected cells; group treated with stable pcDNA3.1 transfected cells; and group treated with PBS. Stable transfected BHT-101 and KMH-2 cells (1 × 107 cells/mouse) were injected subcutaneously into the right flanks of the mice. Tumors were formed 2–3 weeks after inoculation. The length (L) and width (W) of xenograft tumors were measured every week using calipers. The volume of xenograft tumors was calculated using following formula: V (cm3) = (L × W2)/2. On the 7th week, mice were sacrificed and xenograft tumors were weighted (g).
Cell cycle analysis
To determine the effect of BASP1 on the cell cycle of anaplastic thyroid cancer, BHT-101 and KMH-2 cells were seeded in a 6-well plate overnight, then pcDNA-BASP1 was added and incubated with cells for 48 h. After washed twice by PBS, the cells were fixed in 70% precooling ethanol overnight. The cells were then treated with RNaseA (50 μg/ml) and stained with propidium iodide (PI) (Sigma-Aldrich Co.) as per manufacturer's instructions. Samples were run on an FACSCalibur and analyzed their DNA content using Flowjo software (Tree Star Inc., Ashland, OR, USA).
Transwell assays
Transwell test (Corning, NY, USA) was used to detect the changing migration ability of BHT101 and KMH-2 cells transfected pcDNA-BASP1. Briefly, cells were then trypsinized and resuspended in serum-free medium. A cell suspension (5 × 104 cells in 200 μl of medium) was added to the Transwell inserts. After 24 h of incubation in complete medium, cells that passed through the filters were stained with 0.1% crystal violet for at least 5 min. The noninvading cells were then wiped from the inside of the inserts with a cotton swab. Cells on the underside of the membranes that had invaded the Matrigel were counted under a microscope.
Apoptosis analysis
Apoptosis was detected using FITC-Annexin V/PI double staining assay (BD Biosciences, CA, USA). FITC-Annexin V (green fluorescence) and the nonvital dye PI (red fluorescence) allowed the discrimination of intact cells (FITC– PI–), early apoptotic (FITC+ PI−), and late apoptotic or necrotic cells (FITC+ PI+). Here, we used them for apoptosis analysis of BHT101 and KMH-2 cells transfected pcDNA-BASP1. Briefly, cells transfected pcDNA-BASP1 for 72 h were trypsinized, then rinsed with precooled PBS and resuspended in Annexin V-FITC binding buffer, Annexin V-FITC and PI were added to the cell suspension. The cell suspension was gently vortexing and then incubated for 5 min at 4°C protected from light. After that, samples were analyzed by an FACSCalibur within 1 h.
Western blotting analysis
Western blotting was used to analysis protein expression levels. Cells were extracted according to the manufacturer's instructions, and the concentration was determined by the Bradford assay. The 30 μg protein samples were separated on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a 0.2 μm polyvinylidene difluoride membrane. The membrane was blocked in 5% nonfat milk. Membranes were probed with primary antibody (1:1000) (Santa Cruz Biotechnology, CA, USA) overnight at 4°C, washed 3 times in Tris-buffered saline (TBST), incubated with anti-mouse or anti-rabbit horseradish peroxidase antibody (1:5000) (Santa Cruz Biotechnology) for 2 h at room temperature, and then washed 3 times in TBST. The signal was visualized by an enhanced chemiluminescence solution (Amersham Pharmacia Biotech, Uppsala, Sweden) and was exposed to X-Omat LS film (Eastman Kodak Co., Rochester, NY, USA).
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA), and data were expressed as a mean ± standard deviation (SD). The Student's t-test was used to compare differences between the means of two groups. One-way analysis of variance was used to compare differences between the means of three groups. Kaplan-Meier analysis was used to analyze survival between groups. All experiments reported in this study were performed independently at least 3 times. Statistical differences were considered significantly if the P < 0.05.
RESULTS
Low expression of brain acid soluble protein 1 in thyroid cancer tissues and cell lines
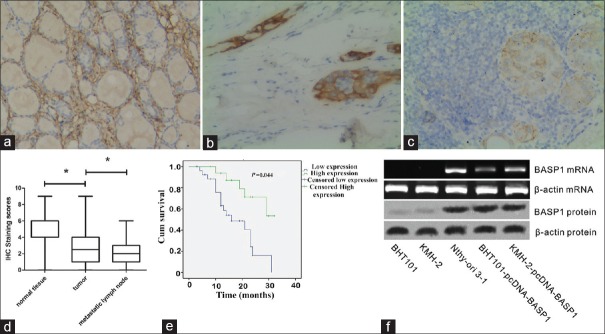
BASP1 expression was analyzed in thyroid cancer tissues and cell lines by immunohistochemistry, RT-PCR, and Western blotting. As shown in Figure 1a–1d, immunohistochemistry scores of BASP1 in thyroid carcinoma and lymph node tissues were significantly lower than that of adjacent nontumor tissues (F = 52.155, P = 0.000). In addition, a positive correlation between BASPA1 expression and prognosis was observed. Kaplan-Meier analysis showed that survival of patients with high BASP1 expression was significantly longer than those with low BASP1 expression (χ2 = 4.052, P = 0.044) [Figure 1e].
Figure 1.
IHC staining of BASP1 in normal thyroid tissues (a), thyroid cancer tissues (b), and metastatic lymph nodes (c; original magnification ×100). (d) IHC scores of BASP1 in normal tissues, cancer tissues, and metastatic lymph nodes. *P < 0.05. (e) The Kaplan-Meier analysis of overall survival of thyroid cancer patients according to the expression of BASP1 level. (f) Western blotting and reverse transcription-polymerase chain reaction analyses confirmed the BASP1 expression in Nthy-ori 3-1 cells, while no expression of BASP1 in BHT101 and KMH-2 cells. pcDNA-BASP1 restored the expression of BASP1. BASP1: Brain acid soluble protein 1; IHC: Immunohistochemical.
As shown in the results of RT-PCR and Western blotting analysis [Figure 1f], the expression of BASP1 mRNA and protein levels were absent in BHT101 and KMH-2 cells. Gene transfection with pcDNA-BASP1 significantly increased the expression of BASP1 mRNA and protein levels in BHT101 and KMH-2 cells.
Overexpression of brain acid soluble protein 1 significantly inhibited the proliferation of BHT101 and KMH-2 cells in vitro and in vivo
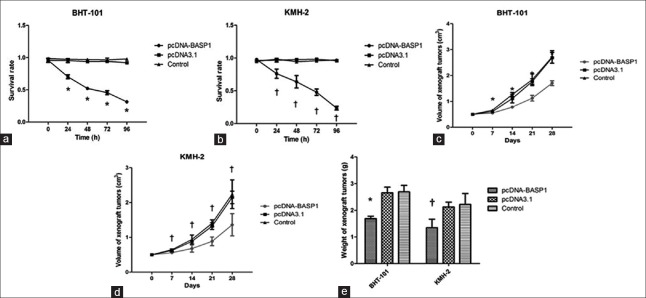
The effects of BASP1 overexpression on proliferation in vitro were examined using WST-1 assay. As shown in Figure 2, pcDNA-BASP1 showed significant time-dependent inhibitory activities against proliferation. Cell viabilities of BHT101 and KMH-2 cells were significantly decreased after treatment with pcDNA-BASP1 (BHT-101: F = 10.988, P = 0.002; KMH-2: F = 7.608, P = 0.007), while pcDNA3.1 did not affect proliferation (P > 0.05).
Figure 2.
Overexpression of BASP1 significantly inhibited the proliferation of BHT101 and KMH-2 cells in vitro and in vivo. (a) Overexpression of BASP1 significantly inhibited the proliferation of BHT101 cells in vitro. (b) Overexpression of BASP1 significantly inhibited the proliferation of KMH-2 cells in vitro. (c) In vivo tumor volume of transplanted BASP1 overexpressed BHT101 cells. (d) In vivo tumor volume of transplanted BASP1 overexpressed KMH-2 cells. (e) In vivo tumor weight of transplanted BASP1 overexpressed BHT101 and KMH-2 cells. *P < 0.05, pcDNA-BASP1 versus pcDNA3.1; †P < 0.05, pcDNA-BASP1 versus the controls. BASP1: Brain acid soluble protein 1.
Meanwhile, overexpression of BASP1 also showed apparent inhibitory activities against proliferation and tumorigenicity in vivo. The numbers of xenograft tumors formed by pcDNA-BASP1-transfected cells were less than those formed by the cells transfected with pcDNA3.1 or control (7/12, 12/12, and 12/12). In addition, the size and weight of tumors in pcDNA-BASP1-transfected group were much less than those of control groups (size: BHT-101: F = 53.894, P = 0.000; KMH-2: F = 13.848, P = 0.000. Weight: BHT-101: F = 42.386, P = 0.000; KMH-2: F = 9.235, P = 0.000). These results suggested that overexpression of BASP1 could inhibit thyroid tumor growth in vitro and in vivo.
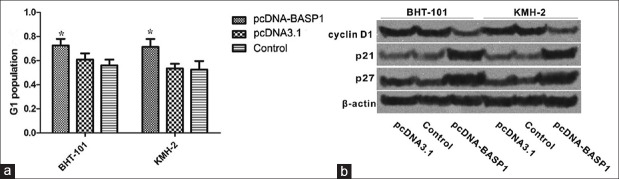
Overexpression of brain acid soluble protein 1 induces G1 Phase Arrest in BHT101 and KMH-2 cells
As shown in Figure 3, after transfected with pcDNA-BASP1 for 48 h, G1 populations of BHT101 and KMH-2 cells were significantly increased compared with those of control group (BHT-101: F = 38.103, P = 0.000; KMH-2: F = 42.222, P = 0.000), indicating that overexpression of BASP1 could induce G1 arrest. In addition, the expressions of p21 and p27 which play the important roles in G1/S transition were increased after treatment with pcDNA-BASP1, while the expression of cyclin D1 was significantly decreased.
Figure 3.
Overexpression of BASP1 induced G1 phase arrest in BHT101 and KMH-2 cells. (a) G1 population of BHT101 and KMH-2 cells after treatments analyzed by FACS. Overexpression of BASP1 induced G1 phase arrest. *P < 0.05, pcDNA-BASP1 versus the controls. (b) Western blotting analysis for the expressions of cyclin D1, p21, and p27. BASP1: Brain acid soluble protein 1.
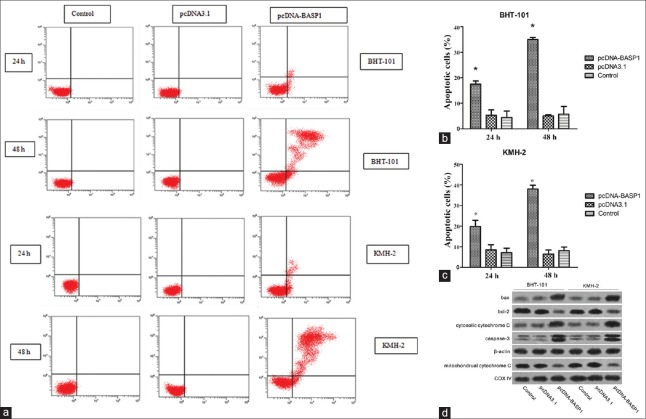
Overexpression of brain acid soluble protein 1 induced apoptosis in BHT101 and KMH-2 cells
The effects of BASP1 overexpression on apoptosis of BHT101 and KMH-2 cells were further examined using Annexin V/PI assay. As shown in Figure 4, BASP1 overexpression caused apoptosis induction in a time-dependant manner. After transfected with pcDNA-BASP1 for 24 h, early apoptotic population was significantly increased in both BHT-101 and KMH-2 cells (BHT-101: F = 26.722, P = 0.001; KMH-2: F = 21.867, P = 0.002), and late apoptotic population was significantly increased after treatment for 48 h (BHT-101: F = 270.663, P = 0.000; KMH-2: F = 276.767, P = 0.000). In addition, the expressions of several apoptosis-associated proteins were detected using immunoblotting. The expressions of bax, cleaved caspase-3, and cytosolic cytochrome C were increased, while the expressions of bcl-2 and mitochondrial cytochrome C were significantly decreased.
Figure 4.
(a) The fluorescence intensity of cells staining with annexin V/FITC and propidium iodide. (b) The proportion of apoptotic cell death significantly increased in BHT-101 cells transfected with pcDNA-BASP1 for 24 h or 48 h. (c) The proportion of apoptotic cell death significantly increased in KMH-2 cells transfected with pcDNA-BASP1 for 24 h or 48 h. (d) Overexpression of BASP1 induced the expression of bax and cytosolic cytochrome C, while decreased bcl-2 and mitochondrial cytochrome C expression. PARP, the substrate of caspase-3, was also detected. *P < 0.05, pcDNA-BASP1 versus the control. BASP1: Brain acid soluble protein 1; PARP: Poly ADP-ribose polymerase.
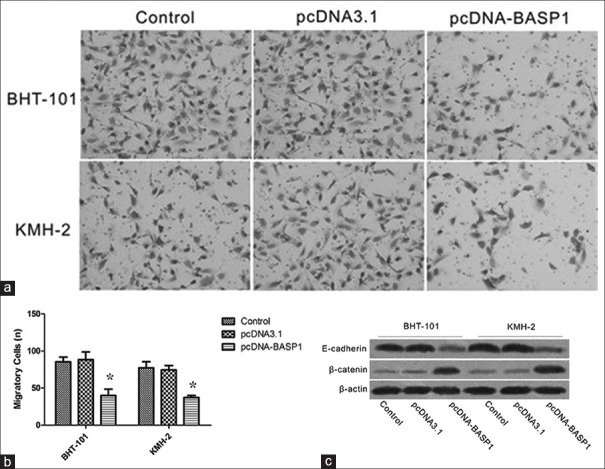
Overexpression of brain acid soluble protein 1 attenuates BHT101 and KMH-2 cell migration
The effects of BASP1 overexpression on migration were examined using Transwell assay. The results showed that the numbers of migratory cells transfected with pcDNA-BASP1 were much less than those of control group, indicating that expression of BASP1 could attenuate BHT101 and KMH-2 cells migration [Figure 5]. In addition, expression of E-cadherin protein was induced after treatment with pcDNA-BASP1, while the expression of β-catenin was significantly decreased.
Figure 5.
Overexpression of BASP1 attenuated BHT101 and KMH-2 cells invasion. (a) Cells stained with crystal violet. (b) Less pcDNA-BASP1 transfected BHT101 and KMH-2 cells migrated and invaded to the bottom chamber, suggested decreased invasiveness. *P < 0.05, pcDNA-BASP1 versus the control. (c) Western blotting showed the reduced β-catenin and increased E-cadherin expression in BASP1 overexpressed BHT101 and KMH-2 cells. BASP1: Brain acid soluble protein 1.
DISCUSSION
Thyroid cancer accounts for approximately 90% of all malignant endocrine tumors and its incidence is increasing worldwide. It was reported that the incidence of thyroid cancer increased by 6.6%, ranking first among all malignant tumors during 2002–2009.[16,17] It is of great importance to investigate the molecular mechanisms underlying thyroid cancer tumorigenesis, which might pave the way to establish effective targeted therapies for advanced thyroid cancers. Increasing evidence showed that inactivation of tumor suppressor genes such as p16 and RASSF1A may play important roles in tumorigenesis of thyroid cancer, and these genes are being paid more and more attention and becoming a hot field for cancer research.[18,19] In the present study, we investigated the biological activities of BASP1 in thyroid cancer through in vitro and in vivo functional studies. We hoped that this investigation might provide a potential novel target for gene therapy in thyroid cancer.
BASP1 expression is found to be downregulated in several cancers including hepatocellular carcinoma, prostate cancer, and melanoma.[20,21,22] In the present study, we also found BASP1 expression was downregulated in thyroid cancer tissues and this downregulation was correlated with poorer prognosis, which indicated BASP1 acted as tumor suppressor in thyroid cancer. Neither BASP1 mRNA nor protein was detected in BHT101 and KMH-2 cells, indicating that the absence of BASP1 expression may be attributed to pretranscriptional deregulation. As we know, eukaryotic pretranscriptional deregulation includes gene loss, amplification, rearrangement, integration, and DNA modification. For tumor suppressor genes, promoter hypermethylation is the most common cause for inactivation of tumor suppressor gene and it has been documented that BASP1 promoter was aberrantly methylated in hepatocellular carcinoma and melanoma tissues. Although we have not detailly revealed the mechanism for BASP1 downregulation in thyroid cancer, it is undeniable that its downregulation was associated with development and prognosis of thyroid cancer. In the future study, we will explore the mechanisms for downregulation of BASP1 in thyroid cancer, which may help to find an effective way to re-activate its function and exert its antitumor activities.
Subsequently, we conducted a series of functional study to evaluate its antitumor activities. For one thing, overexpression of BASP1 can inhibit the expression of cyclin D1, promote the expression of p21 and p27, and induce G1 phase arrest. For another, it can increase the ratio of bax/bcl-2 expressions, promote cytochrome C release from cytoplasm to mitochondria, and activate caspase-3, which finally result in mitochondrial apoptosis. At present, the exact mechanisms for antitumor activities of BASP1 had not been clarified. Some researchers found that BASP1 could inhibit v-Myc-mediated cell transformation, and block its pro-oncogenic effects on down-stream target.[23] Other researchers found BASP1 regulated gene transcription through acting as a co-suppressor for WT1, which is usually abnormally high expressed in a variety of malignant tumors and identified as an oncogene.[24] The exact mechanism for apoptosis induction and cell cycle arrest caused by BASP1 overexpression was not examined in the present study and will be clarified in our next study.
In addition, it is documented that WT1 could activate the Wnt/β-catenin signaling pathway and promote epithelial-mesenchymal transition (EMT) as well as migration and invasion in cancers, while BASP1 could block these biological activities.[25] Therefore, we examined the effect of BASP1 on the migration of BHT-101 and KMH-2 cells. Moreover, we found that BASP1 can inhibit the migration, suppress the expression of β-catenin, and induce the expression of E-cadherin. Downregulation of E-cadherin is usually observed in many cancers, which is a key step for the initiation of EMT.[26] These results suggested that BASP1 may inhibit the migration by modifying the expression of β-catenin and E-cadherin. However, whether this inhibition was mediated by its interaction with WT1 are supposed to be clarified in future studies.
In summary, BASP1 gene was involved in thyroid cancer cell proliferation, cell cycle, apoptosis, and migration. Restoration of BASP1 expression exerted inhibitory activities against thyroid cancer growth and migration, which suggested BASP1 may serve as an effective target for thyroid cancer therapy.
Financial support and sponsorship
This study was supported by a grant from the Shanghai Medical Key Specialist Construction Plans (No. ZK2015B10).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: Why is incidence increasing? Curr Opin Oncol. 2015;27:1–7. doi: 10.1097/CCO.0000000000000148. doi: 10.1097/CCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: Diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:955–69. doi: 10.1016/j.beem.2008.09.017. doi: 10.1016/j.beem.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLellis RA. Pathology and genetics of thyroid carcinoma. J Surg Oncol. 2006;94:662–9. doi: 10.1002/jso.20700. doi: 10.1002/jso.20700. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Mao H, Lv Z. MicroRNA role in thyroid cancer pathogenesis. Front Biosci (Landmark Ed) 2013;18:734–9. doi: 10.2741/4135. doi: 10.2741/4135. [DOI] [PubMed] [Google Scholar]
- 6.Iurlaro R, León-Annicchiarico CL, Muñoz-Pinedo C. Regulation of cancer metabolism by oncogenes and tumor suppressors. Methods Enzymol. 2014;542:59–80. doi: 10.1016/B978-0-12-416618-9.00003-0. doi: 10.1016/B978-0-12-416618-9.00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Toska E, Shandilya J, Goodfellow SJ, Medler KF, Roberts SG. Prohibitin is required for transcriptional repression by the WT1-BASP1 complex. Oncogene. 2014;33:5100–8. doi: 10.1038/onc.2013.447. doi: 10.1038/onc.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter B, Hill KJ, Charalambous M, Wagner KJ, Lahiri D, James DI, et al. BASP1 is a transcriptional cosuppressor for the Wilms’ tumor suppressor protein WT1. Mol Cell Biol. 2004;24:537–49. doi: 10.1128/MCB.24.2.537-549.2004. doi: 10.1128/MCB.24.2.537-549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C, Wang Y, Xia Y, He S, Wang Z, Chen Y, et al. Wilms’ tumor 1 enhances cisplatin-resistance of advanced NSCLC. FEBS Lett. 2014;588:4566–72. doi: 10.1016/j.febslet.2014.10.026. doi: 10.1016/j.febslet.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Oji Y, Miyoshi Y, Koga S, Nakano Y, Ando A, Nakatsuka S, et al. Overexpression of the Wilms’ tumor gene WT1 in primary thyroid cancer. Cancer Sci. 2003;94:606–11. doi: 10.1111/j.1349-7006.2003.tb01490.x. doi: 10.1111/j.1349-7006.2003.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi XW, Zhang F, Yang XH, Fan LJ, Zhang Y, Liang Y, et al. High Wilms’ tumor 1 mRNA expression correlates with basal-like and ERBB2 molecular subtypes and poor prognosis of breast cancer. Oncol Rep. 2012;28:1231–6. doi: 10.3892/or.2012.1906. doi: 10.3892/or.2012.1906. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Ottosson S, Wang S, Jernberg E, Boldrup L, Gu X, et al. Wilms’ tumor gene 1 regulates p63 and promotes cell proliferation in squamous cell carcinoma of the head and neck. BMC Cancer. 2015;15:342. doi: 10.1186/s12885-015-1356-0. doi: 10.1186/s12885-015-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiyama H. WT1 (Wilms’ tumor gene 1): Biology and cancer immunotherapy. Jpn J Clin Oncol. 2010;40:377–87. doi: 10.1093/jjco/hyp194. doi: 10.1093/jjco/hyp194. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese M, Fortunati N, Germano A, Asioli S, Marano F, Palestini N, et al. Histone deacetylase inhibition affects sodium iodide symporter expression and induces 131I cytotoxicity in anaplastic thyroid cancer cells. Thyroid. 2013;23:838–46. doi: 10.1089/thy.2012.0359. doi: 10.1089/thy.2012.0359. [DOI] [PubMed] [Google Scholar]
- 15.Sekiguchi M, Shiroko Y, Arai T, Kishino T, Sugawara I, Kusakabe T, et al. Biological characteristics and chemosensitivity profile of four human anaplastic thyroid carcinoma cell lines. Biomed Pharmacother. 2001;55:466–74. doi: 10.1016/s0753-3322(01)00087-7. [DOI] [PubMed] [Google Scholar]
- 16.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99. doi: 10.1038/nrc3431. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Pei R, Lu Z, Rao X, Liu B. Methylation of p16 CpG islands correlated with metastasis and aggressiveness in papillary thyroid carcinoma. J Chin Med Assoc. 2013;76:135–9. doi: 10.1016/j.jcma.2012.11.007. doi: 10.1016/j.jcma.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Brown TC, Juhlin CC, Healy JM, Prasad ML, Korah R, Carling T. Frequent silencing of RASSF1A via promoter methylation in follicular thyroid hyperplasia: A potential early epigenetic susceptibility event in thyroid carcinogenesis. JAMA Surg. 2014;149:1146–52. doi: 10.1001/jamasurg.2014.1694. doi: 10.1001/jamasurg.2014.1694. [DOI] [PubMed] [Google Scholar]
- 20.Moribe T, Iizuka N, Miura T, Stark M, Tamatsukuri S, Ishitsuka H, et al. Identification of novel aberrant methylation of BASP1 and SRD5A2 for early diagnosis of hepatocellular carcinoma by genome-wide search. Int J Oncol. 2008;33:949–58. doi: 10.3892/ijo_00000082. [PubMed] [Google Scholar]
- 21.Kaehler KC, Politz O, Henderson D, Ulbrich HF, Hauschild A, Mund C, et al. Novel DNA methylation markers with potential prognostic relevance in advanced malignant melanoma identified using COBRA assays. Melanoma Res. 2015;25:225–31. doi: 10.1097/CMR.0000000000000150. doi: 10.1097/CMR.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 22.Lin HY, Kuo YC, Weng YI, Lai IL, Huang TH, Lin SP, et al. Activation of silenced tumor suppressor genes in prostate cancer cells by a novel energy restriction-mimetic agent. Prostate. 2012;72:1767–78. doi: 10.1002/pros.22530. doi: 10.1002/pros.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartl M, Nist A, Khan MI, Valovka T, Bister K. Inhibition of Myc-induced cell transformation by brain acid-soluble protein 1 (BASP1) Proc Natl Acad Sci U S A. 2009;106:5604–9. doi: 10.1073/pnas.0812101106. doi: 10.1073/pnas.0812101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi Y, Wang L, Bian S, Meng Q, Chen G, Wang J. Effect of WT1 gene expression on cell growth and proliferation in myeloid leukemia cell lines. Chin Med J. 1999;112:705–8. [PubMed] [Google Scholar]
- 25.Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2015;54(Suppl 1):E148–61. doi: 10.1002/mc.22221. doi: 10.1002/mc.22221. [DOI] [PubMed] [Google Scholar]
- 26.Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI, et al. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatology. 2015;15:217–25. doi: 10.1016/j.pan.2015.02.011. doi: 10.1016/j.pan.2015.02.011. [DOI] [PubMed] [Google Scholar]